S6K1 deficiency protects against apoptosis in hepatocytes†
Potential conflict of interest: Nothing to report.
Abstract
The mammalian target of rapamycin (mTOR)/S6K1 signaling pathway controls cell growth and proliferation. To assess the importance of S6K1 in the balance between death and survival in the liver, we have generated immortalized hepatocyte cell lines from wild-type and S6K1-deficient (S6K1−/−) mice. In S6K1−/− hepatocytes, caspase-8 and the pro-apoptotic protein Bid were constitutively down-regulated as compared with wild-type. Moreover, S6K1−/− hepatocytes failed to respond to the apoptotic trigger of death receptor activation. Neither caspase-8 activation nor FLIPL degradation in response to tumor necrosis factor alpha (TNF-α) or anti-Fas antibody (Jo2) was observed in cells lacking S6K1. Downstream events such as Bid cleavage, cytochrome C release, caspase-3 activation, DNA laddering, as well as the percentage of apoptotic cells were attenuated as compared with wild-type. In addition, the anti-apoptotic protein BclxL was down-regulated in TNF-α–treated or Jo2-treated wild-type hepatocytes, but this response was abolished in S6K1−/−cells. In vivo, S6K1-deficient mice were protected against concanavalin A–induced apoptosis. The withdrawal of growth factors strongly induced apoptosis in wild-type, but not in S6K1−/− hepatocytes. S6K1 deficiency did not decrease BclxL/Bim ratio on serum withdrawal, thereby protecting cells from cytochrome C release and DNA fragmentation. At the molecular level, the lack of S6K1-mediated negative feedback decreased insulin receptor substrate-1 (IRS-1) serine phosphorylation, resulting in activation of survival pathways mediated by phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase (ERK). However, S6K1−/− hepatocytes underwent apoptosis on serum withdrawal in combination with phosphatidylinositol 3-kinase (PI3K) or ERK inhibitors. Conclusion: This finding might explain the mechanism of resistance to mTOR inhibitors in cancer treatments and strongly suggests that the inhibition of S6K1 could protect against acute liver failure and, in combination with inhibitors that abrogate the sustained activation of Akt and ERK, could improve the efficacy of hepatocarcinoma (HCC) treatment. (HEPATOLOGY 2009.)
Apoptosis mediated by extrinsic or intrinsic pathways is essential for maintaining cellular homeostasis in the liver. In this tissue, binding of tumor necrosis factor (TNF) receptor superfamily, member 6, ligand (FasL) or inflammatory cytokines, such as TNF-α, to death receptors on the cell surface (Fas, TNF-R1) induces the extrinsic apoptotic pathway by the recruitment of adapter proteins (Fas-associated protein with death domain, TNF receptor–associated death domain) and procaspase-8 and procaspase-10 at the intracellular domain of the receptor to form the death-inducing signaling complex (DISC).1 The signal generated at the DISC by activated caspases leads to cell death, which, depending on the cell type, may or may not require mitochondrial involvement for its execution.2 The intrinsic pathway is triggered by extracellular and intracellular stress signals, such as growth factors withdrawal, hypoxia, DNA damage, and oncogene induction resulting in the permeabilization of the outer mitochondrial membrane and the release of cytochrome C and other proapoptotic molecules. These events lead to the formation of the apoptosome followed by activation of the executer caspase-3.3 Deregulation of apoptosis pathways contributes to diseases such as hepatocellular carcinoma (HCC), viral and autoimmune hepatitis, ischemia and reperfusion injury, toxic liver damage, and acute liver failure.4
Among the most important survival factors in the liver are several receptors of tyrosine kinases activated by growth factors, such as epidermal growth factor, fibroblast growth factors, and hepatocyte growth factor. In addition, the protective role of insulin against apoptosis induced by both extrinsic and intrinsic pathways has been reported in neonatal hepatocytes.5, 6 At the molecular level, phosphatidylinositol 3-kinase (PI3K)/Akt signaling represents a major survival pathway. Its activation has been associated with malignant transformation and apoptotic resistance.7 Downstream of Akt, the mammalian target of rapamycin (mTOR) plays a central role in regulating cell growth, proliferation, and survival, in part by regulation of translation initiation.8
MTOR resides in two different multiprotein complexes: the rapamycin-sensitive mTOR complex 1 and the rapamycin-insensitive mTOR complex 2. In mTOR complex 1, mTOR interacts with the regulatory-associated protein of mTOR (raptor), G-protein beta-subunit–like protein (Gβl), and proline-rich protein kinase B/Akt substrate 40 kDa. In response to mitogen stimulation, mTOR phosphorylates and activates S6K1, which in turn phosphorylates the 40S ribosomal protein S6, leading to the enhancement of translation of messenger RNAs (mRNAs) with a 5′-terminal oligopyrimidine, including mRNAs that encode for ribosomal proteins and elongation factor-1. Rapamycin and its derivatives RADD001 (Novartis) and CCI-779 (Wyeth Ayerst) are undergoing clinical trials for the treatment of solid tumors, including HCC.9 These compounds block mTOR signaling by forming an inhibitory complex with the immunophilin FKBP12, which binds to and inhibits the ability of mTOR to phosphorylate downstream targets such as S6K1. The importance of mTOR as a therapeutic target in the treatment of HCC is based on the strong antitumoral effect of mTOR inhibition in an experimental model of HCC10 and, more importantly, on the activation of the mTOR/S6K1 pathway observed in human HCC.11 However, the specific role of S6K1 in the regulation of the balance between death and survival in cells of the liver remains unexplored.
