The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders†
Potential conflict of interest: Nothing to report.
Abstract
TGR5 (Gpbar-1) is a plasma membrane-bound, G protein–coupled receptor for bile acids. TGR5 messenger RNA (mRNA) has been detected in many tissues, including rat cholangiocytes and mouse gallbladder. A role for TGR5 in gallstone formation has been suggested, because TGR5 knockout mice did not develop gallstones when fed a lithogenic diet. In this study, expression and localization of TGR5 was studied in human gallbladders. TGR5 mRNA and protein were detected in all 19 gallbladders. Although TGR5 mRNA was significantly elevated in the presence of gallstones, no such relation was found for TGR5 protein levels. In order to study the localization of TGR5 in human gallbladders, a novel antibody was generated. The receptor was localized in the apical membrane and the rab11-positive recycling endosome of gallbladder epithelial cells. Furthermore, the TGR5 staining colocalized with the cyclic adenosine monophosphate–regulated chloride channel cystic fibrosis transmembrane conductance regulator (CFTR) and the apical sodium-dependent bile salt uptake transporter, suggesting a functional coupling of TGR5 to bile acid uptake and chloride secretion. Stimulation with bile acids significantly increased cyclic adenosine monophosphate concentration in human gallbladder tissue. Incubation of gallbladder epithelial cells with a TGR5 agonist led to a rise of N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE)-fluorescence, suggestive of a decrease in intracellular chloride concentration. The TGR5 agonist–dependent increase in MQAE-fluorescence was absent in TGR5 knockout mice or in the presence of a CFTR inhibitor, indicating that TGR5 mediates chloride secretion via activation of CFTR. The presence of the receptor in both the plasma membrane and the recycling endosome indicate that TGR5 can be regulated by translocation. Conclusion: The data suggest a role for TGR5 in bile acid–induced fluid secretion in biliary epithelial cells. (HEPATOLOGY 2009.)
The gallbladder stores and concentrates bile during fasting and excretes fluid after food intake.1-3 Alterations in absorption and secretion are associated with the development of gallstone disease.2, 4, 5 In early stages of cholesterol gallstone formation, an increased absorption of water and sodium has been observed, increasing the biliary lipid and solute concentration thereby promoting crystal formation.6, 7 In chronic cholelithiasis, fluid absorption decreases5 and inflamed gallbladders may secrete sodium salts.4
Recently, the plasma membrane–bound bile acid receptor TGR5 (Gpbar-1) has been linked to gallstone formation in mice.8 TGR5 is coupled to a stimulatory G protein, and activation of TGR5 by bile acids leads to an increase in intracellular cyclic adenosine monophosphate (cAMP).9, 10 TGR5 messenger RNA (mRNA) has been detected in a variety of human and rodent tissues, including rat cholangiocytes and mouse gallbladder epithelial cells.8-14 TGR5 knockout mice did not develop cholesterol gallstones when fed a lithogenic diet.8 Furthermore, the TGR5 knockout mice showed an increased expression of the cholesterol 7a-hydroxylase (cyp7al), the key regulatory enzyme of bile acid synthesis in hepatocytes, indicating that TGR5 is important for bile acid homeostasis.8, 13 In human tissues, TGR5 expression has only been studied at the mRNA level, and localization of the human TGR5 protein has not yet been investigated due to the lack of reliable antibodies. In the present study, TGR5 expression and localization was analyzed in gallbladders from patients with chronic gallstone disease and compared to normal gallbladders. TGR5 mRNA expression was significantly higher in gallbladders with gallstones, but no association was found between TGR5 protein levels and the presence of gallstones. Using a newly generated antibody against human TGR5, the receptor could be detected in the epithelium of all human gallbladders. Here, it was localized to the apical membrane and the rab11-positive, subapical recycling endosome. Stimulation of gallbladder tissue with bile acids led to a rapid increase in cAMP, demonstrating functional activity of TGR5 in human gallbladder epithelium. In isolated murine gallbladder epithelial cells, a TGR5 agonist led to an increase of N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) fluorescence, indicating a decrease of the intracellular chloride concentration. These findings suggest a role for TGR5 in promoting chloride and fluid secretion in gallbladder epithelial cells in response to bile acids.
Abbreviations
ASBT, apical sodium-dependent bile salt uptake transporter; cAMP, cyclic adenosine monophosphate; CFTR, cystic fibrosis transmembrane conductance regulator; DPPIV, dipeptidyl peptidase IV; GB, gallbladder; GD, gallstone disease; MQAE, N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide; MRP, multidrug resistance protein; NAC, no amplification control; NTCP, sodium-taurocholate cotransporting polypeptide.
Materials and Methods
Taurolithocholic acid and taurocholic acid were obtained from Sigma-Aldrich (Taufkirchen, Germany). AccuPrime-Taq DNA polymerase, LipofectAMINE, MQAE, and Hoechst stain were from Invitrogen (Karlsruhe, Germany). EcoRI and BamHI restriction endonucleases were from New England BioLabs (Beverly, MA). The TGR5 agonist ((4-[[3,5-bis(trifluoromethyl)phenyl]methyl]-6-(2-fluorophenyl)-4,5-dihydro-pyrido[3,2-f]-1,4-oxazepin-3(2H)-one; CAS877052-79-4; patent JP2006056881) was kindly provided by F. Hoffmann-La Roche (Basel, Switzerland). The cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor-172 was from Calbiochem (Darmstadt, Germany).
