Fenofibrate differentially regulates plasminogen activator inhibitor-1 gene expression via adenosine monophosphate–activated protein kinase–dependent induction of orphan nuclear receptor small heterodimer partner†
Potential conflict of interest: Nothing to report.
Abstract
Plasminogen activator inhibitor type I (PAI-1) is a marker of the fibrinolytic system and serves as a possible predictor for hepatic metabolic syndromes. Fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) agonist, is a drug used for treatment of hyperlipidemia. Orphan nuclear receptor small heterodimer partner (SHP) plays a key role in transcriptional repression of crucial genes involved in various metabolic pathways. In this study, we show that fenofibrate increased SHP gene expression in cultured liver cells and in the normal and diabetic mouse liver by activating the adenosine monophosphate–activated protein kinase (AMPK) signaling pathway in a PPARα-independent manner. Administration of transforming growth factor beta (TGF-β) or a methionine-deficient and choline-deficient (MCD) diet to induce the progressive fibrosing steatohepatitis model in C57BL/6 mice was significantly reversed by fenofibrate via AMPK-mediated induction of SHP gene expression with a dramatic decrease in PAI-1 messenger RNA (mRNA) and protein expression along with other fibrotic marker genes. No reversal was observed in SHP null mice treated with fenofibrate. Treatment with another PPARα agonist, WY14643, showed contrasting effects on these marker gene expressions in wild-type and SHP null mice, demonstrating the specificity of fenofibrate in activating AMPK signaling. Fenofibrate exhibited a differential inhibitory pattern on PAI-1 gene expression depending on the transcription factors inhibited by SHP. Conclusion: By demonstrating that a PPARα-independent fenofibrate-AMPK-SHP regulatory cascade can play a key role in PAI-1 gene down-regulation and reversal of fibrosis, our study suggests that various AMPK activators regulating SHP might provide a novel pharmacologic option in ameliorating hepatic metabolic syndromes. (HEPATOLOGY 2009.)
Small heterodimer partner (SHP; NR0B2) is an atypical orphan nuclear receptor lacking the classical DNA binding domain but is classified as a nuclear receptor because of the presence of a putative ligand-binding domain.1 SHP is predominantly expressed in liver and functions mainly as a transcriptional corepressor of a large variety of nuclear receptors and other transcription factors and regulates key enzyme genes involved in various metabolic processes as well as in ameliorating hepatic fibrosis and insulin sensitivity.2, 3 Inducers of SHP such as bile acids and AMP-activated protein kinase (AMPK) activators have been shown to down-regulate cholesterol 7α-hydroxylase, the rate-limiting enzyme in cholesterol biosynthesis, and key gluconeogenic genes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, respectively, through repression of transcription factors regulating these gene promoters,2-5 thereby highlighting its significance in maintenance of metabolic homeostasis.
Plasminogen activator inhibitor-1 (PAI-1), a serine proteinase inhibitor, is the main physiological inhibitor of the fibrinolytic system regulating intravascular fibrinolysis and thereby controlling thrombus dissolution along with invasion and migration of cells through the extracellular matrix.6-9 Studies with transgenic mice suggest a major role of PAI-1 in metabolic disturbances such as obesity, insulin resistance, and metabolic syndrome.6 Recent reports suggest a direct correlation between plasma PAI-1 levels and steatosis in human subjects.10 Various reports demonstrate induction and transcriptional regulation of the PAI-1 gene by cytokines and inflammatory mediators such as endotoxins, interleukin-1 (IL-1), transforming growth factor beta (TGF-β), insulin, tumor necrosis factor alpha (TNF-α), hepatocyte growth factor (HGF), and phorbol 12-myristate 13-acetate.11
Fenofibrate, an agonist of the nuclear receptor peroxisome proliferator-activated receptor α (PPARα), is mainly used to treat hyperlipidemia in patients at risk of cardiovascular disease.12 Recent studies suggest beneficial effects of fenofibrate on insulin resistance13 and in normalizing biochemical abnormalities associated with the metabolic syndromes more effectively when used in combination with metformin.14 AMPK is a ubiquitous, heterotrimeric, serine/threonine protein kinase, mediating cellular adaptation to nutritional and environmental variations/stress by acting as a “metabolic switch” in regulating key genes involved in glucose and lipid metabolism.15 Because of its favorable widespread metabolic effects in the activated state, AMPK stands out to be a tempting therapeutic target in prevention of metabolic syndromes related to hepatic steatosis, insulin resistance, and other hepatic disorders.
Here, we elucidate a novel role of fenofibrate in activating the AMPK signaling pathway to induce SHP gene expression and to regulate PAI-1 gene expression. Using in vivo and in vitro studies, we demonstrate that SHP functions as a mediator of fenofibrate inhibition of PAI-1 gene expression and possibly functions as a key negative regulator of liver fibrosis and steatohepatitis, suggesting that targeting SHP with AMPK activators might provide us with a new therapeutic approach for the treatment of metabolic syndromes.
Abbreviations
ACC, acetyl coenzyme A carboxylase; AML12, nontransformed mouse hepatocytes; AMPK, adenosine monophosphate–activated protein kinase; ATP, adenosine triphosphate; HGF, hepatocyte growth factor; IL-1α, interleukin-1α; IL-6, interleukin-6; MCD, methionine-deficient and choline-deficient; mRNA, messenger RNA; NF-κB, nuclear factor kappa B; p-ACC, phosphorylated acetyl coenzyme A carboxylase; PAI-1, plasminogen activator inhibitor 1; p-AMPKα, phosphorylated adenosine monophosphate–activated protein kinase; PPARα, peroxisome proliferator-activated receptor α; RLU, relative luciferase units; SD, standard deviation; SHP, small heterodimer partner; siRNA, small interfering RNA; SREBP-1c, sterol regulatory element binding protein 1c; t-AMPKα, total adenosine monophosphate–activated protein kinase; TGF-β, transforming growth factor β; TIMP-1, tissue inhibitor of metalloproteinase 1; TNFα, tumor necrosis factor α; USF-1, upstream stimulatory factor 1.
