Toll-like receptor 3 signaling attenuates liver regeneration†
Potential conflict of interest: Nothing to report.
Abstract
The current model for liver regeneration suggests that cell damage triggers Toll-like receptor (TLR) signaling via MyD88, leading to the induction of nuclear factor κB (NF-κB) and secretion of inflammatory cytokines that in turn prime liver regeneration. TLR3 is unique among TLRs in that it signals through TRIF (TIR domain-containing adaptor-inducing interferon-β), not through MyD88, and may lead to activation of either the inflammatory or apoptotic pathway. The inflammatory pathway leads to NF-κB activation, whereas the apoptotic pathway, believed to be mediated by Rip3, leads to caspase-8 activation. In this study, we explored the role of TLR3 in liver regeneration by comparing the response to 70% partial hepatectomy of TLR3wt and TLR3−/− mice. We found that following partial hepatectomy, TLR3−/− mice demonstrated earlier hepatocyte proliferation. Furthermore, within the first hours, we observed a dramatic TLR3-dependent NF-κB activation and an increase in Rip3 levels in hepatocytes, accompanied by caspase-8 activation but without an apoptotic outcome. Conclusion: TLR3 plays an inhibitory role in the priming of liver regeneration, thus reinforcing the role of the innate immune system in balancing tissue regeneration. (HEPATOLOGY 2009.)
The liver has a unique regeneration capacity to recover from up to 70% (and in some cases 90%) partial hepatectomy (PHx). According to the current concept, the innate immune system senses liver damage and leads to the secretion of inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, and hepatocyte growth factor (HGF), which subsequently prime hepatocytes to progress from G0 through G1 to S phase.1, 2
Toll-like receptors (TLR) act as innate immune sensors by recognizing pathogen-associated molecular patterns, thus playing a central role in host defense. Upon engagement with an appropriate ligand, TLRs initiate a canonical signaling cascade, mediated by MyD88, that results in the induction of NF-κB and secretion of inflammatory cytokines, such as IL-6 and TNF-α. The TLR/MyD88 signaling pathway was shown to be required for the initial phase of liver regeneration.1-4 However, the identity of the TLR and the ligand that triggers the MyD88 signaling cascade is not yet known.
TLR3 recognizes double-stranded RNA, messenger RNA (mRNA), and the synthetic ligand polyinosinic:polycytidylic acid [poly(IC)]5, 6 and is unique among TLRs in that it does not signal through MyD88; rather, it uses a distinct adaptor protein, TRIF (TIR domain-containing adaptor-inducing IFN-β). TLR3 signaling may induce two downstream pathways—namely, the inflammatory or the apoptotic pathway. The inflammatory pathway is mediated mainly by Rip1 and leads to NF-κB activation. The apoptotic pathway, on the other hand, was shown to be mediated by Rip3 and results in caspase-8 activation.7, 8 We have shown that in human hepatoma cell lines, unlike white blood cells, TLR3 signaling is skewed towards the apoptotic pathway.9 A similar observation was also reported in other tumor cells.10, 11
In this study, we explored the role of TLR3 in liver regeneration by comparing the regeneration process in TLR3−/− and TLR3wt mice following 70% PHx. We show that following PHx, in the absence of TLR3, hepatocyte proliferation was accelerated, while the levels of IL-6 and soluble interleukin-6 receptor (sIL-6R) were significantly lower. We further demonstrate that at the early time points after PHx, TLR3 signaling is induced in hepatocytes, resulting in activation of NF-κB, in an increase in the levels of Rip3 and activation of caspase-8, with no evidence of apoptosis. In addition, we found that the presence of active TLR3 in Kupffer cells inhibits NF-κB activation. Based on our results, we propose that following PHx, TLR3 signaling attenuates the initiation of liver regeneration.