Antibodies.
The M39 polyclonal antisera were raised in guinea pigs against amino acids 298-318 of human TGR5 (reference sequence NP_733800). The anti–multidrug resistance protein 3 (MRP3) (FDS) antibody was a gift of Dr. Keppler (Heidelberg, Germany). The anti-MRP2(M2I4), anti–dipeptidyl peptidase IV (anti-DPPIV), anti-Na+/K+ adenosine triphosphatase (ATPase), and anti-rab11 antibodies were from Alexis (Grünberg, Germany), BD Biosciences (Heidelberg, Germany), Sigma, and Zymed (San Francisco, CA), respectively. The anti-CFTR(H-182) and anti–apical sodium-dependent bile salt uptake transporter (ASBT) antibodies were from Santa Cruz (Heidelberg, Germany).
Tissue Samples.
The study was performed according to the guidelines of the declaration of Helsinki, and informed written consent was obtained from all patients. Gallbladder tissue was obtained from patients undergoing cholecystectomy for chronic gallstone disease, as part of a resection of a pancreatic or gastric cancer, or as part of a hepatectomy for metastasis resection (Table 1). Tissue was obtained immediately after cholecystectomy and directly washed with ice-cold phosphate-buffered saline (PBS) and divided as follows. Part of the tissue was shock-frozen in liquid nitrogen and stored at −80°C until used for RNA isolation and cryosectioning. For cAMP assays, pieces of about 2 mm × 2 mm × 2 mm were cut and incubated in William's medium, supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, 1% L-glutamine, 100 nM insulin, and 100 nM dexamethasone. Gallstone composition was analyzed by infrared spectrometry and classified as described.15
| GB | Patient Data | Serum Biochemistry | Histopathology | TGR5 mRNA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Diagnosis | Gallstones | AP | γ-GT | Bili | |||
| 1 | 69 | m | Liver metastasis (colon cancer) | None | 103 | 42 | 0.71 | Normal | 6.6 |
| 2 | 53 | m | Liver metastasis (melanoma) | None | 70 | 39 | 0.48 | Normal | 6.6 |
| 3 | 27 | m | Gastric cancer | None | 66 | 32 | 0.14 | Normal | 5.7 |
| 4 | 64 | f | Liver metastasis (breast cancer) | None | 112 | 31 | 0.36 | Normal | 3.7 |
| 5 | 77 | m | Gastric cancer | None | n.d. | 154 | 0.87 | Chronic cholecystitis, mild | 5.1 |
| 6 | 66 | m | Cholangiocarcinoma | None | n.d. | n.d. | 18.94 | Chronic cholecystitis, moderate | 5.4 |
| 7 | 61 | m | Pancreatic cancer | None | 215 | 553 | 8.7 | Chronic cholecystitis, moderate | 5.1 |
| 8 | 66 | m | HCC | Present | 207 | 197 | 2.74 | Chronic cholecystitis, mild | 4.9 |
| 9 | 59 | f | Chronic GD | Present | 92 | 25 | 0.66 | Chronic cholecystitis, mild | 5.9 |
| 10 | 77 | f | Acute pancreatitis | Present | 246 | 190 | n.d. | Chronic cholecystitis, mild | 5.9 |
| 11 | 79 | f | Liver metastasis (colon cancer) | Present | 81 | 158 | 0.39 | Chronic cholecystitis, moderate | 7.3 |
| 12 | 66 | f | Chronic GD | Present | 123 | 363 | 0.52 | Chronic cholecystitis, moderate | 8.6 |
| 13 | 20 | m | Chronic GD | Present | 100 | 97 | 0.32 | Chronic cholecystits, moderate | 9.0 |
| 14 | 58 | f | Chronic GD | Present | n.d. | 21 | 0.62 | Chronic cholecystitis, severe | 5.5 |
| 15 | 32 | f | Cholesterol GD | Present | 60 | 11 | 0.22 | Chronic cholecystitis, mild | 7.2 |
| 16 | 68 | f | Cholesterol GD | Present | 102 | 174 | 0.49 | Chronic cholecystitis, mild | 9.2 |
| 17 | 23 | m | Cholesterol GD | Present | 79 | 119 | 0.55 | Chronic cholecystitis, moderate | 7.6 |
| 18 | 82 | m | Cholesterol GD | Present | 116 | 78 | 0.28 | Chronic cholecystitis, moderate | 8.0 |
| 19 | 70 | f | Cholesterol GD | Present | n.d. | 187 | n.d. | Chronic cholecystits, moderate | 9.7 |
- AP, alkaline phosphatase (normal range: females 35–104 U/L; males 40–129 U/L); bili, total bilirubin (normal range: <1 mg/dL); GB, gallbladder; GD, gallstone disease; γ-GT, γ-glutamyl transferase (normal range: females < 38 U/L; males < 55 U/L); HCC, hepatocellular carcinoma. TGR5 mRNA expression was measured by quantitative real-time PCR and is expressed as difference in threshold cycle numbers between the cDNA and the “no amplification control” of the respective sample. Gallstones from patients 15–19 were analyzed for constituents by infrared spectrometry.