Materials and Methods
Animals and Experimental Protocols.
Male C57BL/6J, congenic SHP null mice (B6.129/Sv-Shptm1, described in Supporting reference 1) at 12 weeks of age were injected intraperitoneally with 25 μg/kg TGF-β or fed with methionine-deficient and choline-deficient (MCD) diet for 4 weeks. C57BL/6J and SHP null mice were then divided into five groups for each model and fed (1) MCD diet for 3 days, (2) MCD diet with fenofibrate (100 mg/kg/day) for 3 days, (3) MCD diet with WY14643 (50 mg/kg/day) for 3 days, (4) TGF-β for 4 hours, and (5) TGF-β with fenofibrate (100 mg/kg/day) for 3 days. Another group was fed the control chow diet for 4 weeks. Male C57BLKS-leptin receptor–deficient mice and PPARα null mice (B6.129/Sv-Pparαtm1Gonz described in Supporting reference 2) at 8 to 9 weeks of age were fed with fenofibrate (100 mg/kg/day) for the indicated time. Animal studies were conducted in accordance with the institutional guidelines for care and use of laboratory animals.
Materials and Methods.
Information on the materials and methods used in this study is given in the Supporting Methods section.
Results
Fenofibrate Induces SHP Gene Expression in Hepatic Cell Lines and Rat Primary Hepatocytes.
Previous reports suggest repressive effects of fenofibrate16 and SHP17 on PAI-1 gene expression. To understand the molecular mechanism of fenofibrate action on PAI-1 gene expression, the effect of fenofibrate on SHP gene expression was assessed. Treatment of various hepatoma cell lines (HepG2 and H4IIE), nontransformed mouse hepatocytes (AML12), and rat primary hepatocytes with fenofibrate (50 μM) for different times (1-24 hours) and different doses of fenofibrate (10-100 μM) for 24 hours caused a significant induction of SHP messenger RNA (mRNA) expression by approximately fivefold to 10-fold in all hepatic cell lines tested and primary hepatocytes (Fig. 1A and Supporting Fig. 1A). In an attempt to determine whether the increase of SHP mRNA level by fenofibrate treatment was attributable to the increase in transcription or protein synthesis, HepG2 cells were pretreated with the transcription inhibitor actinomycin D, alone or preceding fenofibrate treatment, resulting in a drastic decrease in SHP mRNA levels (Fig. 1B). However, the protein synthesis inhibitor cycloheximide showed no significant effect on fenofibrate-induced SHP mRNA levels, thereby suggesting that fenofibrate induces SHP gene expression at the transcriptional level and does not require de novo protein synthesis. Finally, to determine whether fenofibrate treatment increases SHP gene promoter activity and SHP protein synthesis, transient transfection assays and immunoblotting analysis were performed. Fenofibrate treatment resulted in a threefold to fourfold increase in both human and mouse SHP gene promoter activity in HepG2 cells and AML12 cells, respectively (Fig. 1C), and the SHP protein level was significantly increased in a dose-dependent manner in HepG2 cells (Fig. 1D). Taken together, these results indicate that fenofibrate induces SHP gene expression in hepatic cell lines and primary hepatocytes.
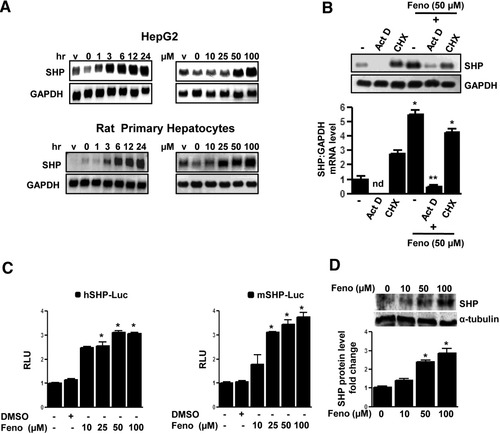
Induction of SHP gene expression by fenofibrate. (A) HepG2 cells and rat primary hepatocytes were treated with fenofibrate (50 μm) or vehicle (dimethylsulfoxide) for the time indicated and in the concentrations indicated for 24 hours. Total RNA was isolated for northern blot analysis of SHP mRNA expression and was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. Data represent mean ± standard deviation (SD) of three individual experiments. (B) HepG2 cells were treated with actinomycin D or cycloheximide alone for 1 hour or pretreated after fenofibrate treatment for 24 hours at the indicated concentration. Total RNA was isolated for northern blot analysis of SHP mRNA expression and was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. Data represent mean ± SD of three individual experiments. nd: not detectable. *P < 0.05 and **P < 0.01 compared with untreated control and fenofibrate-treated cells. (C) The wild-type human (h) and mouse (m) SHP-luciferase reporters were transfected into HepG2 and AML12 cells, respectively, and treated with fenofibrate (Feno, 50 μM) or vehicle (dimethylsulfoxide) for 24 hours under serum-starved conditions. All experiments were done in triplicate; data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control, representing mean ± SD of three individual experiments. *P < 0.05 compared with untreated control. (D) HepG2 cells were treated with fenofibrate at the indicated concentrations for 6 hours, and whole cell extracts (50 μg/lane) were analyzed by immunoblotting with SHP and α-tubulin antibodies. Data represent mean ± SD of three individual experiments. *P < 0.05 compared with untreated control.
PPARα-Independent Induction of SHP Gene Expression by Fenofibrate Is Mediated by the AMPK-Signaling Pathway.