Abbreviations
ALT, alanine aminotransferase; BrdU, bromodeoxyuridine; HGF, hepatocyte growth factor; IL, interleukin; mRNA, messenger RNA; NF-κB, nuclear factor κB; PHx, partial hepatectomy; poly(IC), polyinosinic:polycytidylic acid; sIL-6R, soluble interleukin-6 receptor; STAT3, signal transducer and activator of transcription 3; TLR, Toll-like receptor; TLR3, Toll-like receptor 3; TNF-α, tumor necrosis factor α; TRIF, TIR domain-containing adaptor-inducing interferon-β.
Materials and Methods
Animals.
Twelve-week-old male C57Bl/6 (TLR3wt) mice were purchased from Harlan Israel. TLR3−/− mice (C57Bl/6 background) were a gift from R. Flavel's laboratory. All mice were kept in a specific pathogen-free facility and handled according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health.
Partial Hepatectomy.
Resection of 70% of the total liver mass was performed in mice around 8 A.M. according to Higgins and Anderson12 under light anesthesia with intraperitoneally administered xylazine at 10 mg/g body weight (Chanelle Pharmaceuticals Manufacturing, Loughrea, Galway, Ireland) and ketamine at 450 mg/g body weight (Fort Dodge Animal Health, Fort Dodge, IA). In brief, the abdominal cavity was opened with a longitudinal incision and the median and left lateral lobes were ligated, followed by excision to remove the three lobes. After closing the abdominal wall by suture, animals were returned to cages and fed ad libitum. At the indicated times, mice were sacrificed, serum was taken, and liver tissues were harvested. (n = 5 mice/group).
Immuonhistochemistry Stainings.
For bromodeoxyuridine (BrdU) incorporation, 85 μg BrdU (Sigma, Cat#B5002) per gram body weight were injected intraperitoneally 3 hours before the mice were sacrificed.
Immunohistochemistry was performed on 4-μm-thick formaline-fixed paraffin-embedded liver tissue sections by the standard procedure. Deparaffinization and rehydration were followed by Antigen retrieval using a pressure cooker with Citrate buffer (pH 6.0) for BrdU and Rip3 and with Glycin buffer (pH 9.0) for CDC47 and NF-κB. Primary antibodies were diluted 1:50: Mo744, DAKO for BrdU; AP7819b, ABGENT for Rip3; CM137b, Biocare Medical (Pharmatrade) for CDC47 and sc-372, Santa Cruz for NF-κB p65 detection. Secondary antibodies were from DAKO. Staining was developed with diamonobenzine using a kit from Zymed (Rip3 and CDC47) or with 3-amino-9-ethylcarbazone (BrdU) using a kit from DAKO.
Terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining was performed using the manufacturer's protocol for in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany, catalog #11 684 795 910). As a positive control, sequential liver sections were treated with 1 U/mL DNase I for 30 minutes.
Plasma Aminotransferase Activity Assay.
Serum alanine aminotransferase (ALT) was measured using the Reflotron GPT (ALT) Test (Roche, Manheim, Germany) according to the manufacturer's instructions.
Measurements of Serum Cytokine Levels.
Commercially available enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) were used to measure serum levels of IL-6 (catalog #DY406), sIL-6 (catalog #DY1830), and HGF (catalog #DY2207) cytokines.
Semiquantitative Reverse-Transcription Polymerase Chain Reaction.
Total RNA isolated from frozen liver tissues with Tri-reagent (Sigma) was treated with DNaseI (DNaseI Kit, Ambion). The RNA was then reverse-transcribed (RT) using the Moloney murine leukemia virus reverse transcriptase (Promega) with random hexamer primers (Promega). The resultant complementary DNA was then subjected to polymerase chain reaction (PCR) using the following primer sets: IL-22 sense 5′-ACCTTTCCTGACCAAACTCA-3′ and antisense 5′-AGCTTCTTCTCGCTCAGACG-3′; IL-22R sense 5′-GTTCTGCAACCTGACTATGGAG-3′ and antisense 5′-GTACAGGTGGCTTGGTCATG-3′; IL-2β sense 5′-GGCTGTCCTGATGAGAGCAT-3′ and antisense 5′-AGCTCATATGGGTCCGACAG-3′; glyceraldehyde 3-phosphate dehydrogenase sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-TCCACCACCCTGTTGCTGTA-3′. Band intensity was determined by the TINA computer program and normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Western Blotting.