Cloning of Human TGR5.
Total RNA from human liver was reverse-transcribed into complementary DNA (cDNA). The forward primer contained an EcoRI restriction site (5′-cggaattcatgcacttggtccttgtgctct-3′), and the stop codon in the reverse primer was replaced by a BamHI site (5′-cgggatccgcgtagttcaagtccaggtcga-3′). The polymerase chain reaction (PCR) product was cloned into the pEYFP-N1 vector (Clontech, Palo Alto, CA). The sequence of TGR5–yellow fluorescent protein (YFP) was confirmed by sequencing (reference sequence NM_170699).
Quantitative Real-Time PCR.
Following deoxyribonuclease digestion, 1 μg of total RNA was reverse-transcribed with the QuantiTect kit (Qiagen). To measure the level of genomic DNA per samples, a minus-reverse transcriptase control designated as “no amplification control” (NAC) was included for each sample. The NAC contained all reverse transcription PCR reagents except the reverse transcriptase. The level of gene expression was measured by real-time PCR over 40 cycles using SYBR-Green (Applied Biosystems, Foster City, CA) as reporter dye and an ABI-7500 real-time PCR system. Data were produced in duplicates. TGR5 mRNA levels were determined as follows: the mean values of cycle number (Ct-values) of the NAC of each sample were subtracted from the Ct-values of the cDNA of the same sample. The specificity of the amplification was verified by sequencing of the PCR products. Expression of MRP2(ABCC2), MRP3(ABCC3), CFTR(ABCC7), ASBT(SLC10A2), ABCG5/8, SHP, and farnesoid X receptor (FXR) was determined in relation to hypoxanthine phosphoribosyltransferase-1, which served as housekeeping gene (primer sequences on request).
Western Blot Analysis.
Tissue samples were lysed using a tight-fitting potter (1000 rpm, 10 strokes) in a buffer containing 10 mM Tris (pH 7.4), 1% Triton X-100, 150 mM NaCl, 10 mM Na4P2O2, 1 mM ethylene diamine tetraacetic acid (EDTA), 20 mM NaF, 1 mM sodium vanadate, 20 mM β-glycerophosphate, and complete protease inhibitors (Roche). Equal protein amounts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes. Proteins were visualized by enhanced chemiluminescence. Primary antibodies anti-TGR5(M39) and anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were diluted 1:2500 and 1:10,000, respectively. Densitometry was performed using the TotalLab100 software (Nonlinear Dynamics, Durham, NC). Intensity of TGR5 bands was calculated in relation to intensity of GAPDH bands of the respective sample.
Immunofluorescence Staining.
TGR5-YFP–transfected human embryonic kidney 293 (HEK293) cells (grown on glass coverslips) and cryosections (5 μm) of gallbladders were fixed in 100% methanol (−20°C, 5 minutes) and incubated with the following antibodies, and dilutions: M39(TGR5), 1:1000; FDS, 1:100; M2I4, 1:25; Na+/K+ ATPase, 1:100; rab11, 1:50; CFTR, 1:50; ASBT, 1:10. For peptide competition experiments, the primary antibodies were incubated with the corresponding peptide (1 mg/mL in PBS) for 1 hour at room temperature (RT). The peptide-antibody complexes were cleared from the solution by centrifugation (13,000g, 1 minute at RT). The supernatant was applied instead of the primary antibody solution. Fluorescent-labeled secondary antibodies were from Dianova (Hamburg, Germany). Images were obtained with a LSM510-META confocal microscope and analyzed for colocalization using ImageJ, version 1.37 (Wayne Rasband, National Institutes of Health, Bethesda, MD). For quantification, images were taken with the same settings.
cAMP Measurement.
After a 3-hour incubation (William's medium, 37°C, 5% CO2), small pieces of gallbladder tissue were put into cell sieves (Falcon) and transferred into six-well plates (Falcon) containing 8 mL medium with the respective substance (bile acids/vehicle). After 5 minutes, the cell sieves were transferred onto Falcon tubes and washed with ice-cold PBS. The tissue was homogenized in Tris-EDTA buffer (0.05 M Tris [pH 7.5], 4 mM EDTA) using a tight-fitting potter (1000 rpm, 10 strokes). Aliquots from each sample were used for protein determination and the sample was incubated at 80°C for 2 minutes and centrifuged at 12,000g for 1 minute. The supernatant was analyzed for cAMP using the cAMP(3H) assay from Amersham Biosciences. Total amount of cAMP (picomole per milligram protein) was calculated. Relative increase in intracellular cAMP per sample was determined in relation to controls.
Isolation of Mouse Gallbladder Epithelial Cells.