To evaluate the potential signaling pathways involved in the induction of SHP gene expression by fenofibrate, HepG2 cells were pretreated with several specific protein kinase inhibitors followed by fenofibrate treatment for 24 hours. Northern blot analysis indicated that pretreatment of compound C (an AMPK inhibitor) completely abolished fenofibrate-mediated SHP induction, and SP600125 (a Janus kinase inhibitor) showed significant repressive effects. However, there was no significant effect of PD98059 (a mitogen-activated protein kinase inhibitor), Wortmannin (a PI3 kinase inhibitor), and H-89 (a protein kinase A inhibitor) (Fig. 2A). To confirm the involvement of the AMPK signaling pathway in fenofibrate-mediated SHP gene regulation, the phosphorylation levels of AMPK and its direct downstream target acetyl coenzyme A carboxylase (ACC) by fenofibrate was assessed using immunoblotting analysis with antibodies specifically detecting the phosphorylated as well as the total AMPK (t-AMPKα) and ACC levels in HepG2 cells (Fig. 2B). Fenofibrate treatment phosphorylated AMPK (p-AMPKα) and ACC (p-ACC) at 3 hours, followed by a gradual decrease to the basal levels at the 6-hour time point, confirming that fenofibrate activated the AMPK signaling pathway. To elucidate further the mechanism of AMPK-mediated induction of SHP by fenofibrate, transient transfection assays were performed in HepG2 cells using serial deletion construct of the human SHP gene promoter (Fig. 2C). Fenofibrate treatment significantly induced SHP promoter activity, and pretreatment with compound C dramatically abolished the fenofibrate-induced SHP promoter activity in all of these constructs, suggesting that the fenofibrate and AMPK-responsive factors involved in SHP gene regulation reside within the 100–base pair (bp) region upstream of the SHP gene promoter.
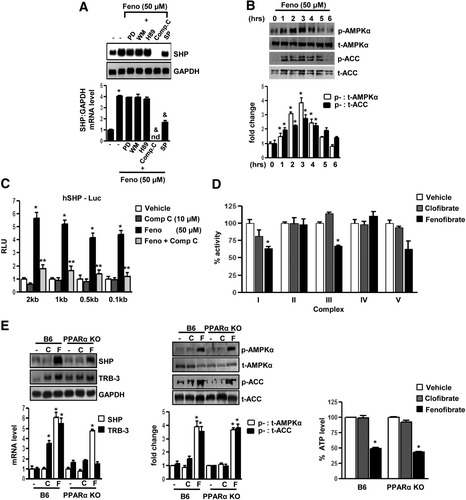
AMPK mediates PPARα-independent induction of SHP gene expression by fenofibrate. (A) HepG2 cells were pretreated with protein kinase inhibitors PD98059 (20 μM), wortmannin (0.1 μM), H-89 (10 μM), compound C (10 μM), and SP600125 (25 μM) for 1 hour (left) or with compound C alone (middle) and then treated with fenofibrate (Feno, 50 μM) for 24 hours. Total RNA was isolated for northern blot analysis of SHP mRNA expression and was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. HepG2 cells were treated with fenofibrate at the indicated time (right) under serum-starved conditions, and whole cell extracts (50 μg/lane) were analyzed by immunoblotting with p-AMPKα, t-AMPKα, p-ACC, and total ACC (t-ACC) antibodies. Data represent mean ± SD of 3 individual experiments. *P < 0.05 and &P < 0.005 compared to untreated control and fenofibrate treated cells. nd; not detectable. (B) HepG2 cells were treated with fenofibrate under serum-starved conditions for the indicated time period and concentration. Whole cell extracts (50 μg/lane) were analyzed by immunoblotting with phospho-AMPKα (p-AMPKα), total-AMPKα (t-AMPKα), phospho-ACC (p-ACC) and total ACC antibodies. Data represent mean ± SD of three individual experiments. *P < 0.05 compared to untreated control. (C) The human (h) SHP-luciferase reporter constructs were transfected into HepG2 cells and treated with compound C alone for 1 hour or pretreated with compound C preceding treatments with fenofibrate or vehicle (DMSO) for 24 hours under serum-starved conditions. All experiments were done in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control, representing mean ± SD of three individual experiments.*P < 0.05 and **P < 0.05 compared to vehicle and fenofibrate-treated cells. bp, base pair. (D) Mitochondrial respiratory enzyme activities were performed as described in Materials and Methods. Fenofibrate (50 μM) and clofibrate (50 μM) were mixed with the mitochondrial fraction in each reaction buffers and enzymatic activities were calculated. Data were normalized with respect to the specific activities in corresponding DMSO control and presented as mean ± SD of three independent experiments. *P < 0.05 and **P < 0.001 compared to control. (E) C57BL/6 mice (B6, n = 3 per group) and PPARα null mice (PPARα null mice, n=3 per group) were fed with fenofibrate (100 mg/kg/day) or clofibrate (500 mg/kg/day) for the 2 days and liver samples were obtained for total RNA isolation for Northern blot analysis of SHP and Trb3 mRNA expression and normalized to GAPDH expression. Tissue extracts (100 μg/lane) were analyzed by immunoblotting with p-AMPKα, t-AMPKα, p-ACC and total ACC antibodies. Total intracellular ATP was quantified by luminescence according to manufacturer's protocol. Results are expressed as the percent decrease in levels of intracellular ATP, setting control liver extract samples as 100, and are normalized to the total protein level. Data represent mean ± SD. *P < 0.005 compared to control.