Tissues were homogenized in lysis buffer A (0.25 M sucrose; 20 mM Tris [pH 7.6], 1.5 mM MgCl2, 10% glycerol, 1 mM ethylene diamine tetraacetic acid, and the Complete Mini protein inhibitor cocktail obtained from Roche Diagnostics, catalog #11836153001) incubated on ice for 30 minutes, and centrifuged at 5,000 rpm for 10 minutes at 4°C to obtain cytoplasmic fractions. To obtain nuclear fractions, pellets were dissolved in B buffer (25% glycerol, 40 mM Tris [pH 7.6], 0.42 M NaCl, 0.2 mM ethylene diamine tetraacetic acid, and Complete Mini protein inhibitor cocktail) and incubated for an additional 30 minutes on ice. The lysates were centrifuged at 13,000 rpm for 20 minutes at 4°C, and the supernatant containing the nuclear fraction was saved. Samples were loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel and subjected to western blot analysis. The primary antibodies used were anti–NF-κB (Santa Cruz Biotechnology, catalog #sc-372), anti-Rip3 (ABGENT, catalog #AP7819b), anti–caspase-8 (Santa Cruz Biotechnology, catalog #sc-7890), anti–β-actin (MPI, catalog #691001), anti–signal transducer and activator of transcription 3 (STAT3) (Santa Crus Biotechnology, catalog #sc-8019), anti–P-STAT3 (Santa Cruz Biotechnology, catalog #sc-8059), anti-Rip1 (Santa Cruz Biotechnology, catalog #sc-7881). As a secondary antibody, Dako EnVision System labeled Polymer-HRP anti-mouse (catalog #K4001) or anti-rabbit (catalog #K4003) were used. Proteins were visualized using the EZ-ECL chemiluminescence detection kit for HRP (Biological Industries, Israel).
Caspase-8 Immunopurification.
Crude protein extracts (0.5 mg) were diluted in 1 mL of immunoprecipitation buffer (30 mM NaCl, 50 nM Tris-HCl, 5 mM ethylene diamine tetraacetic acid, 0.1% Triton, 0.02% Na Azide, and freshly added Complete Mini protein inhibitor cocktail [Roche Diagnostics, catalog #11836153001]). The diluted samples were precleared by Protein A-Sepharose beads (GE Healthcare, catalog #17-0780-01) for 30 minutes at 4°C. The beads were removed by means of centrifugation at 14,000 rpm for 1 minute, and the extract was incubated overnight at 4°C with Protein A-Sepharose beads (GE Healthcare, catalog #17-0780-01) covalently cross-linked to caspase-8 antibodies (caspase-8 p20, Santa Cruz Biotechnology, catalog #sc-7890) using dimethyl pimelimidate (Sigma, catalog #D-8388) as a cross-linking agent by a standard protocol. The complexes thus formed were transferred to spin columns (Handee Spin Columns Snap Cap, Pierce Biotechnologies, catalog #69725), centrifuged at 14,000 rpm for 1 minute, and the flow-through was discarded. The bound caspase-8 was eluted from the antibody-bead complexes by 1 M glycine (pH 3.0). The pH was then neutralized by the addition of 1.5 M Tris (pH 8.8), and the eluate was subjected to western blotting.
Mice Injection.
TLR3wt mice were injected intravenously with 1.5 mL saline with or without 50 μg poly(IC) per 20 g mice. The mice were sacrificed 4 hours later, the livers were excised, and aliquots were frozen in liquid nitrogen.
Statistical Analysis.
Data are expressed as the mean ± standard deviation (SD). Statistical significance was calculated using a t test. P < 0.05 was considered significant.
Results
TLR3 Attenuates Hepatocyte Proliferation Following PHx.