In the current study, we have investigated the molecular mechanism by which S6K1 deficiency alters susceptibility to apoptosis in hepatocytes. As a cellular model, we have developed immortalized neonatal hepatocyte cell lines from wild-type (S6K1+/+) and S6K1-deficient (S6K1−/−) mice. We have demonstrated that the lack of S6K1 protects against apoptosis induced by two different stimuli: death receptor activation and trophic factors withdrawal. Importantly, in vivo S6K1 deficiency protects against fulminant hepatitis induced by concanavalin A (ConA), a pathological response that depends on TNF-R1 activation. These effects result from the regulation of multiple events involving down-regulation of key pro-apoptotic mediators of the death receptor pathway, as well as the activation of a feedback loop that dampens the activation of PI3K/Akt, a pro-survival pathway. Therefore, modulation of S6K1 might be critical to overcoming the resistance to mTOR inhibitors in HCC therapies.
Abbreviations
ALB, albumin; ConA, concanavalin A; CPS, carbamoyl phosphate synthase; DISC, death-inducing signaling complex; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular signal-regulated kinase; FasL, tumor necrosis factor receptor superfamily, member 6, ligand; FBS, fetal bovine serum; HCC, hepatocellular carcinoma; IRS-1, insulin receptor substrate-1; JNK, Janus kinase; Jo2, anti-Fas antibody; LY, phosphatidylinositol 3-kinase/Akt inhibitor; mRNA, messenger RNA; mTOR, mammalian target of rapamycin; PD, ERK kinase inhibitor; PI3K, phosphatidylinositol 3-kinase; SE, standard error; siRNA, small interfering RNA; TNF, tumor necrosis factor.
Materials and Methods
Immortalized neonatal hepatocyte cell lines were obtained from livers of wild-type (S6K1+/+) and S6K1−/− mice12 3 to 5 days after birth. Hepatocyte apoptosis was analyzed by flow cytometry, DNA laddering, and quantification of cleaved caspase-8 and caspase-3 and their enzymatic activities. ConA was injected in S6K1+/+ and S6K1−/−mice (25 mg/kg) via the tail vein. After 8 hours, livers were removed and samples were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin-eosin. Additional experimental procedures are included in Supporting Materials.
Results
Generation and Characterization of Immortalized Hepatocyte Cell Lines from S6K1+/+ and S6K1−/− Mice.
Immortalized neonatal hepatocyte cell lines were generated from pooled excised livers from wild-type (S6K1+/+) or S6K1-deficient (S6K1−/−) mice at postnatal days 3 to 5. Figure 1A shows the lack of S6K1 protein expression in S6K1−/− immortalized hepatocytes, whereas levels of large T antigen were similar in all cell lines. Cells of both genotypes expressed albumin (ALB) and carbamoyl phosphate synthase (CPS), indicating phenotypical features of hepatocytes on immortalization (Fig. 1B). Moreover, the absence of vimentin staining demonstrated that the maintenance of cell lines in arginine-free medium5 was sufficient to eliminate contaminating fibroblasts in the primary culture. Thus, the characterization of S6K1−/− and S6K1+/+ hepatocytes has demonstrated that this novel cellular model is physiologically relevant.
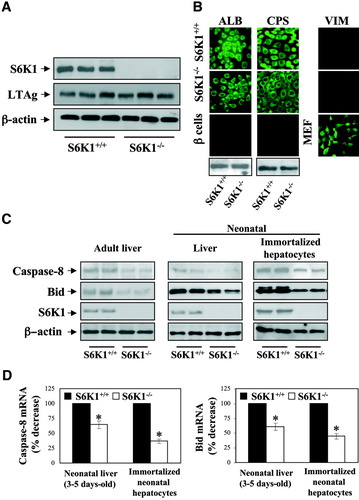
Generation and characterization of immortalized hepatocyte cell lines from S6K1+/+ and S6K1−/− mice. (A) S6K1+/+ and S6K1−/− immortalized hepatocytes cultured under growth-promoting conditions (Dulbecco's modified Eagle medium [DMEM] plus 10% FBS) were lysed and S6K1 and large T antigen expression of three independent cell lines from each genotype was analyzed in whole-cell lysates by western blot. The anti–β-actin antibody was used as a loading control. (B) Immunofluorescence detection of ALB, CPS, and vimentin of growing S6K1+/+ and S6K1−/− immortalized neonatal hepatocytes. As a negative control for ALB and CPS antibodies, immortalized beta cells were used for immunofluorescence. As a positive control for vimentin, mouse embryo fibroblasts were used. Representative images are shown. ALB and CPS were also analyzed by western blot. (C) Liver extracts from six to eight neonatal (3-5 days old) and adult mice (10-12 weeks old) of each genotype (S6K1+/+ and S6K1−/−) and cell lysates from S6K1+/+ and S6K1−/− immortalized hepatocytes cultured under growth-promoting conditions (DMEM plus 10% FBS) were analyzed by western blot with the antibodies against Bid and caspase-8. The anti–β-actin antibody was used as a loading control. A representative experiment is shown. (D) RNA was isolated from livers of S6K1+/+ and S6K1−/− neonatal and adult mice and S6K1+/+ and S6K1−/− immortalized neonatal hepatocytes. Bid and caspase-8 mRNA levels were determined by real-time polymerase chain reaction. The value of wild-type condition was set to 100%. Results are expressed as percentage of decrease of caspase-8 and Bid mRNA and are means ± standard error (SE). Statistical significance was carried out by student t test comparing the S6K1−/− values with the respective values of wild-type controls. *P < 0.05 was considered significant.