TGR5 knockout mice were a generous gift from Schering-Plough Research Institute (Kenilworth, NJ).8 Mice were backcrossed in a C57BL/6 background, and genotyping was performed as described.8 Gallbladder epithelial cells were isolated from 6-week-old male C57BL/6 and TGR5 knockout mice using microdissection. Small pieces of the gallbladder tissue were plated into a collagen gel and cultured in growth factor–containing medium.16, 17 After 8-10 days, biliary epithelial cells grew out of the pieces forming a monolayer. The biliary phenotype was confirmed by detection of mRNA expression of biliary markers such as cytokeratin7 and cytokeratin19, Mrp2, Mrp3, Cftr, and Asbt.
Measurement of Intracellular Chloride.
Changes in intracellular chloride were measured as described.18, 19 Mouse gallbladder epithelial cells were grown in a monolayer on collagen-coated 24-well plates and loaded with the fluorescent chloride indicator MQAE (1 mM)20 in phenol red–free Dulbecco's modified Eagle medium (DMEM; 37°C, 5% CO2). After 6–10 hours, the cells were washed and maintained in MQAE-free, phenol red–free DMEM. MQAE fluorescence was measured using a Fluoroskan-Ascent-FL and an excitation wavelength of 355 nm. Emission was detected at 460 nm. Fluorescence was measured in 1-second intervals for 1 minute to determine the baseline of each experiment. After application of the respective substances, MQAE fluorescence was measured in 2-second intervals for a further 10 minutes. Only experiments with a stable baseline (<2% change) were analyzed. The fluorescence at the beginning of each recording was set to 1.0. An increase in MQAE fluorescence corresponds to a decrease in intracellular chloride concentration.
Statistics.
Data are given as means ± standard error of the mean. Results were analyzed using the two-sided Student t-test and P ≤ 0.05 was considered statistically significant.
Results
Gallbladder tissue was obtained from patients with chronic gallstone disease and patients with cancer who underwent liver resection and cholecystectomy for removal of metastasis or hepatocellular carcinoma. One patient received a cholecystectomy during resection of pancreatic cancer. Histopathological examination showed normal histology in four, mild inflammation in six, moderate inflammation in eight, and severe inflammation with thickening of the gallbladder wall in one of the gallbladders. Gallstones were present in 12 of the gallbladders with mild to severe inflammation (Table 1).
TGR5 mRNA expression in these gallbladders was analyzed by quantitative real-time PCR. The design of TGR5 primer pairs, which distinguish between mRNA and genomic DNA, was not feasible because the coding sequence of TGR5 is derived from only one exon. The real-time PCR results obtained with these primers therefore overestimate the amount of TGR5 mRNA in the samples, because they comprise both the signal from the mRNA as well as from the genomic DNA. In order to quantify the amount of TGR5 mRNA more precisely, real-time PCR was carried out using both cDNA as well as a reverse transcriptase negative control (NAC) as described in Materials and Methods. The PCR products were sequenced to verify the specificity of the amplification. Expression of TGR5 mRNA was observed in all 19 gallbladders, which is in line with the data obtained from mice (Table 1, Fig. 1).8 The mean cycle number needed to reach the threshold (Ct value) obtained with the cDNA samples for TGR5 was 27.0 as compared to 33.6 for the genomic DNA (NAC) samples. The difference in Ct values between cDNA and the genomic DNA (NAC) for TGR5 was 6.7 ± 0.4 and ranged from 3.7 (Gallbladder 4) to 9.7 (Gallbladder 19) (Table 1). TGR5 mRNA expression was 5.5 ± 0.7 in gallbladders without gallstones and 7.4 ± 0.5 in gallbladders with gallstones (n = 12, P < 0.05). Gallbladders from patients with cholesterol gallstones (Gallbladders 15 to 19) showed significantly higher TGR5 mRNA levels as compared to controls (8.3 ± 0.5, n = 5, P < 0.05) (Fig. 1A). The histopathological presence of cholecystitis did not affect TGR5 mRNA expression significantly; however, TGR5 mRNA levels were higher in gallbladder tissue with signs of moderate inflammation (7.4 ± 0.6 versus 5.7 ± 1.0, P = 0.06, Fig. 1B). The mRNA expression of known gallstone-associated genes, such as FXR, ABCG5, and ABCG8, was unchanged in gallbladders from patients with gallstones; however, a significant increase in mRNA levels of the cAMP-regulated chloride channel CFTR and a marked down-regulation of the bile acid uptake transporter ASBT (SLC10A2) were observed in the presence of gallstones (Supporting Fig. 1).