In order to explore the role of TLR3 in liver regeneration, we performed 70% PHx in TLR3wt and TLR3−/− C57Bl/6 mice. Hepatocyte proliferation was assessed through BrdU and CDC47 staining of paraffin-embedded liver sections taken at various time points following PHx. BrdU staining revealed that in TLR3−/− mice, a statistically significant increase in the number of hepatocytes in the S-phase was observed during the first 72 hours (Fig. 1A). However, at 96 to 120 hours, the reverse effect was observed (that is, more hepatocytes in S-phase were counted in the TLR3wt mice). A similar kinetic of hepatocyte proliferation in both mice strains was observed when sequential liver sections were stained for CDC47, a marker for nonquiescent cells (Fig. 1B).
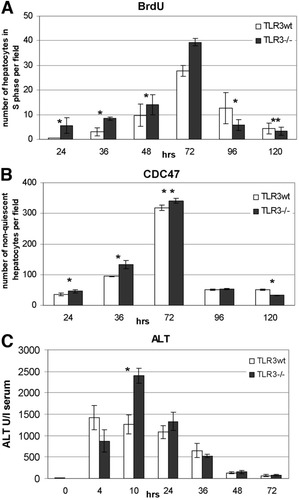
TLR3 inhibits hepatocyte proliferation following PHx. TLR3wt (white bars) and TLR3−/− (black bars) mice were subjected to 70% PHx, and liver regeneration was determined by immunohistochemical staining of paraffin-embedded liver sections for (A) BrdU or (B) CDC47. Stained hepatocytes were counted at a magnification of ×40 in a 0.1-mm2 field. Liver damage was estimated by measuring ALT levels in the serum (C). The results represent mean data of at least five mice per time point. *P < 0.01. **P < 0.05.
Elevated levels of serum ALT are known to correlate with liver injury. Starting from the 10-hour time point following 70% PHx, TLR3−/− mice exhibited higher levels of ALT in their serum compared with TLR3wt mice (Fig. 1C). We ruled out the possibility that the differences in the ALT profile were due to differential sensitivity of the TLR3−/− mice to anesthetics by subjecting mice of both strains to the same anesthetics used during the operations and determining the effect on ALT levels (Supporting Data).
Collectively, these results demonstrate that in the absence of active TLR3, hepatocyte proliferation is significantly more intensive during the first 72 hours following PHx.
The Effect of TLR3 on Cytokine Secretion Following PHx.
IL-6 plays a role in the initiation stage of liver regeneration and was shown to be secreted during the first 24 hours following PHx.13 IL-6 binds to IL-6R, which complexes with the glycoprotein 130 receptor (gp130). The IL-6R may be secreted in its soluble form, sIL-6R, which mediates the response by complexing with glycoprotein 130 in a mechanism named trans-signaling.14
To test whether TLR3 affects the secretion of IL-6, we measured IL-6 levels in the serum of TLR3−/− and TLR3wt mice at 4, 10, and 24 hours following PHx (Fig. 2A). In accordance with previous publications, the level of IL-6 in the serum increased dramatically in TLR3wt mice, reaching approximately 300 pg/mL, 4 hours after PHx, and declined to approximately 200 pg/mL by 10 hours, and remained at this level until the 24-hour time point. Unpredictably, IL-6 secretion was delayed in TLR3−/− mice, peaking at 10 hours post PHx and reaching only a third (100 pg/m) of the maximal level detected in TLR3wt mice.
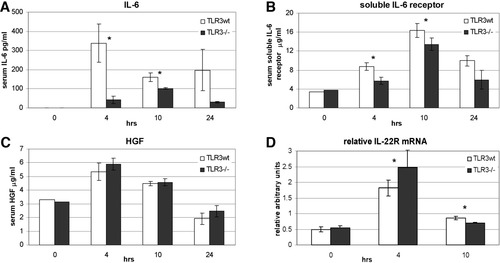
Cytokine levels following PHx. PHx was performed on TLR3wt (white bars) and TLR3−/− (black bars) mice. The serum levels of (A) secreted IL-6, (B) soluble IL-6 receptor, and (C) HGF were measured by ELISA. (D) IL-22R levels were determined through semiquantitative RT-PCR and normalized to the level of GADPH. The results represent the mean data of at least five mice per time point. *P < 0.05.
