Distinct kinetics and dynamics of cross-presentation in liver sinusoidal endothelial cells compared to dendritic cells†
Potential conflict of interest: Nothing to report.
Abstract
Cross-presentation is an important function of immune competent cells, such as dendritic cells (DCs), macrophages, and an organ-resident liver cell population, i.e., liver sinusoidal endothelial cells (LSECs). Here, we characterize in direct comparison to DCs the distinct dynamics and kinetics of cross-presentation employed by LSECs, which promote tolerance induction in CD8 T cells. We found that LSECs were as competent in cross-presenting circulating soluble antigen ex vivo as DCs at a per-cell basis. However, antigen uptake in vivo was 100-fold more pronounced in LSECs, indicating distinct mechanisms of cross-presentation. In contrast to mannose-receptor–mediated antigen uptake and routing into stable endosomes dedicated to cross-presentation in DCs, we observed distinct antigen-uptake and endosomal routing with high antigen turnover in LSECs that resulted in short-lived cross-presentation. Receptor-mediated endocytosis did not always lead to cross-presentation, because immune-complexed antigen taken up by the Fc-receptor was not cross-presented by LSECs, indicating that induction of CD8 T cell tolerance by LSECs is impaired in the presence of preexisting immunity. Conclusion: These results provide a mechanistic explanation how organ-resident LSECs accommodate continuous scavenger function with the capacity to cross-present circulating antigens using distinct kinetics and dynamics of antigen-uptake, routing and cross-presentation compared to DCs. (HEPATOLOGY 2009.)
Presentation of exogenous antigens on major histocompatibility complex class I (MHC-I) molecules, a process termed cross-presentation, is relevant for mounting antigen-specific CD8 T cell immunity to combat infection and cancer.1-4 The mechanisms allowing cross-presentation of particulate and soluble antigens by professional antigen-presenting cells such as dendritic cells (DCs) or macrophages are mechanistically distinct.5, 6 Particulate antigens enter phagosomes where MHC-I–restricted as well as MHC-II–restricted antigen presentation is initiated.7, 8 Recently, a direct link between endocytosis mechanisms and the cell biology of antigen presentation was reported, i.e., delivery of ligands by receptor-mediated endocytosis into endosomal compartments dedicated to cross-presentation or to MHC-II–restricted presentation to CD4 T cells.9, 10 In particular, uptake through the mannose-receptor delivers ligands into an early endosomal antigen 1 (EEA1)-positive stable endosomal compartment which also contained transporter associated with antigen transport (TAP) for loading peptide onto MHC-I molecules.10, 11 Although these reports provide an explanation to the question why different DC subtypes have distinct functional properties12, 13 by correlating receptor expression with cross-presentation, it remained unclear whether other cell populations capable of cross-presentation would use similar mechanisms. We reported previously that liver sinusoidal endothelial cells (LSECs) cross-present soluble exogenous antigens on MHC-I molecules to CD8 T cells.14-16 LSECs line the hepatic sinusoids and are in direct contact with lymphocytes circulating with the blood.17 Given the large number of LSECs (2 × 107 in mice and approximately 1011 in humans),18 it is important to consider the contribution of this cell population to immune regulation toward circulating antigens. Here, we characterize the distinct dynamics and kinetics of cross-presentation in LSECs.
Abbreviations
AcLDL, acetylated low-density lipoprotein; DC, dendritic cell; EEA1, early endosomal antigen 1; ER, endoplasmic reticulum; IL-2, interleukin-2; LSEC, liver sinusoidal endothelial cell; MHC, major histocompatibility complex; OVA, ovalbumin; TAP, transporter associated with antigen processing.
Materials and Methods
Mice.
C57BL/6 (H-2b) mice, mannose-receptor knockout mice, H-2Kb-restricted CD8+ OT-1 T cell receptor transgenic mice recognizing the ovalbumin (OVA)-derived SIINFEKL-peptide, and bm1 mice were bred under specific pathogen-free conditions. For all experiments, mice between 6-20 weeks of age were used in accordance with local animal experimentation guidelines.
Antibodies and Reagents.
Antibodies were purchased from eBiosciences (USA), BD Biosciences (Germany), or Santa Cruz Biotechnology (Santa Cruz, CA); anti-mannose receptor antibody was purchased from Serotec (Germany). Antibody-coated magnetic beads were from Miltenyi (Germany), ovalbumin was from Serva (Germany), fluorescently labeled ovalbumin, acetylated low-density lipoprotein (AcLDL), bovine serum albumin, and transferrin were obtained from Invitrogen (Germany). A peptide derived from the Epstein-Barr virus–encoded TAP-inhibitor BNLF2a20 encompassing its hydrophilic domain (VHVLERALLEQQSSACGLPGSSTETRPSHPCPEDPDVSR) was prepared using standard chemical synthesis.