Next, we measured the expression of pro-apoptotic and anti-apoptotic proteins of the intrinsic and extrinsic apoptotic pathways in growing cells of both genotypes. It is noteworthy that the expression of caspase-8 and Bid was clearly down-regulated in S6K1−/− hepatocytes, and in liver extracts of S6K1−/−-deficient mice at 3 to 5 days or 10 to 12 weeks after birth, as compared with their corresponding wild-type controls (Fig. 1C). As shown in Fig. 1D, both caspase-8 and Bid were down-regulated at the mRNA level, indicating that S6K1 is required for transcription of these genes. By contrast, we did not observe changes in the levels of other pro-apoptotic and anti-apoptotic proteins (Supporting Fig. 1).
Apoptosis Induced by Death Receptor Activation Is Reduced in S6K1-Deficient Neonatal Hepatocytes.
To investigate the role of S6K1 in the susceptibility to apoptosis, we compared the effect of death receptor activation in wild-type and S6K1−/− immortalized neonatal hepatocytes. Cells were stimulated with TNF-α in the presence of actinomycin D or with the Fas agonist Jo2 at the indicated doses for 16 hours. Phase-contrast microscopy revealed many wild-type hepatocytes with morphological characteristics of apoptotic cells. By contrast, only a few S6K1−/− cells displayed apoptotic phenotype (Fig. 2A). Analysis of nuclear morphology confirmed an increase in the incidence of condensed or fragmented nuclei in wild-type hepatocytes (51%) in comparison with S6K1−/− cells (<10%) (Fig. 2B). These results suggest that, in immortalized neonatal hepatocytes, there is a direct link between S6K1 expression and susceptibility to apoptosis on death receptor activation.
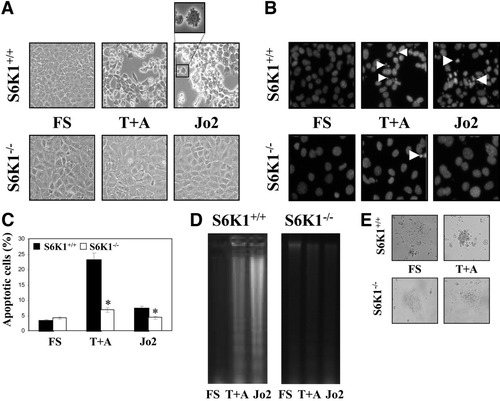
Apoptosis induced by death receptor activation was reverted in S6K1-deficient neonatal hepatocytes. S6K1+/+ and S6K1−/− immortalized hepatocytes were stimulated with TNF-α (30 ng/mL) plus actinomycin D (100 ng/mL) (T+A) or Jo2 (2 μg/mL), for 16 hours in DMEM plus 5% FBS. (A) Representative phase-contrast micrographs of S6K1+/+ and S6K1−/− hepatocytes after apoptotic stimuli. (B) Representative images of the nuclear morphology of untreated and treated S6K1+/+ and S6K1−/− hepatocytes after staining of DNA with 4′,6-diamidino-2-phenylindole and visualization by fluorescence microscopy. (C) The percentage of cells with DNA lower than 2C (apoptotic cells) was determined by flow cytometry. Results are means ± SE. Statistical significance was determined by student t test comparing the S6K1−/− condition with the respective values for wild-type controls. *P < 0.05 was considered significant. (D) Extranuclear DNA obtained from untreated and treated S6K1+/+ and S6K1−/− hepatocytes was electrophoresed and visualized by ultraviolet fluorescence. A representative experiment is shown. (E) Representative phase-contrast micrographs of S6K1+/+ and S6K1−/− primary hepatocytes after exposure to the apoptotic stimuli TNF-α (30 ng/mL) plus actinomycin D (100 ng/mL) (T+A) for 16 hours.
Next, we measured the percentage of cells undergoing apoptosis by quantification of hypodiploid cells. Treatment with TNF-α receptor (TNF-R1) or Fas activators for 16 hours increased the percentage of hypodiploid wild-type but not S6K1−/− hepatocytes, as compared with untreated cells (Fig. 2C). Importantly, reconstitution of S6K1 expression reversed the apoptotic phenotype of S6K1−/− hepatocytes (Supporting Fig. 2A,B). Moreover, the DNA laddering pattern showed that S6K1 deficiency decreased the percentage of apoptotic cells on death receptor activation (Fig. 2D). Consistent with our data in immortalized cells, primary hepatocytes from S6K1−/− mice were more resistant to TNF-α plus actinomycin D–induced apoptosis when compared with hepatocytes from wild-type mice (Fig. 2E). The lack of response of S6K1−/− cells was not attributable to a general inhibition of protein synthesis because cycloheximide did not block apoptosis in wild-type cells. In addition, rapamycin-treated S6K1+/+ cells showed a significant inhibition of apoptosis induced by death receptor activation (Supporting Fig. 3A,B).
S6K1 Deficiency Protected Neonatal Hepatocytes from Caspase-8 Activation, Bid Cleavage, and Cytochrome C Release by Inhibiting c-Jun N-terminal kinasde–Mediated FLIPL Degradation.
To determine the step in the apoptotic cascade that is blocked as a result of S6K1 deficiency, we studied the activation of caspase-8 in wild-type and S6K1−/− hepatocytes after Jo2 or TNF-α plus actinomycin D treatment. Consistent with our previous data, cleavage of caspase-8, which is activated after Fas or TNF-R1 oligomerization,2 was abrogated in S6K1−/− hepatocytes (Fig. 3A). These results paralleled changes in caspase-8 activity. Because activation of caspase-8 is a proximal event after activation of the Fas or TNF-R1 receptors, our data demonstrate that one of the earliest events in death receptor–induced apoptosis is suppressed in S6K1−/− hepatocytes.