To elucidate whether fenofibrate lowers cellular adenosine triphosphate (ATP) levels, the mitochondrial respiratory chain complex (I-V) activity was assessed after fenofibrate and clofibrate treatment of mitochondrial fractions from normal mice liver homogenates (Fig. 2D). Clofibrate showed no significant effect on any of the complexes, whereas fenofibrate treatment significantly lowered respiratory chain complex I and III activity and thereby decreased cellular ATP levels. Treatments with other fibrates or synthetic PPARα agonists showed no significant change in SHP gene expression or AMPK activity in HepG2 cells (Supporting Fig. 1B). Fenofibrate alone showed a significant decrease in cellular ATP levels, compared with other PPARα agonists (Supporting Fig. 1C). However, human homologue of Drosophila tribbles, a PPARα target gene (Supporting reference 14), was significantly induced by all PPARα agonists, highlighting the specificity of fenofibrate in activating AMPK and inducing SHP gene expression. 3-(4,5-Dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay in HepG2 cells demonstrated that fenofibrate or clofibrate have no significant effect on cell viability at these concentrations (Supporting Fig. 1D), as was reported previously (Supporting reference 13).
Finally, we confirmed the PPARα-independent effect of fenofibrate in vivo, using fenofibrate and clofibrate treatments to wild-type (B6) and PPARα null mice (Fig. 2E). Fenofibrate showed a significant induction of SHP mRNA levels (left) along with activation of AMPK (middle) and a decrease in ATP levels (right) in vivo. Treatment with clofibrate showed no significant change in any of these parameters ascertained. However, human homologue of Drosophila tribbles mRNA levels were significantly induced on treatments with either fenofibrate or clofibrate in wild-type mice and, expectedly, no changes in human homologue of Drosophila tribbles mRNA levels were observed in PPARα null mice on these treatments. Collectively, these results suggest that fenofibrate decreases cellular ATP levels via inhibition of mitochondrial respiratory chain complexes I and III, consequently activating AMPK signaling to induce SHP gene expression in a PPARα-independent manner both in vitro and in vivo.
Fenofibrate Represses TGF-β–Induced PAI-1 Gene Expression via SHP In Vivo.
To confirm in vitro observations in the animal model, wild-type mice fed with fenofibrate clearly demonstrated a significant induction of SHP mRNA levels from 12 hours to 7 days (Fig. 3A left). Immunoblotting analysis of AMPK and ACC activity (Fig. 3A middle) from liver extracts of these animals demonstrated a significant increase in phosphorylation of AMPK and ACC on fenofibrate treatment, and this pattern of AMPK activation was inversely correlated to the cellular ATP levels, which showed a significant decrease during fenofibrate treatment (Fig. 3A right). Fenofibrate feeding for 3 days showed a similar pattern of increase in SHP gene expression and subsequent decrease in PAI-1 mRNA levels (left), along with a significant decrease in PAI-1 intracellular protein levels (middle) and an increase in AMPK and ACC phosphorylation (right) in the C57BLKS-leptin receptor–deficient mice model, a unique model of diabetic dyslipidemia (Supporting Fig. 2A). PAI-1 levels are significantly elevated by TGF-β treatments.18 The effect of TGF-β injection (T) in wild-type mice and SHP null mice in the presence or absence of fenofibrate treatments (Fig. 3B) was studied. Wild-type mice injected with TGF-β for 4 hours showed a dramatic increase in PAI-1 mRNA and protein levels as well as hepatic collagen α1(I) gene expression. The expression of various other marker genes that were previously reported to be elevated in fibrosis conditions was checked. Treatment with fenofibrate for 3 days (T+F) was associated with significant reduction of PAI-1 mRNA and protein levels (Supporting Fig. 2B left) (by 80%-90%), collagen α1 (I), TGF-β, interleukin-6 (IL-6), and TIMP-1 mRNA levels, along with a subsequent increase in SHP gene expression (Fig. 3B). However, TNFα gene expression showed no significant changes either on TGF-β injection or with fenofibrate treatments. Interestingly, SHP null mice showed increased basal mRNA levels of all genes involved in fibrosis in comparison with wild-type (C) and did not respond to fenofibrate treatments, suggesting a role of SHP in mediating effects of fenofibrate. As expected, AMPK and ACC phosphorylation status is similar in both wild-type and SHP null mice (Supporting Fig. 2B). Overall, these results suggest that activation of SHP by fenofibrate may reverse TGF-β–induced hepatic fibrosis directly by repression of PAI-1 and other pro-inflammatory cytokine and profibrogenic marker gene expression.
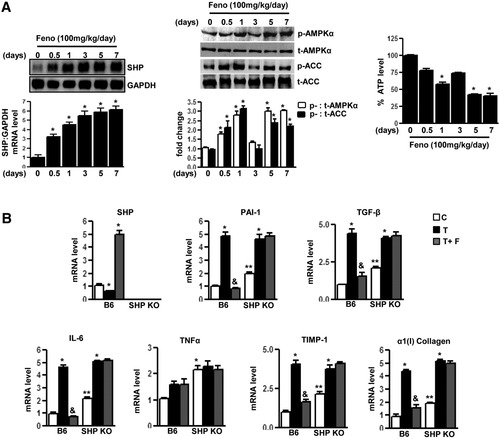
SHP null mice reverse inhibitory effects of fenofibrate on TGFβ-induced PAI-1 gene expression. (A) C57BL/6 mice (B6, n=4 per group) were fed with fenofibrate (100 mg/kg/day) for the indicated time period and liver samples were obtained for total RNA isolation for Northern blot analysis of SHP mRNA expression and normalized to GAPDH expression. Tissue extracts (100 μg/lane) were analyzed by immunoblotting with phospho-AMPKα (p-AMPKα), total-AMPKα (t-AMPKα), phospho-ACC (p-ACC), and total ACC antibodies. Total intracellular ATP was quantified by luminescence according to manufacturer's protocol. Results are expressed as the percent decrease in levels of intracellular ATP, setting control liver extract samples as 100, and are normalized to the total protein level. Data represent mean ± SD. *P < 0.05 compared with control. (B) C57BL/6 mice (B6, n = 4 per group) and SHP null mice (n = 4 per group) were fed with chow (C) or injected with TGF-β (T, 25 μg/kg) intraperitoneally followed by treatment with fenofibrate (100 mg/kg/day) (T+F) for an additional 3 days. Liver samples were obtained for total RNA isolation for semiquantitative reverse transcription polymerase chain reaction analysis of PAI-1, α(1) I collagen, TNFα, IL-6, TIMP-1, TGF-β, and SHP mRNA expression and normalized to actin expression. Data represent mean ± SD. *P < 0.05, &P < 0.005, and **P < 0.005 compared with chow-fed mice, TGF-β–fed mice, and B6 chow-fed mice.