Cells and Cell Isolation.
LSECs were isolated as described before.14, 16 Isolated LSECs were usually 98% pure as determined by AcLDL uptake and CD146 staining. To further increase purity, we isolated LSECs by fluorescence-activated cell sorting (FACS) (DIVA, Becton Dickinson) of CD146+ cells with high scavenger activity for fluorochrome ligands in vivo. Purity of FACS was determined by in vitro uptake of AcLDL. For direct comparison of different cell populations, LSECs or splenic CD8α+CD11c+CD3− DCs or CD8α−CD11c+CD3− DCs were directly sorted into 96-well plates. Splenic DCs were isolated after collagenase digestion by anti-CD11c+-mediated selection. Bone marrow–derived DCs were generated as described9 and used on day 7 for experiments following αCD11c-based immuno-magnetic purification.
Determination of Cross-Presentation.
LSECs or DCs were pulsed with OVA (gradeV, Serva, Germany) free of contaminating peptides after PD-10 column purification, fixed (0.008% glutardialdehyde), and after washing were incubated with OVA-specific CD8 T cells, B3Z T cell hybridomas, and OT-1 T cells that recognize H-2Kb-SIINFEKL. Inhibitors of cross-presentation were added to LSECs 15–30 minutes prior to addition of OVA. Cross-presentation was determined by interleukin-2 (IL-2) release from T cells by enzyme-linked immunosorbent assay (ELISA).
Flow Cytometry.
Cells were stained with saturating concentrations of fluorochrome-labeled antibodies for 30 minutes in FACS buffer (1% fetal bovine serum, 2 mM ethylene diamine tetraacetic acid) on ice after blocking Fcγ receptor (FcγR) using aCD16/32 (10 μg/mL, clone 2.4G2). Dead cells were excluded from analysis by Hoechst-33258 staining (Sigma, Germany). Measurements were conducted with an LSRII or CantoII (BD Biosciences) cytometer, and data were analyzed using FlowJo software (TreeStar Inc., Ashland, OR).
Immunofluorescence.
LSECs seeded on collagenR-coated slides were pulsed for 15 minutes with fluorochrome-labeled OVA (5 μg/mL), transferrin (10 μg/mL), and AcLDL (2 μg/mL) and were chased for 0 minutes to 3 hours with medium, before fixation with 4% wt/vol paraformaldehyde. Cells were permeabilized with 0.1% Triton and stained with specific antibody. Nuclei were visualized with DAPI (4′,6-diamidino-2-phenylindole; 0.5 μg/mL). Analysis was performed with an IX71 microscope or FV-1000 confocal microscope (Olympus, Germany). For quantitative determination of colocalization, automated analysis with the ScanR software was performed (Olympus, Germany).
Statistical Analysis.
The Student t test was used for both in vitro and in vivo experiments. Results are shown as mean ± standard error of the mean (SEM). P values < 0.05 were considered significant; *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Rapid Scavenging of Circulating Antigen by Liver Cells.
We investigated which cell population eliminated soluble bloodborne antigen. Following intravenous application, we quantified cellular uptake of Alexa Fluor 647 conjugated with ovalbumin (Alexa647-OVA) in various organs. Clearly, bloodborne antigen accumulated in hepatic cells, whereas little accumulation in spleen or lung and almost no accumulation in lymphatic tissue were observed (Fig. 1A). Antigen uptake by liver cells was two log-steps more efficient compared to that by cells in other organs (Fig. 1A). Confocal analysis of perfusion-fixed liver tissue revealed antigen uptake by cells lining the liver sinusoids (Fig. 1B). Further characterization revealed that antigen-positive cells were not CD11c+ or CD11b+ (Fig. 1C), but instead were positive for the endothelial cell marker CD146.19 However, not all CD146+ cells showed pronounced scavenger activity (Fig. 1C), suggesting that microvascular LSECs rather than macrovascular hepatic endothelial cells take up circulating antigen. Direct comparison of LSECs with DCs from liver and spleen revealed that LSECs contained far more Alexa647-OVA after intravenous application (Fig. 1D), which must be due to different efficiency in antigen uptake.