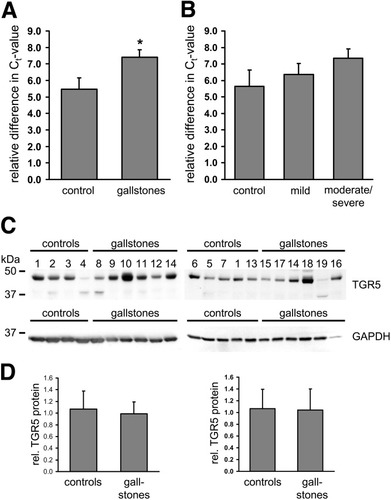
TGR5 mRNA expression in human gallbladder tissues was analyzed by quantitative real-time PCR. Data are expressed as relative difference in Ct values between the cDNA and the “no amplification control” of the respective sample. (A) TGR5 mRNA was detected in all 19 gallbladders. TGR5 mRNA levels were significantly higher in the presence of gallstones (control, n = 7; gallstones, n = 12). (B) There was no significant difference in the TGR5 mRNA expression between gallbladders with normal histology (control, n = 4) and gallbladders with mild (n = 6) or moderate to severe cholecystitis (n = 9). (C) Western blot analysis of TGR5 in human gallbladder tissue. The anti-TGR5 antiserum detected a broad band around 48 kDa in all 19 gallbladders, which corresponds to the glycosylated form of the TGR5 protein. The unglycosylated form of the TGR5 protein was visible just below 37 kDa in some gallbladders (2,4,8,5,19). Detection of GAPDH served as loading control for densitometric analysis. (D) Densitometric analysis of TGR5 protein levels in relation to GAPDH of the respective sample. No difference in TGR5 protein levels was observed between controls and gallbladders from patients with gallstone disease.
The previously described anti-TGR5 antibodies (M38, K36) are directed against rat TGR5 and do not recognize human TGR5.11, 12 Therefore, a new polyclonal antibody (M39) was raised against human TGR5. This novel antibody to TGR5 (M39) detected a broad band of approximately 48 kDa on immunoblots of gallbladder tissue lysates (Fig. 1C). In some of the samples, a second band of about 36 kDa was visible and may represent the unglycosylated form of the TGR5 protein. There was no significant difference in TGR5 protein levels between gallbladders with gallstones and controls, as measured by densitometric analysis. These findings were unexpected because knockout of TGR5 in mice prevented cholesterol gallstone formation.8
For immunofluorescence analysis, the anti-TGR5 antiserum (M39) was tested on HEK293 cells, which were transiently transfected with a construct containing human TGR5 fused to YFP (TGR5-YFP). TGR5-YFP was clearly detected with the anti-TGR5 antiserum, whereas untransfected cells showed no fluorescence staining (Fig. 2A,B). Furthermore, the staining of TGR5-YFP with the M39 serum was completely abolished by preincubation of the antiserum with the M39 peptide (Fig. 2G,H). The preimmune serum of the same animal did not show any specific labeling (Fig. 2D,E).
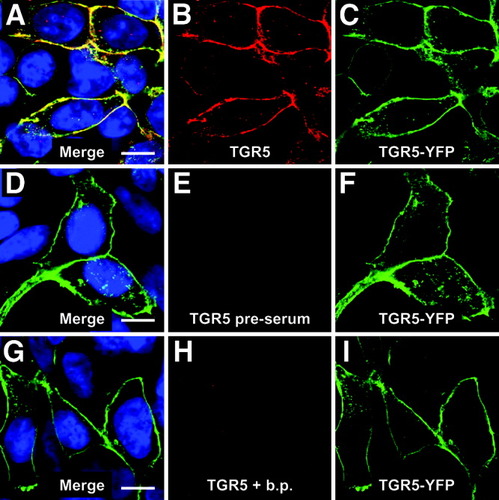
Characterization of the anti-TGR5 antiserum. In transiently transfected HEK293 cells, human TGR5-YFP is localized in the plasma membrane and some intracellular vesicles (green fluorescence in panels C,F,I). Staining with the M39 anti-TGR5 antiserum (red) detected the receptor both in the plasma membrane as well as intracellularly (A-C). The preimmune serum from the same animal did not show any specific staining (D,E) and preincubation of the serum with the peptide (b.p.), which was used to immunize the animal, abolished the staining of the transfected cells completely (G,H). Bars = 10 μm.
Immunofluorescence staining of the gallbladder cryosections with the anti-TGR5 (M39) serum showed a strong signal in the epithelium (Fig. 3A,B). Colabeling using antibodies against MRP2 (ABCC2) and MRP3 (ABCC3), which are localized in the apical and basolateral membranes of gallbladder epithelial cells,21 respectively, revealed that the TGR5 immunofluorescence pattern was similar to that obtained for MRP2 (Fig. 3C). Preincubation of the M39 serum with the respective peptide completely abolished the TGR5 staining, whereas the MRP2 and MRP3 fluorescence signals were unaffected, indicating the specificity of the detection (Fig. 3E-H). Higher magnification demonstrated that the TGR5 immunoreactivity only partially colocalized with the MRP2 fluorescence within the apical membrane (Fig. 4C,F,I) and that TGR5 was also present in a subapical compartment. Use of an antibody against DPPIV, which is found in the apical membrane of the gallbladder epithelium,21 confirmed that TGR5 is detected in both the apical membrane as well as in the cytoplasm below the apical membrane (Fig. 4J-L). The TGR5-positive, subapical compartment could be identified as the apical recycling endosome by the use of an antibody against rab11, which is a marker protein of this compartment22, 23 (Fig. 5).