In parallel to IL-6, we measured the level of sIL-6R and detected significantly higher levels of sIL-6 in TLR3wt compared with TLR3−/− mice, at all time points following PHx (Fig. 2B). Taken together, the results suggest that TLR3 signaling affects IL-6 and sIL-6R secretion following PHx.
IL-6 family members signal via STAT3,15 which is phosphorylated upon activation to P-STAT3, and was shown to be elevated during the first 24 hours after PHx.13 We measured the relative levels of P-STAT3 in liver lysates at 4 and 24 hours after PHx by western blot analysis, and found no difference between TLR3−/− and TLR3wt mice (data not shown).
STAT3 is known to be activated by other cytokines, including HGF, which is essential for liver regeneration following PHx.16 Measurement of HGF serum levels at different time points after PHx revealed an approximately two-fold increase at 4 hours in both mice strains which declined thereafter (Fig. 2C). In the TLR3−/− mice, the levels of HGF following PHx were slightly (but not significantly) higher than those in TLR3wt mice.
The IL-22 cytokine also signals via STAT317 and was shown to protect hepatocytes upon liver injury.18 Whereas IL-22 secretion is limited to T cells, natural killer cells, and natural killer T cells, its receptor, IL-22R, is expressed in peripheral tissues and was shown to be strongly expressed in the liver.18 In an attempt to explain the inconsistency between the differential levels of IL-6 secretion and the lack of significant differences in the levels of activated STAT3 (Fig. 2A,B), we measured the levels of IL-22R mRNA at 4 and 10 hours post-PHx by way of semiquantitative RT-PCR. As shown in Fig. 2D, IL-22R mRNA levels increased at 4 hours and subsided to approximately basal levels at 10 hours. The increase at the 4-hour time point was significantly more pronounced in TLR3−/− mice. The higher level of IL-22R mRNA in TLR3−/− mice could contribute to STAT3 activation and thus may explain, at least in part, the similarity in P-STAT3 levels in both mouse strains.
Overall, these results suggest that neither IL-6 nor HGF is the culprit for the enhanced regeneration observed at the early phase after PHx in the TLR3−/− mice.
Partial Hepatectomy Induces the TLR3 Signaling Pathway.
TLR3 signals via the TRIF adaptor protein which can induce two divergent pathways. One pathway is mediated mainly by Rip1 and leads to activation of NF-κB and its translocation to the nucleus. The second pathway involves the binding of Rip3 to TRIF and the recruitment of FADD, which results in the activation of caspase-8.
To study TLR3 signaling following PHx, we first measured the amount of Rip1 protein in the liver during the first 24 hours through western blot analysis. The results revealed no difference between TLR3wt and TLR3−/− mice (data not shown).
We next tested the activation of NF-κB by immunohistochemical staining (Fig. 3A). As seen in the representative tissue sections, NF-κB was dramatically activated in hepatocytes of TLR3wt mice compared with TLR3−/− mice. This difference in the level of staining was apparent at 10 hours but was more pronounced at the 24-hour time point. Importantly, at these time points hepatocyte staining of NF-κB–positive nuclei in the TLR−/− mice was very weak. Counting the number of stained hepatocyte nuclei verified the significant difference between the two mice strains at the 10-hour time point (Fig. 3B).