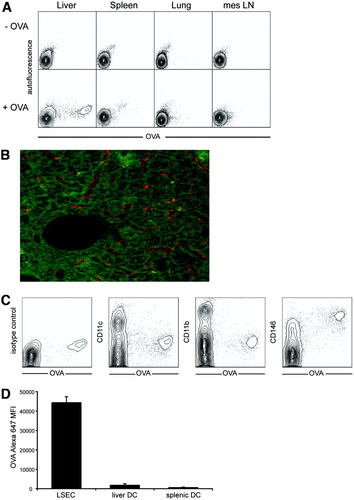
Preferential uptake of circulating soluble antigen by scavenger cells in the liver. (A) Alexa647-OVA (4 μg/mouse) was intravenously injected and (A,C) cells from various organs were analyzed after 1 hour by flow cytometry, or (B) 80 μm thick slices from perfusion-fixed livers were analyzed by confocal laser scanning microscopy. (C) Hepatic cells taking up OVA from the circulation were stained for various surface markers for phenotypical analysis by flow cytometry. (D) LSECs and splenic or hepatic DCs were isolated from mice 1 hour after intravenous injection of Alexa647-OVA (20 μg/mouse) and fluorescence was analyzed by flow cytometry.
Different Kinetics of Cross-Presentation in LSECs Compared to DCs.
We next asked whether efficient antigen uptake also allowed LSECs to efficiently cross-present soluble antigen to CD8 T cells. LSECs constitute a homogenous cell population with respect to the ability to cross-present soluble antigens shown by staining with an antibody recognizing OVA-derived peptides on H-2Kb (data not shown).14 Comparing similar numbers of LSECs and DCs in antigen dose titration experiments in vitro revealed that LSECs were more efficient than DCs in cross-presenting soluble OVA to H2Kb-SIINFEKL specific CD8 T cells (Fig. 2A) and in priming naïve CD8 T cells for proliferation (Fig. 2B). The more prominent cross-presentation by LSECs was accompanied by more pronounced antigen-uptake in vitro compared to bone marrow–derived DCs (Fig. 2C). Furthermore, OVA-uptake by LSECs did not depend on binding to serum-proteins arguing for specific receptor-mediated uptake (Supporting Fig. 1). Specificity of OVA cross-presentation to CD8 T cells was determined by using LSECs from bm1 mice, the latter of which carry a point mutation in the H-2Kb molecule abrogating MHC-I restricted presentation of SIINFEKL. Bm1-derived LSECs did not cross-present OVA to CD8 T cells, demonstrating that antigen-processing and presentation by LSECs was required for the effects observed in our experiments. To further study the efficiency of LSEC cross-presentation of circulating antigen, we developed a novel isolation procedure that is based on immunomagnetic separation in combination with flow cytometric cell sorting (FACS) of cells with high scavenging activity. This procedure yielded a purity of more than 99% for CD146+ LSECs (Fig. 2E) and enabled us to directly characterize cross-presentation ex vivo. LSECs and CD8α+ or CD4+ splenic DCs were isolated 1 hour after intravenous challenge with 1 mg OVA and directly sorted into 96-well plates for assessment of cross-presentation. Clearly, LSECs showed more pronounced cross-presentation of circulating antigen than splenic CD8α+ DC (Fig. 2E), whereas CD8α− DCs did not show cross-presentation capacity (Fig. 2F).
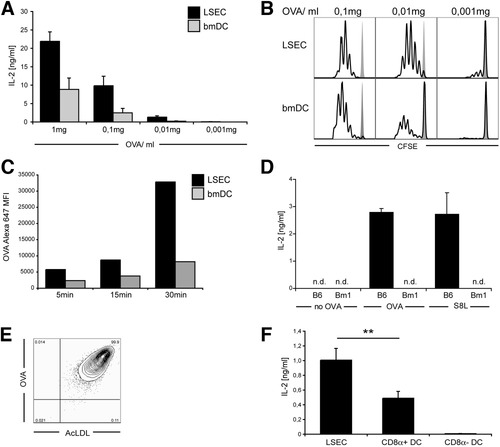
More pronounced early cross-presentation by LSECs compared to DCs. (A) LSECs or bone marrow-derived DCs (bmDCs) were incubated with different concentrations of soluble OVA in vitro for 1 hour, cultured with H-2Kb-SIINFEKL-specific B3Z T cells over night, and cross-presentation was determined by measuring IL-2 release into the cell culture supernatant. (B) LSECs and bmDCs pulsed with different OVA concentrations were incubated with carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve OVA-specific OT-I T cells, and proliferation was determined on day 3 by CFSE dilution. (C) LSECs and bmDCs were incubated for different time periods with 1 μg/mL Alexa647-OVA. Cells were washed and analyzed for OVA uptake by flow cytometric analysis. Background fluorescence was subtracted. (D) LSECs from C57Bl/6 or from mutant bm1 mice (unable to present OVA-derived SIINFEKL on H-2Kb) were pulsed with OVA or SIINFEKL and presentation to B3Z T cells was determined. (E) CD146+ LSECs isolated from mice intravenously injected with Alexa647-OVA were sorted by FACS according to high OVA uptake, and purity of the sorted cell population was determined by further in vitro incubation with Alexa488-AcLDL (1 μg/mL) followed by flow cytometric analysis for OVA and AcLDL uptake. (F) LSECs, or CD8α+ or CD8a− splenic CD11c+ DCs were isolated from mice intravenously injected 1 hour before with OVA (1 mg), and equal numbers of cells were sorted by FACS into 96-well plates for direct ex vivo comparison of cross-presentation to B3Z T cells.