Fenofibrate Represses MCD Diet–Induced PAI-1 Gene Expression via SHP In Vivo.
Next, wild-type and SHP null mice were treated with an MCD, which causes progressive liver fibrosis, pathologically similar to human metabolic steatohepatitis.19 Wild-type mice fed with MCD diet (M) for 4 weeks showed a dramatic increase in PAI-1 mRNA and protein levels (Supporting Fig. 2C) along with collagen α1(I) gene expression (Fig. 4A, B). Expression of genes involved in fatty acid synthesis (sterol regulatory element binding protein [SREBP-1c] and FAS), proinflammatory cytokines (TNFα and IL-6), profibrogenic marker (TGF-β), and tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA levels (Fig. 4A, B) were significantly increased in MCD diet–fed wild-type mice. Interestingly, treatment with fenofibrate (M+F) (Fig. 4A) showed significant induction of SHP gene expression, whereas WY (M+WY, Fig. 4B) had no significant effect on SHP gene expression in wild-type mice. Both fenofibrate and WY significantly repressed the PAI-1 mRNA levels in wild-type mice; however, fenofibrate failed to repress PAI-1 in SHP null mice. However, WY repressed PAI-1 gene expression in SHP null mice, suggesting a PPARα-dependent mechanism of this action. Interestingly, fenofibrate significantly repressed expression of proinflammatory cytokine genes (TGF-β, TNF-α, and IL-6) in wild-type mice, and this repression was abrogated in SHP null mice. In contrast, WY had no significant effect on these cytokines' gene expression in both wild-type and SHP null mice. These results suggest an SHP-dependent specific repressive effect of fenofibrate on proinflammatory cytokine gene expression.
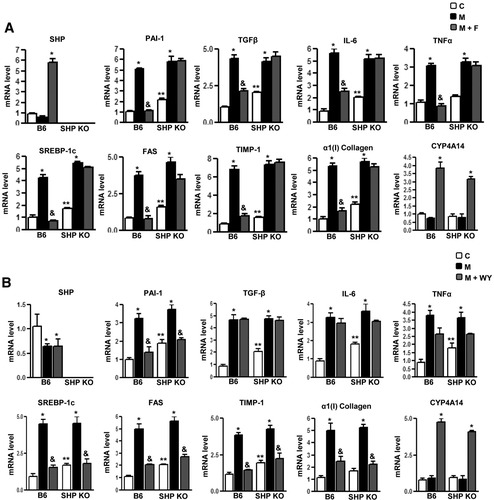
SHP null mice reverse inhibitory effects of fenofibrate on MCD diet–induced PAI-1 gene expression. (A, B) C57BL/6 mice (B6, n = 4 per group) and SHP null mice (n = 4 per group) were fed with chow (C) or with an MCD diet (M) for 4 weeks and compared with MCD diet–fed mice followed by treatment with fenofibrate (100 mg/kg/day) (M+F) or WY14643 (50 mg/kg/day) (M+WY) for an additional 3 days. Liver samples were obtained for total RNA isolation for semiquantitative reverse transcription polymerase chain reaction analysis of PAI-1, SREBP-1c, FAS, α(1) I collagen, TNFα, IL-6, TIMP-1, TGF-β, CYP4A14, and SHP mRNA expression, and normalized to actin expression. Data represent mean ± SD. *P < 0.05, &P < 0.005, and **P < 0.005 compared with chow-fed mice, MCD-diet–fed mice, and B6 chow-fed mice. nd, not detectable.
Furthermore, Wy significantly repressed the expression of genes involved in fatty acid synthesis (SREBP-1c and FAS) along with fibrotic marker genes (collagen α1[I], TIMP-1) in both wild-type and SHP null mice, confirming the previously reported effect of WY on fibrotic marker genes expression.19 Fenofibrate showed similar repressive effects in wild-type mice but failed to repress these genes in SHP null mice. This implicated a predominantly PPARα-dependent action in the case of WY, whereas regulation of these genes' expression by fenofibrate occurs in an SHP-dependent manner. Interestingly, both fenofibrate and WY induced expression of PPARα-target gene CYP4A14 (fatty acid o oxidation marker gene) (Fig. 4A, B) in an SHP-independent manner. As expected, AMPK and ACC were activated in a similar manner in both wild-type and SHP null mice on fenofibrate treatment (Supporting Fig. 2C). Overall, these results demonstrate that fenofibrate repression on various target genes expression up-regulated during the diet-induced progressive fibrosis model (MCD diet) is mediated by the induction of SHP in a PPARα-independent manner, thereby highlighting the significance of the fenofibrate-AMPK-SHP pathway in partial reversal of progressive liver fibrosis.
Fenofibrate Differentially Regulates Stimulatory Factor-Induced PAI-1 Gene Expression by AMPK and SHP.