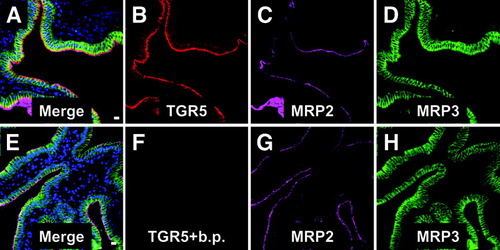
Immunofluorescence staining of TGR5 in human gallbladder tissue. Cryosections were triple-labeled for TGR5 (red), MRP2 (purple), and MRP3 (green) which are marker proteins of the apical and basolateral membrane of the gallbladder epithelium, respectively. The TGR5 staining (A,B) was restricted to the epithelium and showed a similar fluorescence pattern as the MRP2 immunoreactivity (C,G). Preincubation of the anti-TGR5 antiserum with the corresponding peptide abolished the TGR5 staining of the epithelium completely (E,F), whereas the MRP2 fluorescence (G) and MRP3 fluorescence (H) remained unaffected. Nuclei were stained with Hoechst (blue). Bars = 10 μm.
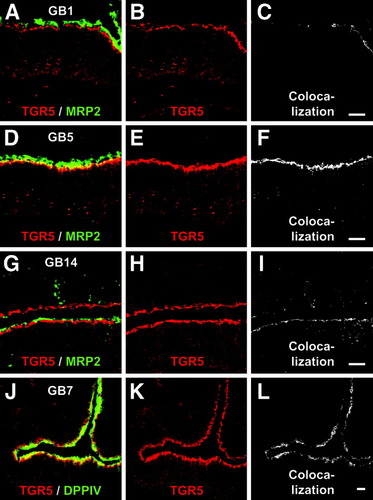
Localization of TGR5 in the gallbladder epithelium. Gallbladder tissue from gallbladders with normal histology (GB1), mild cholecystitis (GB5), or severe cholecystitis (GB14) was stained for MRP2 (green) and TGR5 (red). TGR5 partially colocalized with MRP2 in the apical membrane domain as demonstrated by the yellow color (A,D,G) and the extraction of the colocalized pixels using ImageJ (C,F,I). Most of the TGR5 protein is localized in a subapical compartment below the MRP2-stained membrane. Dipeptidyl peptidase IV (DPPIV) is localized in the apical membrane and also only partially colocalized with the TGR5 immunoreactivity (J-L). Bars = 10 μm.
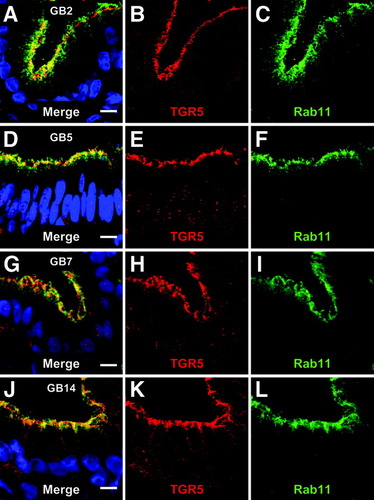
TGR5 is localized in the apical recycling endosome. An antibody against rab11 was used to stain the recycling endosome, which is localized below the apical membrane in polarized epithelial cells. The TGR5 immunoreactivity colocalized with the rab11-staining, indicating that TGR5 is present in the recycling endosome. No difference between gallbladders from patients with gallstone disease (GB7, GB14) or control gallbladders (GB2, GB5) was observed. Bars = 10 μm.
The cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7)24 is a cAMP-regulated chloride channel25 in the apical membrane of polarized epithelial cells, such as airway epithelial cells, cholangiocytes, and gallbladder epithelial cells.26-29 CFTR-mediated chloride secretion depends on the total amount of CFTR within the apical membrane, which is regulated through retrieval from and insertion into the membrane and involves different endosomal compartments, including rab11- containing recycling endosomes.30 Double-labeling experiments with antibodies against TGR5 and CFTR revealed colocalization of both antigens in the apical compartment (Fig. 6A-L). The apical sodium-dependent bile salt transporter (ASBT, SLC10A2)31 can also be found in the apical membrane and the subapical compartment of the biliary epithelium,32-34 where the ASBT staining colocalized with the TGR5 immunoreactivity (Fig. 6M-T). A small amount of TGR5 was also detected in the basolateral membrane as shown by colocalization of the TGR5 immunoreactivity with the Na+/K+ ATPase staining, a marker protein of the basolateral membrane (data not shown).
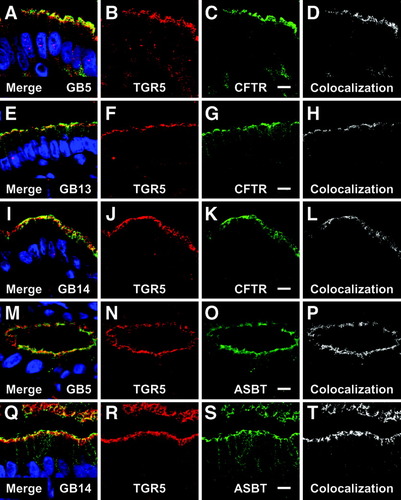
Localization of apical transport proteins and TGR5 in the gallbladder epithelium. The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated chloride channel. Double-labeling for TGR5 (red) and CFTR (green) revealed the colocalization of both proteins in the apical domain (A-I), as demonstrated by the colocalized pixels (D,H,L). The apical sodium-dependent bile acid transporter (ASBT) also colocalized with TGR5 in the apical compartment (M,Q,P,T). No relation of transporter localization to the presence of gallstones (GB13, GB14) as compared to controls (GB5) was noted. Bars = 10 μm.