Given the enormous scavenging activity, we reasoned that antigen uptake needed to be accompanied by rapid antigen elimination to allow for continuous scavenging activity. Therefore, we characterized how long LSECs would retain endocytosed OVA in vivo. By isolating cells at various time points after Alexa647-OVA injection, we found that maximal fluorescence intensity in LSECs was rapidly lost with a biological half-life (t1/2) of 6 hours (Fig. 3A). Detection of OVA by immunoblotting from isolated LSECs confirmed rapid uptake and turnover of antigen (Fig. 3B). Importantly, rapid antigen turnover in LSECs was accompanied by decreased cross-presentation 24 hours after antigen uptake in vivo, whereas no such decrease was observed for DCs isolated from spleen (Fig. 3C). Taken together, these data demonstrate potent cross-presentation by LSECs and indicate that high antigen turnover restricts the duration of cross-presentation.
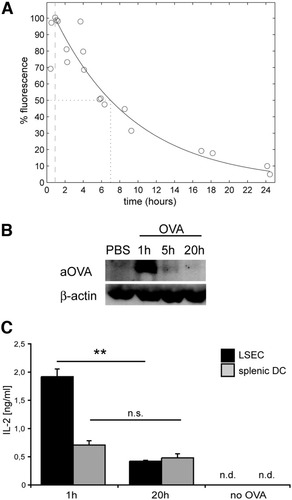
Rapid turn-over of endocytosed antigen in LSECs. (A) Mice were intravenously injected with Alexa647-OVA (4 μg), LSECs were purified at various time points after injection and analyzed by flow cytometry. Maximal uptake of OVA by LSECs determined as increase in mean fluorescence intensity (MFI) was observed 1 hour after injection and was set to 100%. The nonlinear correlation coefficient of antigen clearance from LSECs is 0.967, and the t1/2 is calculated to 6 hours. (B) LSECs were isolated at different time points after intravenous antigen injection and OVA was detected by western blot. (C) Cross-presentation of LSECs ex vivo to B3Z cells was determined at 1 hour or 20 hours after intravenous OVA injection. n.d., not detected.
The Mannose Receptor Is not Essential for Cross-Presentation of OVA in LSECs.
Uptake via the mannose receptor has been shown to determine cross-presentation in DCs.9, 10 LSECs expressed the mannose receptor at high levels both at the cell surface and in intracellular compartments (Fig. 4A,B). The mannose receptor colocalized with endocytosed OVA (Fig. 4C), but in contrast to DCs, which strictly require expression of the mannose receptor for OVA cross-presentation, LSECs from mannose receptor knockout animals retained their ability to cross-present OVA in vitro, indicating that the mannose receptor was not essential (Fig. 4D). However, dose titration experiments in vitro revealed that lack of mannose receptor expression diminished the ability of LSECs to initiate proliferation of naïve OT-1 T cells at low antigen concentrations (Fig. 4E). Also under limiting antigen concentration in vivo, the mannose receptor contributed to cross-presentation because LSECs from mannose receptor knockout compared to wild-type animals showed less cross-presentation ex vivo (Fig. 4F), which was accompanied by reduced antigen uptake (Fig. 4G).