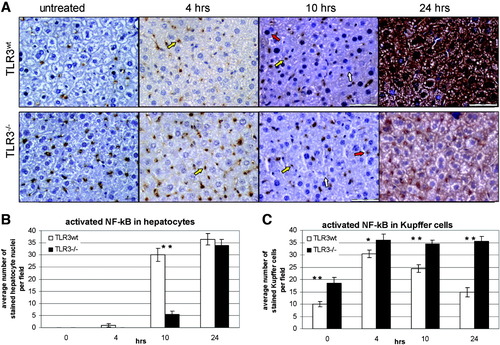
Partial hepatectomy induces TLR3-dependent NF-κB activation in hepatocytes. TLR3wt and TLR3−/− mice were subjected to 70% PHx. Paraffin-embedded liver sections were immunostained for NF-κB. (A) Representative results at magnification ×40. NF-κB activation was quantified by determining the number of (B) stained hepatocyte nuclei or(C) Kupffer cells counted in magnification ×40 in a 0.1-mm2 field. White, red, and yellow arrows indicate unstained hepatocyte nuclei, positively stained hepatocyte nuclei, and positively stained Kupffer cells, respectively. *P < 0.01. **P < 0.05.
In contrast to hepatocytes, significantly more NF-κB positive Kupffer cells were counted in TLR3−/− compared with TLR3wt mice, at all time points following PHx (Fig. 3C). To a lesser extent, the same phenomenon was observed in nontreated mice of both strains (Fig. 3A,C).
The data shown in Fig. 3B,C were confirmed by performing automatic blind counting of stained sections by the Ariol computer program (data not shown). These results demonstrate for the first time that TLR3 is required for the activation of NF-κB in hepatocytes during the first 24 hours following PHx. The results also suggest that TLR3 attenuates NF-κB activation in Kupffer cells.
We next performed experiments to assess the activation of the second TLR3 signaling branch, the TLR3–TRIF–Rip3–caspase-8 pathway. Western blot analysis of liver sections revealed that during the first 10 hours after PHx, Rip3 levels in TLR3−/− mice were significantly lower than in TLR3wt mice (Fig. 4A). Similar results were obtained by immunohistochemical staining of Rip3 protein at the 10-hour time point (Fig. 4B,C). It is noteworthy that Rip3 staining in the liver was restricted to hepatocytes in zone 3 around the central vein (Fig. 4B); however, following PHx its expression was expanded in TLR3wt but not TLR3−/− mice.
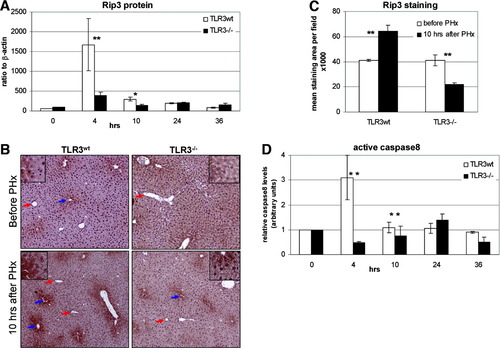
Partial hepatectomy induces TLR3-dependent Rip3 expression and caspase-8 activation. TLR3wt and TLR3−/− mice were subjected to 70% PHx. (A) Western blot analysis of Rip3 in lysates from frozen liver sections. Rip3 expression levels were normalized to β-actin levels. (B) Paraffin-embedded liver sections immunostained for Rip3. Red arrows indicate zone 1, blue arrows indicate zone 3. (C) Stained areas were measured using Ariol software. (D) Anti–caspase-8 antibody was cross-linked to protein A sepharose beads and used to immunoprecipitate caspase-8 from 0.5-mg liver protein lysates. Western blot analysis was performed to assess active caspase-8 levels. Shown are caspase-8 levels after PHx normalized to the levels of nontreated mice. *P < 0.01. **P < 0.05.
Measurements of active caspase-8 levels following PHx closely paralleled those of Rip3, showing a substantial increase during the first 10 hours in TLR3wt mice, with only a negligible increase in the TLR3−/− mice (Fig. 4D).
Caspase-8 is a known initiator of apoptosis that upon activation cleaves effector caspases, which in turn leads to apoptosis and cell death. However, using TUNEL staining, we could not detect any apoptotic cells in liver sections taken at all time points after PHx, from both TLR3−/− and TLR3wt mice (data not shown). Thus, caspase-8 probably plays a nonapoptotic role in this system.