The PAI-1 gene has been reported to be regulated at the transcriptional level by various physiologically important factors.7-11 To determine the involvement of fenofibrate-mediated induction of SHP gene expression in the regulation of various stimulatory factor-induced PAI-1 gene expression, the effects of TGF-β, TNF-α, HGF, and IL-1α on PAI-1 gene expression in HepG2 cells (Fig. 5) were examined. Adenoviral overexpression of SHP (Ad-SHP) or AMPKα showed significant repression of TGF-β and TNF-α–induced PAI-1 mRNA levels (Fig. 5A, B), consistent with dose-dependent fenofibrate-treated HepG2 cells (Supporting Fig. 3A, B). However, fenofibrate, adenoviral overexpression of SHP, or adenoviral overexpression of AMPK-α showed no significant effect on HGF-mediated and IL-1α–mediated induction of PAI-1 mRNA (Fig. 5C, D). HGF treatments showed a significant increase in SHP mRNA levels (Fig. 5C). Similar effects were observed with AML12 cells treated with either TGF-β or HGF (Supporting Fig. 3C, D). These results suggest that fenofibrate differentially inhibits PAI-1 gene expression by repressing specific stimulatory factor effects via AMPK-mediated up-regulation of SHP gene expression.

Inhibition of cytokine-induced PAI-1 mRNA levels by fenofibrate is mediated by AMPK-dependent induction of SHP. (A-D) HepG2 cells were treated with mock virus, adenovirus SHP, and adenovirus AMPKα at indicated concentrations for 24 hours, after which cells were treated with TGF-β (A), TNF-α (B), HGF (C), or IL-1α (D) at indicated concentrations for another 4 hours under serum-starved conditions. Total RNA was isolated for northern blot analysis of PAI-1 and SHP mRNA expression and was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. Data represent mean ± SD of three individual experiments. *P < 0.05 and &P < 0.005 compared with untreated control and cytokine-treated cells.
Fenofibrate Represses Cytokine-Induced PAI-1 Promoter Activity via SHP.
Next, to elucidate the differential regulation of PAI-1 gene expression by fenofibrate via SHP at the promoter level, the effects of TGF-β, TNF-α, HGF, and IL-1α on human PAI-1 promoter activity in HepG2 cells (Fig. 6A -D) were examined. Fenofibrate significantly repressed TGF-β (Fig. 6A), and TNF-α (Fig. 6B) induced PAI-1 promoter (−800 bp) activity; however, consistent with the previous observations (Fig. 5C, D), it failed to repress HGF-induced (Fig. 6C) and IL-1α–induced (Fig. 6D) activation of the PAI-1 promoter. The repression pattern of fenofibrate was significantly similar to that of cells cotransfected with the SHP expression vector and treatment with metformin, a known activator of SHP,5 for all four factors tested. Endogenous knockdown of AMPK and SHP expression by adenovirus dominant negative AMPK or the expression vector for small interfering RNA (siRNA), SHP, and adenovirus siRNA SHP significantly reversed the fenofibrate-mediated repression of PAI-1 promoter activity and mRNA levels in TGF-β (Fig. 6A,E) and TNF&-agr; (Fig. 6B, F) treated cells. Alpha1 (I) collagen was used as a positive control for TGF-β treatment (Fig. 6E). These results further confirmed that SHP-mediated repression of PAI-1 gene transcription by fenofibrate is a stimulatory factor–specific (TGF-β and TNF-α) phenomenon rather than a general inhibition of various other factors (HGF and IL-1α) regulating PAI-1 gene transcription.
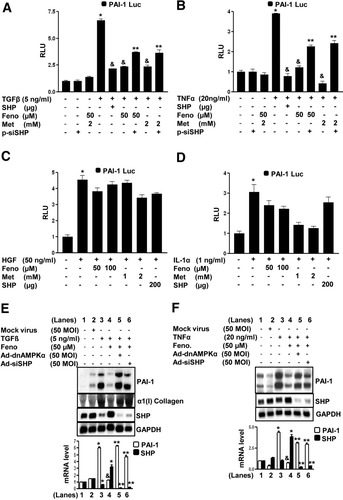
Inhibition of cytokine-induced PAI-1 promoter activity by fenofibrate is SHP dependent. (A-D) HepG2 cells were transfected with the human PAI-1 gene promoter (−800 bp) for 18 hours followed by treatments with TGF-β (A), TNF-α (B), HGF (C), and IL-1α (D) at indicated concentrations for 24 hours in the presence or absence of SHP (200 μg), pSuper siRNA SHP (200 μg), fenofibrate, and metformin for another 24 hours under serum-starved conditions. All experiments were done in triplicate, and data are expressed in RLU and as the fold activation relative to the control, representing mean ± SD of three individual experiments. *P < 0.05, &P < 0.05 and **P < 0.005, compared with untreated control, individual cytokine treatments, and fenofibrate-treated or metformin-treated cells. (E, F) HepG2 cells were treated with mock virus, adenovirus siRNA SHP, and adenovirus dominant negative AMPKα for 48 hours, followed by fenofibrate (Feno) treatment at the indicated concentration for an additional 24 hours, after which cells were treated with TGF-β (E), TNF-α (F), at indicated concentrations for another 4 hours under serum-starved conditions. Total RNA was isolated for Northern blot analysis of PAI-1, collagen type I, and SHP mRNA expression and was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. Data represent mean ± SD of three individual experiments. *P < 0.05, &P < 0.005, and **P < 0.005 compared with untreated control, cytokine-treated cells, and fenofibrate-treated cells.
Fenofibrate Represses Transcription Factor–Mediated Activation of PAI-1 Promoter via AMPK and SHP.