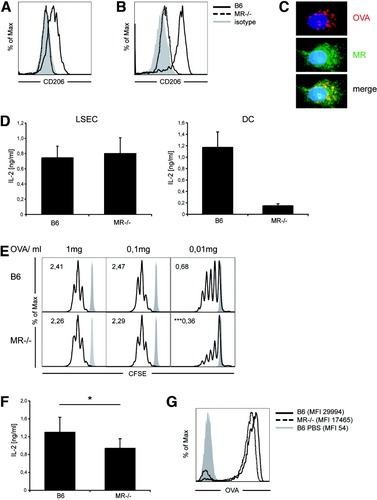
The mannose-receptor (CD206) is not essential for cross-presentation in LSECs. (A,B) LSECs were stained for (A) surface or (B) intracellular expression of CD206. (C) Immunofluorescence microscopy of LSECs after endocytosis of OVA stained for the mannose receptor (MR). (D) LSECs from MR−/− and wild-type B6 were pulsed with OVA (1 mg/mL), and cross-presentation to B3Z cells was determined by IL-2 release. (E) Proliferation of CFSE-labeled naïve OT-I T cells 72 hours after priming by MR−/− or wild-type LSECs pulsed with different OVA concentrations; numbers denote division indices. (F) Ex vivo cross-presentation by MR−/− and wild-type LSECs 1 hour after intravenous injection of OVA (1 mg); mean of five independent experiments is shown. (G) Uptake of fluorochrome-labeled OVA by B6 or MR−/− or wild-type LSECs in vivo after intravenous injection of 4 μg OVA, mean fluorescence intensities are shown in brackets.
Unique Antigen Shuttling After Receptor-Mediated Endocytosis in LSECs.
In macrophages and DCs, uptake via different cell-surface receptors leads to delivery into distinct endosomal compartments.9 In LSECs, uptake through the scavenger-receptor (for AcLDL and bovine serum albumin), mannose receptor (for OVA), or transferrin receptor (for transferrin) all resulted in ligand delivery into the same early endosomal compartment within several minutes (Fig. 5A), which indicates existence of a common endosomal trafficking pathway for receptor-mediated endocytosis. This compartment was characterized as an early endosomal compartment by expression of the marker EEA1 (Fig. 5B). We did not detect colocalization of endocytosed antigen with Rab7+ late endosomal or lysosomal-associated membrane protein 1 (LAMP1+) lysosomal compartments for up to 4 hours after antigen uptake (not shown). Macropinocytosis was not involved, because amiloride failed to influence antigen uptake and cross-presentation in LSECs (not shown). Cross-presentation was rapidly achieved within 45-60 minutes after OVA uptake (not shown),14 and OVA colocalized with EEA1+ compartments at early time-points after receptor-mediated uptake (Fig. 5C). At 60 minutes after antigen uptake, however, there was almost no colocalization of OVA with EEA1+ compartments (Fig. 5D), suggesting that the endosomal compartment, into which endocytosed OVA was initially delivered, was not stable over time. Further support for a continuous endosomal transport of endocytosed antigen in LSECs came from the observation that OVA taken up 45 minutes after a first OVA challenge was not delivered into the same compartment and did not colocalize with OVA taken up earlier (Fig. 5E,F). We conclude from these observations that shuttling of antigen taken up by receptor-mediated endocytosis in LSECs is fundamentally different from that in DCs or macrophages.
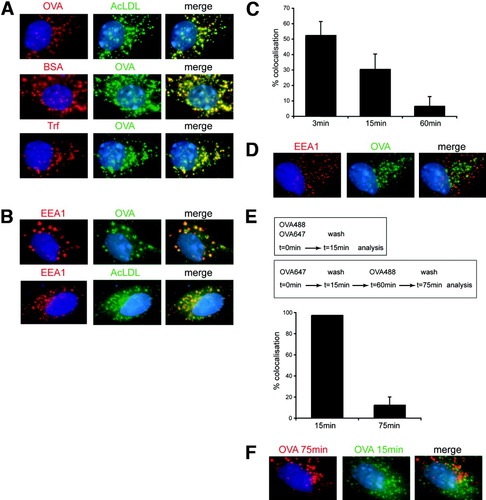
Unique in vitro antigen-routing in LSECs. (A) LSECs were incubated in vitro with fluorochrome-labeled ligands for 15 minutes before fixation. (B) Immunofluorescence microscopy of LSECs after endocytosis of OVA or AcLDL stained for EEA1. (C) Percent colocalization of endocytosed OVA with EEA1 quantified at different time points after endocytosis. (D) Immunofluorescence microscopy of LSECs 1 hour after endocytosis of OVA, stained for EEA1. (E) Quantification of colocalization of differently-labeled OVA in LSECs either after simultaneous application or after separate application (45 minutes apart). (F) Immunofluorescence microscopy of LSECs incubated for 75 minutes with Alexa647-OVA and for 15 minutes with Alexa488-OVA.