Active caspase-8 was shown to activate the pro–IL-1β cytokine, which is a known suppressor of hepatocyte proliferation after PHx.19, 20 We found that in TLR3−/− mice, the mRNA level of IL-1β was significantly and consistently lower than in TLR3wt mice before and after PHx (data not shown).
Taken together, these results clearly show that following PHx, TLR3 signaling is activated, leading to the induction of NF-κB in hepatocytes and the activation of caspase-8.
Discussion
In this study, we have identified a role for TLR3 in liver regeneration. We provide several lines of evidence for an inhibitory role of TLR3 in the initiation phase of liver regeneration. We demonstrate that in mice lacking functional TLR3, the initiation of hepatocyte proliferation after PHx was accelerated. Furthermore, during the first hours following PHx, TLR3 signaling induced both the activation of NF-κB and the elevation in Rip3 levels in hepatocytes, and was accompanied by activation of caspase-8, with no apparent cell death.
Our finding that TLR3 signaling leads to a delay in hepatocyte proliferation following PHx is supported by the data of Sun and Gao,21 who showed that intraperitoneal injection of the TLR3 ligand poly(IC) negatively regulated liver regeneration. Stimulation of TLR3 by poly(IC) was also shown to have an inhibitory role in the division of nonhepatic cells including axonal growth, choroidal neovascularization, and—indirectly (via activation of dendritic cells)—proliferation of CD4+T cells.22-24 It is noteworthy that in our system there was no administration of an external TLR3 ligand, and we therefore assume that following PHx, TLR3 was triggered by an endogenous ligand that has not yet been elucidated. To date, some TLRs were shown to respond to endogenous ligands in addition to their known pathogen-associated molecular patterns (reviewed by Wagner25). For example, TLR7 and TLR9 were shown to respond to small U1 nuclear ribonucleoproteins and self chromatin DNA, respectively,26, 27 and TLRs 2 and 4 were shown to respond to fibrinogen, heat shock proteins, or β-defensins.28, 29 The endogenous ligands for TLR2 are of particular interest, because TLR2 was shown to be capable of synergizing with TLR3.30 Indeed, TLR3 was shown to respond to RNA released from necrotic cells.31-34
IL-6 was shown to be involved in the initiation of liver regeneration, although data pertaining to its effect on hepatocyte proliferation range from a negative to a positive role.20, 35-38 Here we demonstrate that following PHx, the level of IL-6 and sIL-6R secreted to the serum of TLR3−/− mice was significantly lower than that of TLR3wt mice, whereas hepatocyte proliferation rates were increased. Published data supporting our finding that TLR3 signaling participates in IL-6 induction were obtained when mice of the same TLR3−/− strain were treated with known TLR3 ligands, such as poly(IC), or infected with influenza A virus or West Nile virus.5, 39, 40 However, IL-6 induction per se is not impaired in these TLR3−/− mice, because it was shown that administration of lipopolysaccharide (a TLR4 ligand) resulted in the same level of IL-6 induction in both TLR3wt and TLR−/− mouse strains.5 Thus, we suggest that TLR3 signaling is important for IL-6 induction in the initial stage of liver regeneration. Our finding that in the TLR3−/− mice there was less IL-6 secreted, but that more hepatocytes were dividing, could point to a negative effect of IL-6 on hepatocyte proliferation in the early phase following PHx.
TLR3 was shown to signal by way of two main pathways: the inflammatory and the noninflammatory (or apoptotic) pathway. The inflammatory pathway was shown to be activated in white blood cells and the HEK293 cell line,5, 41 leading to NF-κB activation and cytokine secretion. The apoptotic pathway was shown to be induced in breast cancer and melanoma cell lines,10, 11 and we have demonstrated that in the HepG2 hepatoma cell line, TLR3 signaling is skewed toward apoptosis rather than to the inflammatory pathway.9
Our data suggest that TLR3 is required for the activation of NF-κB in hepatocytes during the first hours following PHx. We show that without TLR3, NF-κB activation in hepatocytes was significantly delayed (though it was enhanced in Kupffer cells), and this was accompanied by earlier hepatocyte proliferation. A similar observation was reported by Malato et al.42 and Abshagen et al.,43 who showed that a weaker and delayed NF-κB activation in hepatocytes, accompanied by an enhanced activation of NF-κB in nonparenchymal liver cells, triggers earlier hepatocytes proliferation. Moreover, in agreement with our hypothesis, Malato et al. showed that IL-6 is not responsible for the difference in hepatocyte division rates. They proposed that the difference in hepatocyte proliferation rates may be due to higher levels of TNF-α secreted by overactivated Kupffer cells, which led to earlier and more rapid priming of regeneration.