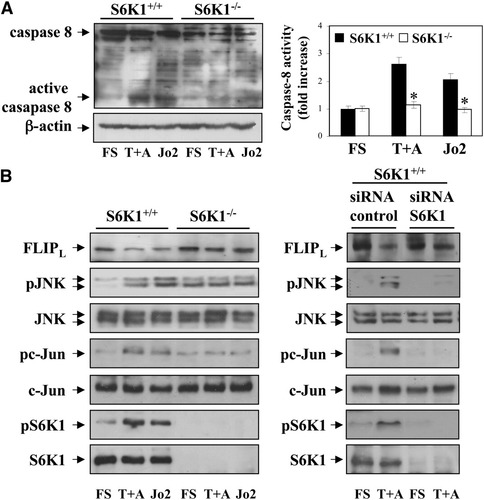
S6K1 deficiency protected neonatal hepatocytes from caspase-8 activation by inhibiting JNK-mediated FLIPL degradation. S6K1+/+ and S6K1−/− immortalized hepatocytes were stimulated with TNF-α (30 ng/mL) plus actinomycin D (100 ng/mL) (T+A) or Jo2 (2 μg/mL) for 16 hours in DMEM plus 5% FBS. (A) (left panel) Total protein (50 μg) was used for western blot analysis with the corresponding antibodies against caspase-8 and anti–β-actin as a control for protein loading. A representative experiment is shown. (Right panel) S6K1+/+ and S6K1−/− immortalized hepatocytes were treated as described, and caspase-8 enzymatic activity was measured. Results are expressed as fold increase of caspase-8 enzymatic activity compared with the S6K1+/+ condition, which was arbitrarily assigned a value of 1, and are expressed as means ± SE from three independent experiments. Statistical significance was determined by student t test comparing the S6K1−/− condition with respective values for wild-type controls. *P < 0.05 was considered significant. (B) (left panel) Apoptosis was induced as described in (A). Total protein (50 μg) was used for western blot analysis, with the corresponding antibodies against FLIP, phospho-JNK (Thr183/Tyr185), total JNK, phospho-c-Jun (Ser73), total c-Jun, phospho-S6K1 (Thr 389), and total S6K1. A representative experiment is shown. (Right panel) S6K1+/+ immortalized hepatocytes were seeded in 6-cm dishes and incubated overnight at 37°C with 5% CO2. When 40% to 50% confluence was reached, cells were transfected with 100 nM control or siRNA oligos following DharmaFECT General Transfection Protocol. After 48 hours, hepatocytes were treated as described. Then, cells were collected and cell lysates were analyzed by western blot with the indicated antibodies. Representative autoradiograms are shown.
Activation of caspase-8 is modulated by the levels of FLIP. Mouse liver expresses mostly the FLIPL isoform that contains a pseudo-caspase domain and is therefore a specific caspase-8 inhibitor.13 FLIPL protein degradation is a key event in FasL and TNF-α–induced cell death. FLIPL levels were consistently reduced after 16 hours' treatment of wild-type hepatocytes with Jo2 and TNF-α plus actinomycin D (Fig. 3B). Of note, degradation of FLIPL by both apoptotic stimuli was inhibited in S6K1−/− cells. This was coincident with an increase in phospho-c-Jun N-terminal kinase (JNK) and phospho-c-Jun in TNF-α plus actinomycin D or Jo2-stimulated wild-type hepatocytes, but not in S6K1−/− cells. Moreover, in S6K1−/− hepatocytes, phosphorylation of JNK and c-Jun was present at higher levels than in wild-type cells under nonapoptotic conditions. This basal increase may be attributable to signaling unrelated to death receptor activation possibly related to increased developmental stress in S6K1−/− hepatocytes. However, in these cells phosphorylated JNK remained unchanged on apoptotic stimulation. Importantly, S6K1 activation, measured by its phosphorylation at Thr 389 residue, was increased in TNF-α plus actinomycin D–stimulated or Jo2-stimulated S6K1+/+ cells.
To further demonstrate that S6K1 expression regulates the susceptibility to death receptor–mediated apoptosis in hepatocytes at the DISC level, we established small interfering RNA (siRNA) assays. As depicted in Fig. 3B, S6K1 expression was reduced by 95% in wild-type cells treated with S6K1 siRNA as compared with the control siRNA. Moreover, reduction of S6K1 in wild-type hepatocytes abolished JNK phosphorylation and FLIPL degradation on treatment with TNF-α plus actinomycin D. To investigate whether activation of JNK is required for TNF-α plus actinomycin D–induced apoptosis in S6K1+/+ cells, JNK1/2 expression was reduced using siRNA. As shown in Supporting Fig. 4, FLIPL degradation, activation of caspase-8 and caspase-3, and the percentage of hypodiploid cells were partly reduced by silencing JNK1/2.
We investigated whether the lack of S6K1 protected neonatal hepatocytes from Bid cleavage and whether S6K1 deficiency affected the mitochondrial pathway. Detectable levels of tBid were observed in Jo2 or TNF-α plus actinomycin D–stimulated wild-type hepatocytes, whereas in S6K1−/− cells tBid was barely detectable (Fig. 4A). Moreover, BclxL, an anti-apoptotic member of the Bcl2 family with a prominent role in hepatocyte survival,5, 14 was down-regulated in wild-type hepatocytes but remained at high levels in S6K1-deficient cells stimulated with death receptor ligands. We next evaluated whether the release of cytochrome C from mitochondria to the cytosol in response to Jo2 or TNF-α plus actinomycin D was affected by deletion of S6K1. Cytochrome C appeared in the cytosol after death receptor stimulation in wild-type cells, but not in the absence of S6K1 (Fig. 4B). Consistent with our previous data, cleavage of the executer caspase-3 after Fas or TNF-R1 oligomerization was abrogated in S6K1−/− immortalized hepatocytes (Fig. 4A). These results paralleled changes in caspase-3 activity (Fig. 4C). Moreover, reconstitution of S6K1 expression in S6K1−/− immortalized hepatocytes reversed the protection against activation of caspase-3 (Supporting Fig. 2A,D). Of note, primary S6K1−/− hepatocytes, but not wild-type cells, were protected against activation of caspase-3 on TNF-α plus actinomycin D treatment, thus ruling out artifactual effects mediated by the immortalization procedure.