Finally, to elucidate the underlying molecular mechanism of fenofibrate-mediated repression of PAI-1 gene promoter activity, we tried to determine the role of SHP on various transcription factors mediating the effects of TGF-β, TNF-α, HGF, and IL-1α on PAI-1 promoter transactivation in HepG2 cells. Transient transfection assays demonstrated that cotransfections with Smad7 (iSmad, inhibitor of Smad activity), dominant negative Nur77, dominant negative upstream stimulatory factor-1 (USF-1), and inhibitory kinase of nuclear factor kappaB (NF-κB) significantly repressed the PAI-1 promoter activity by TGF-β, TNF-α, HGF, and IL-1α (Supporting Fig. 4A). Next, the effect of fenofibrate on these transcription factors and the role played by the SHP and AMPK signaling pathway (Fig. 7A -E) were assessed. Fenofibrate treatment or overexpression of constitutively active AMPK and SHP significantly repressed the PAI-1 promoter activity by Smad3 and Smad4 (TGF-β) and Nur77 (TNF-α), whereas overexpression of a dominant negative AMPK significantly reversed the inhibitory effects of fenofibrate on PAI-1 promoter activity (Fig. 7A,B). Furthermore, SHP competes with coactivator p300 for Smad3 and Smad4, and with cyclic adenosine monophosphate response element binding protein for Nur77-mediated activation of the PAI-1 gene promoter activity (Fig. 7C,D). These results are in accordance with in vivo results demonstrating that the inhibitory effect of fenofibrate on fibrotic genes expression in TGF-β–treated mice was mediated by SHP (Fig. 3B). Treatments with either TGF-β or TNFα in HepG2 cells to induce PAI-1 gene expression were repressed by fenofibrate, and knockdown of AMPK activity or endogenous SHP expression significantly reversed this inhibitory effect of fenofibrate (Fig. 6E,F). As expected, fenofibrate or metformin treatments or overexpression of SHP were unable to repress the transcriptional activation of the PAI-1 promoter by either USF-1 (HGF) or NF-κB p65 subunit (IL-1α) (Fig. 7E). Fenofibrate also exhibited a similar selective repressive effect on the transcriptional activity of liver receptor homolog-1 (LRH-1), a transcription factor repressed by SHP, but not on steroidogenic factor-1, which is not repressed by SHP, in a multicopy liver receptor homolog-1/steroidogenic factor-1 binding reporter (Sft4-Luc) (Supporting Fig. 4B,C). Overall, these results demonstrate that inhibition of PAI-1 promoter activity by fenofibrate depends on the nature of transcription factors mediating the stimulatory factor effect, essentially repressing those effects that involve transcription factors that are repressed by SHP through AMPK activation (Fig. 7F) and a selective but highly effective role of the fenofibrate-AMPK-SHP cascade in repressing major inflammatory and fibrogenic stimuli related to progressive hepatic fibrosis.
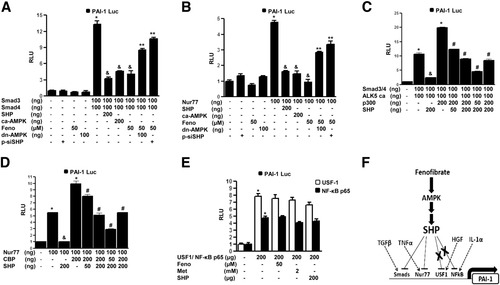
Inhibition of transcription factor–induced PAI-1 promoter activity by fenofibrate is SHP dependent. (A-E) HepG2 cells were cotransfected with the human PAI-1 gene promoter (−800 bp), and Smad3, Smad4 (A, C), Nur77 (B, D), USF1 (E), and NF-κB p65 (E) in the presence or absence of cotransfected constitutively active AMPK, dominant negative MPK (panels A, B, D, E), or with p300 (C), cyclic adenosine monophosphate response element binding protein (D), and SHP (A-E) at indicated concentrations for 18 hours, or the expression vector for small interfering SHP for 48 hrs and fenofibrate (Feno) treatment for an additional 24 h at indicated concentrations. All experiments were done in triplicate, and data are expressed in RLU and as fold activation relative to the control, representing mean ± SD of three individual experiments. *P < 0.05, &P < 0.05, **P < 0.05, and #P < 0.05 compared with untreated control, individual transcription factor cotransfection, fenofibrate treatment, and coactivator cotransfection. (F) Schematic model depicting differential regulation of hepatic PAI-1 gene expression by fenofibrate in an SHP-dependent manner. Fenofibrate represses hepatic PAI-1 transcriptional activity and expression in animals through an AMPK-dependent induction of SHP gene expression. Fenofibrate decreases the cellular ATP/adenosine monophosphate ratio, subsequently leading to AMPK activation and induction of SHP gene expression in liver. Consequently, SHP selectively inhibits transcription factors mediating various stimulatory signaling pathways, which induces PAI-1 gene expression, and as a result can reverse steatohepatitis and liver fibrosis in animals.
Discussion
The pathogenesis of steatohepatitis, which is correlated with a set of metabolic syndromes such as insulin resistance, diabetes, and obesity, or nonalcoholic fatty liver disease, and the underlying molecular mechanisms related to the regulation of genes involved in this process are yet to be elucidated fully. In this study, we demonstrate that reversing the PAI-1 gene expression pattern in liver with fenofibrate through SHP in an AMPK-dependent pathway was rapid and effective both in vitro and in fibrosis-induced in vivo murine models and provides a new insight regarding the molecular cause of steatohepatitis and liver fibrosis at a broader level, with possible therapeutic significance.