Functional activity of TGR5 in human gallbladder was analyzed through measurement of cAMP after stimulation with taurolithocholate (TLC, 25 μM) and taurocholate (TC, 25 μM) for 5 minutes. Both TLC and TC increased cAMP in gallbladder tissue significantly by 2.2-fold ± 0.5-fold and 2.4-fold ± 0.7-fold (n = 5), respectively, as compared to controls (Fig. 7A). The observed effects varied between gallbladders (1.4-fold to 4.0-fold) and may underestimate TGR5 activity and bile acid responsiveness, because the epithelium accounted for only a small part of the tissue used, which also contained serosa, muscle, and submucosa.
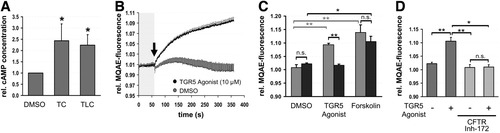
Activation of TGR5 increases intracellular cAMP and chloride secretion in gallbladder epithelium. (A) Cyclic AMP content was measured in small pieces of human gallbladder tissue after incubation with taurocholate (TC) or taurolithocholate (TLC) for 5 minutes. Stimulation with TC and TLC led to a significant increase in cAMP content (n = 5, P ≤ 0.05). DMSO, dimethyl sulfoxide. (B) Measurement of intracellular chloride concentration in isolated murine gallbladder epithelial cells using the chloride-sensitive dye MQAE. After determination of baseline MQAE fluorescence for 1 minute, a TGR5 agonist (10 μM) or vehicle (DMSO) was applied (arrow) and fluorescence was measured in 2-second intervals for 10 minutes. The fluorescence value at the beginning of each recording was set to 1.0. Administration of the TGR5 agonist led to a significant increase in MQAE fluorescence (n = 5; P < 0.01). (C) Measurement of intracellular chloride concentration in isolated gallbladder epithelial cells from TGR5 wild-type (gray) and TGR5 knockout mice (black). Treatment with a TGR5 agonist (10 μM, 10 minutes, n = 5) significantly increased MQAE fluorescence in TGR5 wild-type cells (P < 0.01), whereas no change was observed in cells from TGR5 knockout mice. Forskolin (10 μM, n = 4) lead to a significant rise in MQAE fluorescence independent of TGR5 expression. (D) Treatment of gallbladder epithelial cells with the CFTR inhibitor-172 (10 μM, n = 4) prior to stimulation with the TGR5 agonist (10 μM) completely abolished the increase in MQAE fluorescence. *P < 0.05; **P < 0.01; n.s.= not significantly different.
In isolated murine gallbladder epithelial cells, a TGR5 agonist (10 μM, 10 minutes, Supporting Fig. 2) increased fluorescence of the chloride sensitive dye MQAE significantly by 9.4 ± 0.5% (n = 5, P = 0.006) as compared to controls (dimethyl sulfoxide [DMSO] only), suggesting a decrease in intracellular chloride content (Fig. 7B,C). This effect was abolished in cells derived from TGR5 knockout mice, suggesting that the agonist and the observed changes in MQAE fluorescence are TGR5-specific. Forskolin (10 μM) was used as positive control and induced a significant increase in MQAE fluorescence in cells from both wild-type (13.9% ± 2.9%) and TGR5 knockout mice (10.6% ± 1.9%, n = 4, Fig. 7C). Preincubation of wild-type cells with the cell-permeable inhibitor of CFTR Inh-172 (10 μM, 30 minutes) prior to stimulation with the TGR5 agonist (10 μM, 10 minutes) completely suppressed the increase in MAQE fluorescence observed in the respective control experiments (DMSO + agonist, n = 4, Fig. 7D). These data suggest that TGR5 induces chloride secretion in biliary epithelial cells through activation of CFTR.
Discussion
The present study shows that the membrane-bound bile acid receptor TGR5 is highly expressed in human gallbladder epithelium at both the mRNA and protein level. TGR5 knockout mice did not develop cholesterol gallstones, suggesting that high expression levels of TGR5 or increased TGR5 activity may predispose to cholesterol gallstone formation.8 TGR5 mRNA expression was significantly increased in gallbladders from patients with gallstone disease; however, no association between TGR5 protein levels and the presence of gallstones was observed. TGR5 was localized in the apical compartment of human gallbladder epithelial cells, where it can be activated by TLC and TC, leading to an increased production of cAMP.