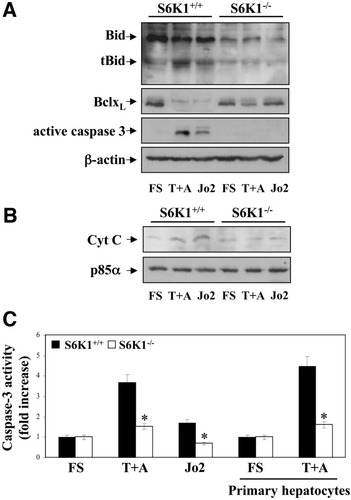
S6K1 deficiency protects neonatal hepatocytes from Bid cleavage and prevents movement of cytochrome C from mitochondria to cytosol. A. S6K1+/+ and S6K1−/− immortalized hepatocytes were stimulated with TNF-α (30 ng/mL) plus actinomycin D (100 ng/mL) (T+A) or Jo2 (2 μg/mL) for 16 hours in DMEM plus 5% FBS. (A) Total protein (50 μg) was used for western blot analysis with the corresponding antibodies against Bid, BclxL, active caspase-3, and anti–β-actin as a protein loading control. A representative experiment is shown. (B) Mitochondria were separated from the cytosol, and cytochrome C content in the cytosolic fraction was analyzed by western blot. Protein loading was visualized with the anti-p85α antibody. A representative experiment is shown. (C) S6K1+/+ and S6K1−/− immortalized and primary hepatocytes were treated as described, and caspase-3 enzymatic activity was measured. Results are expressed as fold change of caspase-3 enzymatic activity compared with the S6K1+/+ condition, which was arbitrarily assigned a value of 1, and are expressed as means ± SE from three independent experiments. Statistical significance was determined by student t test comparing the S6K1−/− condition with the respective values for wild-type controls. *P < 0.05 was considered significant.
S6K1−/− Mice Are Resistant to Fas and TNF-α–Dependent Fulminant Hepatitis.
Our finding that the absence of S6K1 protects hepatocytes from Jo2-induced and TNF-α–induced apoptosis prompted us to analyze this phenomenon in vivo. We asked whether S6K1−/− mice were resistant to acute liver failure. Con A–induced fulminant hepatitis mimics many aspects of human acute liver failure, including Fas/TNF-α–mediated hepatocyte death.15 Accordingly, mice of both genotypes were injected with ConA (25 mg/kg body weight) via the tail vein, and livers were removed at 8 hours postinjection. Whereas the livers from wild-type mice turned dark red after injection, an indication of hepatic hemorrhage, livers from S6K1−/− mice did not show this effect (results not shown). Histological analysis from the ConA-injected wild-type mice showed parenchymal necrosis and hemorrhage (Fig. 5A), whereas livers from most S6K1-null mice showed no pathological histology. As a measure of death receptor–mediated apoptosis, FLIPL degradation, Bid cleavage, and activation of caspase-3 were detected in the livers of wild-type but not S6K1−/− mice on ConA injection (Fig. 5B,C).
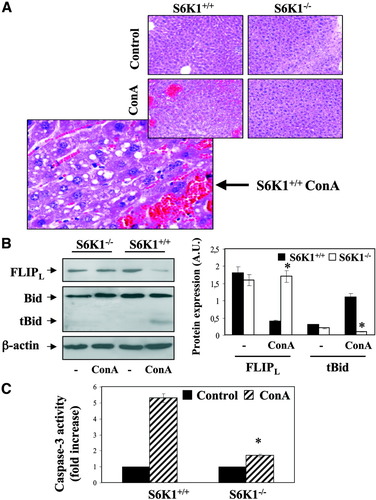
Deletion of S6K1 protects against ConA-induced liver failure. (A) S6K1+/+ and S6K1−/− adult mice were injected with ConA or with PBS. After 8 hours, livers were removed and fixed. Paraffin sections of liver tissue were stained with hematoxylin-eosin. (B) Liver extracts from S6K1+/+ and S6K1−/− adult mice injected with ConA or with PBS were prepared. Total protein (50 μg) was used for western blot analysis with the corresponding antibodies against FLIP and Bid. The anti–β-actin antibody was used as a loading control. A representative experiment is shown. The autoradiograms showing FLIPL levels and Bid cleavage from four to six control or ConA-injected mice of each genotype were quantitated by scanning densitometry. Results are expressed as arbitrary units and are means ± SE. Statistical significance was carried out by student t test by comparison between wild-type and S6K1−/− mice. *P < 0.05 was considered significant. (C) Caspase-3 enzymatic activity was measured in liver extracts analyzed in (B). Results are expressed as fold increase of enzymatic activity compared with the S6K1+/+ condition, which was arbitrary assigned a value of 1, and are means ± SE from three to four liver extracts of each genotype. Statistical significance was determined by student t test comparing the S6K1−/− condition with the respective values for wild-type controls. *P < 0.05 was considered significant.
S6K1 Deficiency Confers Protection Against Apoptosis Induced by Serum Withdrawal in Neonatal Hepatocytes.