NF-κB was shown to induce synthesis of the pro–IL-1β cytokine, which was recently demonstrated to be cleaved by caspase-8 to its active form, IL-1β, in vitro19, 20 (Fig. 5). Active IL-1β is known to suppress hepatocyte proliferation and to terminate the surge of DNA synthesis induced after PHx.20 We reason that the TLR3-dependent activation of NF-κB and caspase-8 in hepatocytes could result in an increase in activated IL-1β, subsequently inhibiting hepatocyte proliferation. In accordance, we found that the level of IL-1β mRNA in the liver of TLR3−/− mice was significantly lower, whereas the hepatocyte proliferation rate was higher.
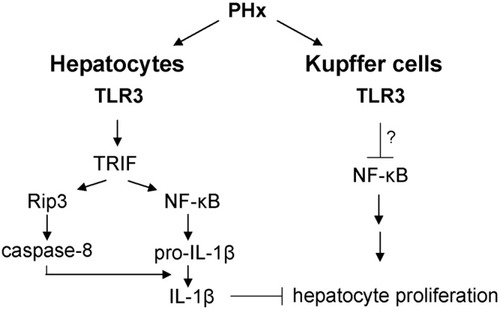
A model for the role of TLR3 in liver regeneration. Following partial hepatectomy, NF-κB is activated in Kupffer cells, leading to the secretion of TNF-α, which in turn primes hepatocyte proliferation. In parallel, TLR3 signaling is induced in hepatocytes and leads to NF-κB and caspase-8 activation. NF-κB induces pro–IL-1β that is cleaved by caspase-8 to yield activated IL-1β, which in turn inhibits hepatocyte proliferation.
The induction of caspase-8 following PHx with no apparent apoptosis may suggest that caspase-8 plays a nonapoptotic role at the initial stage of liver regeneration. Caspase-8 is a known initiator caspase but was also shown to participate in nonapoptotic functions, including hematopoietic progenitor function and cell motility,44, 45 and we have recently demonstrated its nonapoptotic role in cellular response to liver infection and injury.46 On the other hand, there may be several explanations for the absence of apoptosis after PHx: (1) the NF-κB and c-Jun N-terminal kinase pathways, which are induced following PHx, inhibit apoptosis (reviewed by Wullaert et al.47 and Ni et al.48); (2) IL-6, which is induced after PHx, was shown to protect hepatocytes from apoptosis49; and (3) during the first 12 hours following PHx, the antiapototic proteins Bcl-x and Bxl-2 are activated.50
Based on our results and previous publications, we propose a model for the role of TLR3 in the course of liver regeneration. According to this model, during the initial phase following partial hepatectomy, TLR3 signaling is induced in hepatocytes, and leads to activation of NF-κB as well as increased Rip3 protein levels and caspase-8 activation. NF-κB was shown to induce pro–IL-1β expression in hepatocytes, which is then activated by caspase-8, leading to inhibition of hepatocyte proliferation.19, 20 In parallel, TLR3 signaling in Kupffer cells represses NF-κB activation. The outcomes of TLR3 signaling in Kupffer cells and hepatocytes converge to modulate the initial phase of liver regeneration.
Acknowledgements
We thank R. A. Flavell for the gift of the TLR3−/− mice. We thank Lina Mizrachi and Temima Schnitzer Perlman for technical assistance.