Molecular Mechanisms Determining Cross-Presentation in LSECs.
We next addressed the question how LSECs accommodate their scavenger function with the ability to cross-present soluble antigens. Clearly, endosomal acidification was required, because cross-presentation in LSECs was prevented by drugs inhibiting vesicular adenosine triphosphatase such as bafilomycin (Fig. 6A) or chloroquine (data not shown). Inhibition of proteasomal function by epoxomicin abrogated cross-presentation (Fig. 6B) as we have already reported,14 indicating that cross-presentation required transport of antigen from the endosome into the cytosol. Functional TAP was required for cross-presentation, as shown with a TAP-inhibitor derived from Epstein-Barr virus (BNLF2)20 (Fig. 6C), but we did not detect TAP within EEA1+ endosomes (Fig. 6D), which is in contrast to DCs, where localization of TAP to EEA1+ endosomes confers cross-presentation competence to this particular compartment.10 To further validate the independence of cross-presentation from endosomal peptide-loading, we made use of the observation that primaquine, which inhibits endosomal transport to the cell surface, abolished cross-presentation in DCs.9 Primaquine did not influence cross-presentation in LSECs but inhibited cross-presentation in DCs (Fig. 6E), indicating that LSECs did not employ the same endosomal compartment for cross-presentation.
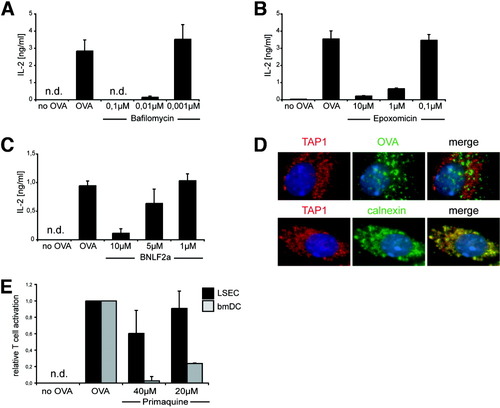
Mechanisms determining cross-presentation in LSECs. ELISA of IL-2 in supernatant of B3Z cells incubated with OVA-pulsed LSECs treated with (A) bafilomycin, (B) epoxomicin, or (C) a TAP-inhibiting peptide, derived from Epstein-Barr virus BNLF2a. (D) Immunfluorescence microscopy of LSECs staining for TAP1 and OVA (upper panel) or TAP1 and calnexin (lower panel). (E) The bmDCs and LSECs treated with primaquine were pulsed with OVA and incubated with B3Z cells. Controls are shown in Supporting Fig. 2.
Because cross-presentation was closely correlated to endocytic activity in LSECs, we wondered whether antigen uptake through receptor-mediated endocytosis in general conferred the capacity for cross-presentation. We therefore tested whether antigen uptake via Fcγ receptors (FcγR) would also lead to cross-presentation in LSECs. Clearly, there was little uptake of fluorochrome-labeled antibodies compared to OVA uptake (Fig. 7A), although LSECs expressed significant levels of FcγRII/III (Fig. 7B). Interestingly, FcγR-mediated antibody uptake was more pronounced in DCs (data not shown) and leads to increased antigen presentation.21, 22 These results demonstrated that FcγR-mediated uptake of antibodies in LSECs was slow compared to uptake through the mannose receptor. Importantly, there was no significant colocalization of endocytosed OVA and antibody taken up by FcγRII in LSECs (Fig. 7C), which suggests distinct endosomal routing. To investigate whether antigen-uptake via FcγR also allowed cross-presentation, we incubated LSECs with different ratios of OVA to anti-OVA antibodies. If OVA-specific antibodies were in excess over OVA, we observed a reduction in cross-presentation by LSECs (Fig. 7D). Collectively, these results demonstrated that FcγR-mediated antigen uptake occurred with low efficiency and that antigen complexed to immunoglobulins was cross-presented less efficiently by LSECs.