Next, we investigated whether S6K1-null hepatocytes were protected against a different apoptotic trigger such as trophic factor deprivation, which elicits its effect mainly via the intrinsic pathway.5, 14 After 16 hours of serum deprivation, most of the S6K1−/− immortalized hepatocytes remained intact, whereas wild-type hepatocytes displayed an apoptotic phenotype (Fig. 6A). Of note, the appearance of condensed or fragmented nuclei was only visible in wild-type cells on serum withdrawal (Fig. 6B). In light of these results, serum withdrawal significantly increased the percentage of hypodiploid cells and the extent of DNA laddering in a time-dependent manner (Fig. 6C,D). Again, reconstitution of S6K1 expression reversed the apoptotic phenotype of S6K1−/− hepatocytes (Supporting Fig. 2A,B). Importantly, primary hepatocytes prepared from S6K1-null mice were completely resistant to serum withdrawal–induced apoptosis, when compared with primary hepatocytes prepared from wild-type mice (Fig. 6E). Moreover, rapamycin significantly decreased the percentage of apoptotic S6K1+/+ hepatocytes on serum withdrawal (Supporting Fig. 3B).
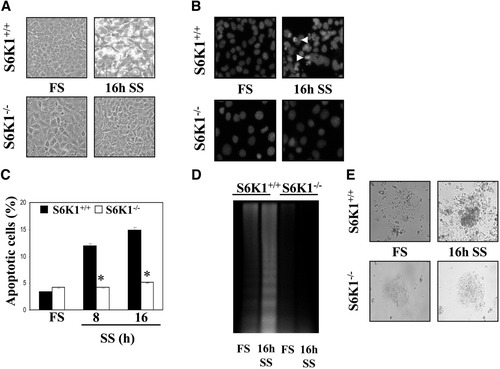
Protection against growth factor withdrawal–induced apoptosis in S6K1-deficient hepatocytes. S6K1+/+ and S6K1−/− immortalized hepatocytes were incubated in serum-free medium for 8 and 16 hours. (A) Representative phase-contrast microscopy images of S6K1+/+ and S6K1−/− hepatocytes after 16 hours of serum withdrawal. (B) Representative images of nuclear morphology of untreated and treated S6K1+/+ and S6K1−/− hepatocytes after staining of DNA with 4′,6-diamidino-2-phenylindole and analysis by fluorescence microscopy. (C) The percentage of cells with DNA lower than 2C (apoptotic cells) was determined by flow cytometry Results are expressed as a percentage of apoptotic cells and are expressed as means ± SE. Statistical significance was determined by student t test comparing the S6K1−/− condition with the respective values for wild-type controls. *P < 0.05 was considered significant. (D) Extranuclear DNA, obtained from untreated and treated S6K1+/+ and S6K1−/− hepatocytes, was electrophoresed and visualized by ultraviolet fluorescence. A representative experiment is shown. (E) Representative phase-contrast micrographs of S6K1+/+ and S6K1−/− primary hepatocytes after 16 hours of serum withdrawal.
S6K1 Deficiency Provoked an Imbalance Between Pro-apoptotic and Anti-apoptotic Protein Expression in Hepatocytes.
To elucidate the molecular basis for reduced apoptosis on growth factor deprivation in S6K1-deficient hepatocytes, we studied the expression of Bim and BclxL, two Bcl2 family members whose expression is modulated by serum withdrawal in neonatal hepatocytes.5, 6, 14 Levels of the pro-apoptotic Bim were low under growth-promoting conditions and gradually increased in serum-deprived wild-type hepatocytes, but not in S6K1−/− cells. By contrast, the expression of the anti-apoptotic BclxL was high under growth-promoting conditions, but gradually decreased after serum deprivation in wild-type but not in S6K1−/− cells (Fig. 7A). Thus, the BclxL/Bim ratio decreased dramatically in 16-hour serum-deprived wild-type hepatocytes but remained unchanged in the absence of S6K1. Consequently, cytochrome C appeared in the cytosol after 16 hours of serum withdrawal in wild-type cells. In contrast, cytochrome C was never detected in the cytosol of S6K1−/− hepatocytes throughout the period of serum withdrawal (Fig. 7B and results not shown). Finally, cleavage and enzymatic activity of caspase-3 were increased in serum-deprived immortalized and primary wild-type hepatocytes, but not in those lacking S6K1 (Fig. 7A,C). Importantly, reconstitution of S6K1 expression in S6K1−/− hepatocytes reversed the protection against serum withdrawal-induced activation of caspase-3 (Supporting Fig. 2A, D).
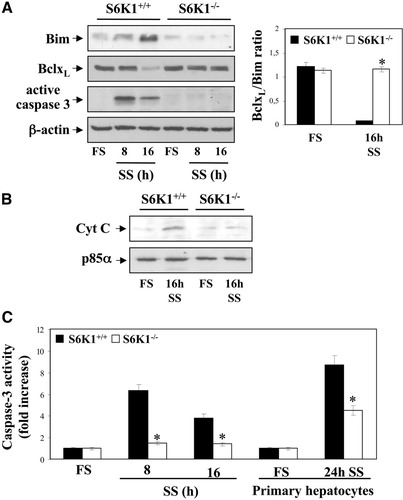
S6K1 deficiency protected neonatal hepatocytes from cytochrome C release and caspase-3 activation on serum withdrawal. S6K1+/+ and S6K1−/− immortalized hepatocytes were incubated in serum-free medium for 8 and 16 hours. (A) (left panel) Total protein (50 μg) was used for western blot analysis with the corresponding antibodies against Bim, BclxL, and active caspase-3. The anti–β-actin antibody was used as a loading control. A representative experiment is shown. The autoradiograms corresponding to three independent experiments were quantitated by scanning densitometry, and the BclxL/Bim ratio was calculated. Results are expressed in arbitrary units. Statistical significance was determined by student t test. *P < 0.05 was considered significant (right panel). (B) Mitochondria were separated from cytosol, and cytochrome C content was analyzed in the cytosolic fraction by western blot. The anti-p85α antibody was used as a loading control. A representative experiment is shown. (C) S6K1+/+ and S6K1−/− primary and immortalized hepatocytes were treated as described, and caspase-3 enzymatic activity was measured. Results are expressed as fold increase of enzymatic activity as compared with the S6K1+/+ condition, which was arbitrary assigned a value of 1, and are means ± SE from three independent experiments. Statistical significance was determined by student t test. *P < 0.05 was considered significant.