Fenofibrate has beneficial effects in amelioration of metabolic syndromes through down-regulation of multiple target genes involved in the pathogenesis of steatohepatitis and fibrosis,12-14 including PAI-1 gene expression, which is critical for ameliorating fibrosis and steatohepatitis.16 Reports from our group have suggested the inhibitory role of SHP in TGF-β–mediated PAI-1 gene transcription.17 However, the exact molecular mechanism of antifibrotic effects of fenofibrate remained unclear. Our study demonstrates that fenofibrate induces SHP gene expression both in vitro and in vivo through AMPK signaling. Our results in wild-type and PPARα null mice clearly suggest that the induction of SHP by fenofibrate was PPARα-independent. Recent reports demonstrated AMPK activation by fenofibrate in a similar PPARα-independent manner.20 Our results demonstrate that, in comparison with other PPARα agonists, fenofibrate specifically activates AMPK by lowering the cellular ATP levels through inhibition of mitochondrial respiratory complexes I and III in PPARα null mice. This result reconfirmed previous reports suggesting inhibition of mitochondrial respiratory chain complexes by fenofibrate, and to a significantly lesser extent by clofibrate, and the mechanism of inhibition as well as the inhibitory function differed considerably.21 We previously demonstrated that metformin also activates AMPK and induces SHP expression.5 Thus, it can be suggested that activation of AMPK by both drugs leads to induction of SHP gene expression, which may be the plausible mechanism underlying the effects.
PAI-1 is a marker of hyperfibrinolysis, and plasma PAI-1 levels are correlated to PAI-1 expression in the liver22 and are directly correlated to the features observed with insulin resistance and visceral obesity as well as steatohepatitis and liver fibrosis.10 By inducing severe and progressive liver fibrosis using TGF-β injection in mice, we elucidated the protective role of fenofibrate via SHP. As previously demonstrated,23 we observed auto-induction of TGF-β expression itself on this treatment as well as an increase in gene expression of α1 (I) collagen IL-6, TIMP-1, and PAI-1, and fenofibrate significantly reversed these TGF-β–mediated effects via SHP. This reversal was completely abolished in SHP null mice, thereby establishing a positive correlation between SHP and its ameliorative effects in liver fibrosis. Furthermore, using the nutritional fibrosis model (MCD diet), we observed that treatment with fenofibrate significantly abrogated mRNA levels of genes involved in fatty acid synthesis (FAS and SREBP-1c), inflammatory response (TNFα and IL-6), inhibitor of fibrosis reversal (TIMP-1), and PAI-1 and profibrogenic markers (α1 [I] collagen and TGF-β). This inhibitory effect of fenofibrate was significantly abrogated in SHP null mice. However, treatments with another PPARα agonist, WY14643, did not show any significant increase of SHP expression in normal mice, consistent with a recent report suggesting no effect of WY on SHP gene expression.24 The effect of WY treatment on the marker genes reflected a pattern independent of SHP. Surprisingly, WY treatment shows no significant effect on the expression of any proinflammatory genes (TGF-β, TNF-α, and IL-6) we tested, whereas fenofibrate significantly represses these gene expressions in an SHP-dependent manner. Our results illustrate a significant difference in mechanism of fenofibrate and WY action in ameliorating hepatic fibrosis. A previous study showed a similar role of SHP in ameliorating bile duct–ligated fibrosis model in rodents.25 Importantly, the fenofibrate-AMPK-SHP cascade provides a broader range of inhibitory effect in the development of fibrosis and signifies the antifibrogenic effects of fenofibrate via induction of SHP.
Our current study demonstrates a selective inhibitory effect of fenofibrate via AMPK and SHP on Smad3 and Smad417 and Nur779 mediated transactivation on PAI-1 promoter activity via competition with coactivators p300 and cyclic adenosine monophosphate response element binding protein, respectively, but no effect on USF-126 or NF-κB p65 subunit11–mediated transactivation on PAI-1 promoter (Fig. 7E). Interestingly, our previous studies demonstrated an inhibitory effect of SHP on Smad3 and Smad417 and Nur77 transactivation (Supporting reference 4) but stimulatory effect on NF-κB p65 in RAW264.7 cells, thus supporting our current observations. However, TGF-β signaling has also been demonstrated to involve transcription factors from the AP-1 family, and SHP also inhibits AP-1 transactivity,23, 25 thereby suggesting the possibility of other transcription factors that might be repressed by the fenofibrate-AMPK-SHP signaling cascade. We are currently investigating the mechanism and possible transcription factors involved in AMPK-mediated induction of SHP gene expression. The effect of fenofibrate on the AMPK-mediated SHP gene expression leads to the possibility that the effects of several other anti-diabetic drugs and adipokines, such as thiazolidinediones, adiponectin, and leptin,27 might be mediated by SHP. Currently, we are investigating the effect of several hepatokines on AMPK signaling and SHP gene regulation. Overall, this selective inhibitory effect of fenofibrate on PAI-1 gene expression provides a possible molecular mechanism for beneficial effects of fenofibrate in the treatment of hepatic metabolic syndromes in addition to lipid-lowering.
To correlate the possible significance of our results in humans, it is important to consider species differences in the case of PPARα activation in rodents and humans. PPARα is expressed at a much lower level in humans compared with rodents, and this is likely to be of functional relevance for fibrate therapy.19 By demonstrating a PPARα-independent mechanism of fenofibrate action and the predominance of SHP gene expression in mammalian species, our study signifies the importance of SHP in liver-related metabolic syndrome. In summary, the current study provides an in-depth analysis of the molecular cause underlying the regulation of PAI-1, a key gene involved in various metabolic syndromes. The pharmacological modulation of SHP gene expression by fenofibrate, metformin, and other AMPK activators in the treatment of metabolic syndromes associated with liver is therefore worthy of further research.
Acknowledgements
The authors thank Dr. David D. Moore (Baylor College of Medicine, Houston, TX) for permitting us to use SHP null mice and Dr. Keun-Gyu Park (Keimyung University School of Medicine, Daegu, South Korea) and Dr. Young-Joo Park (Seoul National University College of Medicine, Seongnam, Republic of Korea) for helpful discussions.