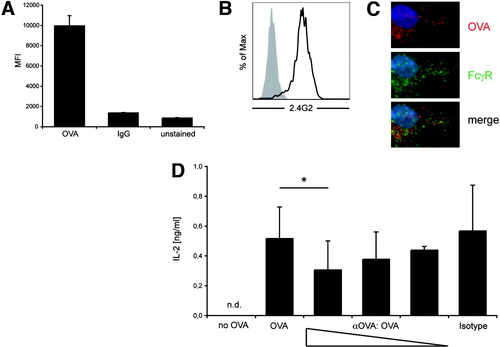
Reduced cross-presentation of immuncomplexed OVA. (A) Flow cytometric analysis of Alexa647-OVA or Alexa488-labeled rat immunoglobulin G uptake at several time points. Mean fluorescence intensity values at 30 minutes are shown. (B) Immunostaining for FcγRII/III on LSECs. (C) Fluorescence microscopy of LSECs after incubation with Alexa647-OVA and Alexa488-labeled rat immunoglobulin G for 15 minutes. (D) ELISA of IL-2 from supernatant of B3Z cells incubated with LSECs pulsed with OVA in the presence of different concentrations of anti-OVA immunoglobulin (OVA 100 μg complexed to 600 μg, 300 μg, or 75 μg mouse anti-OVA immunoglobulin G).
Discussion
Cross-presentation of exogenous antigens by DCs in secondary lymphatic tissue is important for induction of CD8 T cell immunity against infection and tumors.23 Recently, we reported that antigen uptake by the mannose receptor facilitates cross-presentation by delivery of antigen into an EEA1+ compartment where antigen loading onto MHC-I molecules occurs.9 LSECs are a liver-resident cell population also capable of cross-presenting exogenous antigens,14 and which are known for their scavenger activity24 and contribute to the tolerogenic immune function of the liver.25 Here, we characterize the kinetics and dynamics of cross-presentation by LSECs compared to DCs. We found that antigen circulating in the blood was most efficiently taken up by LSECs but to a much lesser extent by DCs or macrophages in the spleen, or by other hepatic cell populations, such as Kupffer cells or liver DCs. This observation confirmed earlier observations that LSECs are highly specialized to eliminate soluble antigens from the circulation.24 Still, it may be that in vivo access to bloodborne antigen differs between cell populations. To determine whether preferential antigen uptake was accompanied by increased ability for cross-presentation, we sorted by FACS LSECs from OVA-injected mice and characterized their ability to cross-present in vivo endocytosed OVA in direct comparison to sorted splenic CD8α+ DCs, which cross-present soluble antigen efficiently.26, 27 Clearly, at a per-cell basis, LSECs showed more cross-presentation to CD8 T cells ex vivo than splenic CD8α+ DCs. Also in vitro, antigen uptake and cross-presentation by LSECs was superior to that of DCs as revealed by antigen dose-titration experiments. Even priming of naïve CD8 T cells and induction of proliferation requiring costimulatory molecules28, 29 was more efficient by LSECs, which express significant levels of CD80 (Supporting Fig. 3), than by DCs at limiting antigen concentration. Cross-presentation of LSECs in vivo had functional relevance, because OVA-specific hepatic adhesion of naïve CD8 T cells was mediated by LSECs cross-presenting OVA,30 supporting the notion that cross-presentation of circulating antigens by LSECs in vivo is at least as prominent as cross-presentation by professional antigen-presenting cells.
However, because antigen uptake in vivo or in vitro was more than 100-fold more pronounced in LSECs but cross-presentation was at best two-fold increased compared to DCs, one has to assume that the efficiency to cross-present endocytosed antigen was lower in LSECs than in DCs. This prompted us to characterize the mechanisms underlying cross-presentation in LSECs. We found a fundamental difference between LSECs and DCs in the handling of endocytosed antigen. In order to function continuously as efficient scavenging cells, LSECs have to balance antigen uptake with antigen elimination. Indeed, we found that endocytosed antigen remained only for a short time within LSECs in vivo with a half-life (t1/2) of 6 hours, whereas antigen taken up by DCs is maintained in these cells over a long period of time.31 This correlated with short-lived cross-presentation by LSECs and long-lived cross-presentation by DCs. The longevity of antigen retention supports the function of DCs to cross-present antigen taken up in peripheral organs after migration into secondary lymphatic tissue, whereas high antigen turnover by organ-resident LSECs accommodates maintenance of scavenger function with cross-presentation. Antigen eliminated from LSECs was most likely transferred to hepatocytes for biliary secretion, because we found OVA in bile at later time points (not shown). Such transcytotic transport across LSEC may also facilitate viral targeting of hepatocytes, as we and others have recently reported.32, 33