S6K1 Deficiency Resulted in Sustained Activation of Akt and Extracellular Signal-Regulated Kinase Survival Pathways on Serum Withdrawal Through the Suppression of a Negative-Feedback Loop.
Apoptotic triggers fail to affect S6K1−/− neonatal hepatocytes, likely because of sustained activation of Akt and extracellular signal-regulated kinase (ERK), two major survival pathways in mammalian cells.7, 16 Because Akt is known to act upstream of mTOR/S6K1, one would expect that S6K1 deficiency would not alter its phosphorylation levels. However, in S6K1−/− hepatocytes, Akt remained highly phosphorylated on 16 hours of serum withdrawal, whereas no phospho-Akt was detected in wild-type cells under these conditions (Fig. 8A). Moreover, FOXO1 was detected in nuclear extracts from serum-deprived wild-type but not S6K1−/− hepatocytes. Similarly, sustained phosphorylation of ERK was observed in serum-deprived S6K1−/− hepatocytes as compared with wild-type controls. A substantial amount of phosphorylated S6K1 was still present in wild-type hepatocytes on 16 hours of serum withdrawal. To directly demonstrate that the activation of Akt and ERK was responsible for the resistance to apoptosis observed in serum-deprived S6K1−/− hepatocytes, we examined the effect of serum withdrawal combined with LY294002 (LY, PI3K/Akt inhibitor) or PD098059 (PD ERK kinase inhibitor) for 16 hours. As shown in Fig. 8B, serum deprivation in combination with either LY or PD increased by twofold the percentage of apoptotic S6K1−/− hepatocytes. Moreover, the combination of both compounds increased the percentage of S6K1−/− apoptotic cells to levels similar to those seen in serum-deprived wild-type cells (Fig. 6C).
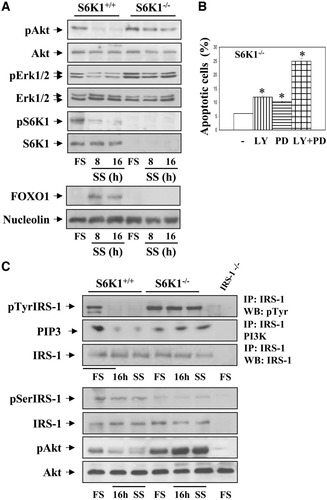
Survival pathways remain intact in S6K1-deficient hepatocytes on growth factor withdrawal. S6K1+/+ and S6K1−/− immortalized hepatocytes were incubated in serum-free medium for 8 and 16 hours. (A) At the end of the culture time, attached and nonattached cells were collected, and total cell lysates and nuclear extracts were prepared. (Upper panel) Total protein (50 μg) was used for western blot analysis with the corresponding antibodies against phospho-Akt (Ser 473), total Akt, phospho-ERK1/2 (Thr 202/Tyr 204), total ERK1/2, phospho-S6K1 (Thr 389), and total S6K1. (Lower panel) Nuclear protein (50 μg) was submitted to western blot analysis with the anti-FOXO1 antibody and the anti-nucleolin antibody as a loading control. A representative experiment is shown. (B) S6K1-deficient hepatocytes were treated with LY 294002 (40 μM) and PD 098059 (20 μM), alone or in combination with serum-free medium for 16 hours. The percentage of cells with DNA lower than 2C (apoptotic cells) was determined by flow cytometry as described in Materials and Methods. Statistical significance was performed by student t test comparing the values of S6K1−/− hepatocytes cultured in serum-free medium plus LY, PD, or LY plus PD with the respective values of S6K1−/− cells cultured in serum-free medium. *P < 0.05 was considered significant. (C) Apotosis was induced as described. (Upper panel) At the end of the culture time, cells were lysed, and 600 μg total protein was immunoprecipitated with anti–IRS-1 antibody and analyzed by western blot with anti-pTyr or used for an in vitro PI3K assay. The conversion of PI to PIP3 in the presence of [γ32-P] adenosine triphosphate was analyzed by TLC (left panel). (Lower panel) Total protein (50 μg) was used for western blot analysis with the corresponding antibodies anti-phospho-IRS-1 (Ser307), anti-total IRS-1, anti-phospho-Akt (Ser 473), and anti-Akt. The anti–β-actin antibody was used as a loading control (right panel). Representative autorradiograms are shown.
Because the phosphorylation of insulin receptor substrate-1 (IRS-1) is a common molecular event that leads to Akt and ERK phosphorylation,17 we investigated its tyrosine phosphorylation in serum-deprived cells of both genotypes. As depicted in Fig. 8C, IRS-1 tyrosine phosphorylation was high in wild-type hepatocytes under growth-promoting conditions and decreased dramatically on growth factor withdrawal. Conversely, S6K1−/− cells retained IRS-1 tyrosine phosphorylation even after long-term (16 hours) serum deprivation. Under these conditions, the activation of PI 3-K paralleled IRS-1 tyrosine phosphorylation in both cell types. Finally, because S6K1 is an IRS-1 serine kinase,17 we analyzed the status of IRS-1 ser-307 phosphorylation under apoptotic and nonapoptotic conditions. IRS-1 Ser307 phosphorylation was detectable in wild-type hepatocytes growing in 10% FBS and was slightly decreased under serum deprivation (Fig. 8C). Conversely, in S6K1−/− hepatocytes, IRS-1 ser- 307 phosphorylation was absent in both growth-promoting and serum-free conditions.