The activity of G protein–coupled receptors can be regulated through endocytic retrieval and exocytic insertion (for reviews, see Ferguson35 and Pierce et al.36). However, regulation of TGR5 has not been studied to date. The localization of TGR5 in a subapical compartment, which could be identified as the apical recycling endosome, indicates that the receptor is regulated through translocation. The retrieval of TGR5 from the plasma membrane may also lead to a desensitization of the receptor toward bile acids. The responsiveness of the endothelin-A receptor (ETA) toward endothelin-1 was shown to decrease from picomolar to nanomolar concentrations through cAMP-dependent phosphorylation and subsequent internalization of the receptor.37 In this respect, it is noteworthy that in both cholangiocytes and gallbladder epithelial cells, which are exposed to millimolar bile acid concentrations, TGR5 is mainly localized in a subapical compartment and only to a smaller extent in the plasma membrane, whereas in sinusoidal endothelial cells and Kupffer cells, i.e., cells normally exposed to low bile acid concentrations, the receptor was predominantly detected within the plasma membrane.11, 12
The function of TGR5 in gallbladder epithelium is unclear. However, both fluid and mucin secretion are stimulated by bile acids and cAMP, suggestive of a role for TGR5 in gallbladder function.38, 39 Secretin1, 40 and vasoactive intestinal polypeptide41, 42 promote fluid secretion into bile through activation of G protein–coupled receptors in the basolateral membrane of gallbladder epithelial cells43 and subsequent elevation of intracellular cAMP. The second messenger cAMP activates the apical CFTR and promotes chloride excretion both through activation of the channel itself as well as through translocation of CFTR into the apical membrane.25, 44-48 Luminal bile acids can induce mucin and chloride secretion and potentiate cAMP-mediated chloride efflux from biliary epithelial cells.38, 39, 49 The TGR5 agonist used in this study induced a significant increase in MQAE fluorescence in gallbladder epithelial cells from wild-type mice, whereas no changes were observed in cells derived from TGR5 knockout mice. Forskolin increased MQAE fluorescence independent of TGR5 expression, indicating that TGR5 may promote cAMP-dependent chloride secretion via activation of CFTR. Preincubation of gallbladder epithelial cells with a CFTR inhibitor prior to addition of the TGR5 agonist completely abolished the increase in MQAE fluorescence, supporting the hypothesis that TGR5 facilitates chloride secretion through activation of CFTR. Furthermore, the colocalization of TGR5 with CFTR in gallbladder epithelial cells may allow for rapid and compartmentalized signal transduction from the receptor to the channel, leading to increased channel opening as well as translocation of intracellular CFTR into the plasma membrane. The assembly of G protein–coupled receptors with their downstream effectors into macromolecular complexes allows localized signaling events and has already been described for the β2-adrenergic receptor together with CFTR as well as with a L-type Ca2+ channel.50, 51
Bile acid–induced chloride and mucin secretion in gallbladder epithelial cells has been linked to the uptake of bile acids into the cells by the ASBT (SLC10A2) and the subsequent activation of intracellular kinase pathways.38, 49 In isolated cholangiocytes, ASBT function is regulated by cAMP, which induces insertion of ASBT into the apical membrane from intracellular stores and enhances uptake of bile acids.32 A similar cAMP-mediated regulation by insertion and retrieval has been described for the basolateral sodium taurocholate cotransporting protein (NTCP, SLC10A1), which is closely related to ASBT.52 After activation through biliary bile acids, TGR5 may, via cAMP, promote the insertion of ASBT into the apical membrane thereby increasing bile acid uptake inside the cell through ASBT-mediated reabsorption, which allows biliary bile acid concentrations to diminish. The subsequent rise in intracellular bile acid concentrations within the biliary epithelium may then increase mucin and fluid secretion.38 Furthermore, through elevation of cAMP, TGR5 may contribute to bile acid–induced fluid and electrolyte secretion directly via activation and translocation of CFTR (Fig. 8). TGR5 could therefore function as a sensor coupling biliary bile acid concentration to bile acid reabsorption and fluid secretion.
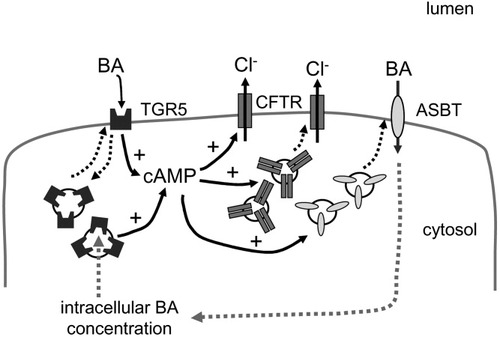
Working model of TGR5 function in biliary epithelium. Bile acids (BA) activate TGR5 in the plasma membrane, which leads to an increase in intracellular cAMP. The rise in cAMP activates the cAMP-regulated chloride channel CFTR directly and also promotes insertion of CFTR into the plasma membrane, thereby increasing chloride secretion. Translocation of the bile acid uptake transporter ASBT from intracellular stores into the membrane in response to cAMP promotes uptake of bile acids into the cell,32 thereby lowering biliary bile acid concentrations. The subsequent rise in intracellular bile acid levels may facilitate fluid and mucin secretion.38 TGR5 may not only be activated by extracellular bile acids but also by intracellular bile acids, which then again leads to the insertion of CFTR and ASBT into the apical membrane and subsequent secretion. Internalization of TGR5 from the membrane desensitizes the receptor for bile acids. Dotted lines represent translocation.
Acknowledgements
Technical assistance by Stefanie Winandy is gratefully acknowledged. TGR5 knockout mice were a generous gift from Dr. Vassileva from Schering-Plough Research Institute (Kenilworth, NJ)