Antigen-uptake through the mannose receptor in DC determines cross-presentation through distinct routing of endocytosed antigen into a stable early endosome and in the absence of the mannose receptor, DCs are unable to take up soluble OVA for cross-presentation.9, 11 LSECs expressed the mannose receptor even at higher levels than DCs but cross-presentation did not depend on mannose receptor expression. This suggested that endocytic receptors with similar carbohydrate-binding affinity such as L-SIGN (liver-specific intercellular adhesion molecule-3–grabbing non-integrin) or other C-type lectins abundantly expressed by LSECs34 (Supporting Table 1) may substitute the mannose receptor for cross-presentation. Cross-presentation in LSECs depended on receptor-mediated endocytosis because inhibition of receptor-mediated endocytosis abrogated cross-presentation (Supporting Fig. 4) whereas blockade of macropinocytosis did not alter cross-presentation (not shown).15 We therefore analyzed the routing of antigens in LSECs taken up by different endocytic receptors. Surprisingly, we found that ligands of the mannose scavenger, or transferrin receptor were all rapidly delivered within minutes into a common endosomal compartment in LSECs, which is in contrast to the strict spatial separation observed for mannose receptor–mediated delivery into an EEA1+ compartment for cross-presentation and scavenger receptor–mediated routing into lysosomal compartments for MHC-II–restricted presentation in macrophages.9, 10 Also, antigens taken up through receptor-mediated endocytosis only transiently colocalized with EEA1+ endosomal compartments in LSECs. Together with the observation that antigens taken up at different time points did not convene in a stable early endosomal compartment but remained separated over time, these observations suggested that antigens were rapidly shuttled “through” LSECs similar to the transport on a conveyor belt. Thus, we identify here a fundamental difference in shuttling of antigen in organ-resident LSECs compared to highly motile DCs, which retain antigen in EEA1+ compartments for at least several hours using this TAP-containing compartment for cross-presentation.10 Short-lived cross-presentation as a function of continuous transcytotic antigen transport through LSECs may account for the rather low efficiency of cross-presentation of endocytosed antigens in LSECs compared to DCs.
How is cross-presentation of soluble antigens in LSECs then achieved? Similar to DCs, cross-presentation in LSECs required endosomal acidification as well as proteasomal degradation, indicating that endocytosed antigens must be shuttled into the cytoplasm. In contrast to DCs, EEA1+ compartments in LSECs did not contain TAP, and the drug primaquine, which blocks endosomal trafficking and cross-presentation in DCs, did not affect the ability of LSECs to cross-present soluble OVA. It is unclear whether after proteasomal degradation of exogenous antigen, loading of peptides onto MHC-I molecules in LSECs occurs within endosomal vesicles or in the endoplasmic reticulum (ER). Using a TAP-inhibitor derived from the Epstein-Barr virus BNLF2a protein, it was shown that TAP is required for cross-presentation of OVA in LSECs. Transport of soluble protein into the ER and MHC-I loading at this site was observed in DCs,35 but we did not observe OVA within the ER in LSECs. The abundant antigen uptake may allow less efficient pathways of peptide loading onto MHC-I molecules to account for cross-presentation in LSECs. Our data suggest that in LSECs exogenous antigens have to compete with endogenous peptides for presentation on MHC-I molecules because they lack a dedicated compartment for cross-presentation, whereas usage of such a compartment in DCs facilitates presentation of exogenous antigens on MHC-I molecules.10 Thus, the extraordinary scavenging activity of LSECs compensates for less selective molecular mechanisms to load MHC-I molecules with peptides derived from soluble exogenous antigens.
However, not all ligands taken up by receptor-mediated endocytosis showed similar intracellular routing and were efficiently cross-presented by LSECs. Despite significant surface expression levels of CD16/32 on LSECs, FcγR-mediated uptake of antibodies was lower compared to uptake of OVA or AcLDL and did not distribute ligands into the same endosomal compartments. Even more importantly, in the presence of excess antibodies to OVA, we observed reduced cross-presentation. In addition to demonstrating that most efficient endocytosis and cross-presentation is not a uniform feature of all endocytic receptors in LSECs, these findings also suggest that antigens, which have already elicited a CD4 T cell and B cell response leading to antibody production, will not be cross-presented by LSECs. Lack of cross-presentation and subsequent lack of induction of CD8 T cell tolerance by LSECs may then support induction of CD8 T cell immunity and help to combat infection by microorganisms such as viruses (including the hepatitis B virus), which release large amounts of viral antigens into the circulation.
Acknowledgements
We thank W.E. Benckhuijsen, P.E. de Koning, and J.W. Drijfhout for peptide synthesis and purification.