Protection of Wild-Type Hepatocytes Against Serum Withdrawal-Induced Cell Death by siRNA-Mediated Reduction of S6K1.
To confirm that S6K1 expression regulates the susceptibility of neonatal hepatocytes to apoptotic signals on growth factor deprivation, we performed siRNA assays. As depicted in Supporting Fig. 5, silencing of S6K1 in neonatal hepatocytes reduced the percentage of hypodiploid cells. In addition, it significantly decreased caspase-3 activity detected on serum withdrawal. Importantly, in siRNA-treated hepatocytes, phosphorylation of Akt and ERK were maintained at higher levels than in the controls, and this effect occurred in parallel with a markedly lower level of IRS-1 serine-307 phosphorylation. Thus, removal of S6K1 elicits a survival effect in serum-deprived hepatocytes.
Discussion
Among the survival pathways triggered by tyrosine kinase growth factors, mTOR has emerged as a therapeutic target for HCC because mTOR/S6K1 signaling plays an important role in angiogenesis, cell cycle progression, and proliferation of liver cancer cells.18 In this regard, overexpression of the activated phosphorylated form of mTOR was observed in 15% of human HCCs, and increased expression of S6K1 was found in 45% of cases and was positively correlated with tumor size.11 To date, systemic chemotherapeutic treatment is ineffective against HCC,19 in part because of the apoptosis resistance observed in HCC cells. This is particularly relevant in therapies with mTOR inhibitors because it has been shown that rapamycin suppresses the feedback loop that dampens the activation of Akt, a pro-survival intermediate.20 In light of these findings, loss of the tuberous sclerosis complex genes leads to constitutive activation of mTORC1 pathway, which confers resistance to insulin-induced activation of PI3K and Akt.21 Although genetic studies have contributed to the discovery of several cytoplasmic modulators of hepatocyte survival, the specific role of S6K1 in the complex signaling networks controlling the balance between death and survival in liver cells has not been investigated. To address this important issue, we have explored the mechanism of cell death in immortalized hepatocyte cell lines generated from wild-type and S6K1-deficient mice. This cellular model has provided insight regarding the molecular mechanism of S6K1 deficiency in modulating the susceptibility of hepatocytes to apoptotic triggers including death receptor activation (mediated by the extrinsic pathway) or growth factor withdrawal (mediated by the intrinsic pathway).
Unexpectedly, we have found that the lack of S6K1 confers protection against apoptosis induced by TNF-α plus actinomycin or Jo2 in both immortalized and primary hepatocytes, ruling out the possibility of collateral effects caused by the immortalization protocol. In light of these results, S6K1 is activated by these apoptotic triggers in wild-type cells. Moreover, rapamycin significantly inhibited death receptor–induced apoptosis in S6K1+/+ hepatocytes. At the molecular level, FLIPL degradation and, subsequently, caspase-8 cleavage and its activation were absent in TNF-α plus actinomycin or Jo2-treated S6K1−/− hepatocytes, indicating that this protection was elicited at the DISC, an early event in the death receptor apoptotic pathway, despite the reduced expression of downstream intermediates such as caspase-8 and Bid. Thus, subsequent molecular events of the apoptotic program, such as cytochrome C release from the mitochondria, activation of the executer caspase-3, and, finally, DNA laddering were totally abolished in S6K1-null hepatocytes. Interestingly, protection against FLIPL degradation on TNF-α plus cycloheximide treatment has recently been reported by Chang et al.22 in fibroblasts lacking JNK1 or the ubiquitin ligase Itch. Thus, one possibility to explain our results could be the absence of JNK1-mediated Itch phosphorylation in S6K1-deficient hepatocytes, resulting in the prevention of FLIPL degradation and the subsequent caspase-8–mediated Bid cleavage. Although our results show the absence of modulation of JNK phosphorylation in S6K1−/− hepatocytes on TNF-α plus actinomycin D or Jo2 treatment, the fact that silencing of JNK1/2 in S6K1+/+ cells did not totally prevent apoptosis induced by these stimuli indicates that JNK-independent signaling also might mediate death receptor–induced apoptosis in hepatocytes, as recently suggested.23 Conversely, it has been reported that in hepatocytes PI 3-K/Akt signaling activated by hepatocyte growth factor-Met coupling ensures high levels of FLIPL and that is a critical step in the protection against its degradation by Fas.24 However, TNF-α plus actinomycin or Jo2 treatments did not decrease Akt or ERK phosphorylation in either kind of cells (results not shown), excluding this mechanism in S6K1 deficiency–induced protection against apoptosis. Moreover, in S6K1−/− hepatocytes, protection against death receptor–mediated apoptosis correlated with the maintenance of high levels of the anti-apoptotic protein BclxL compared with wild-type cells. These data are in agreement with our previous findings in PTP1B−/− hepatocytes, showing a positive correlation between BclxL levels and cell survival.14 Accordingly, the maintenance of high levels of FLIPL and BclxL during stimulation with death receptor ligands confers protection against apoptosis in S6K1-deficient hepatocytes. It remains to be determined exactly where S6K1 downstream signaling interferes with anti-apoptotic gene expression. Nevertheless, the importance of these molecular events has been confirmed in a pathophysiologycally relevant in vivo model in which we showed that S6K1 deficiency protects against acute liver failure associated with hepatocytes apoptosis provoked by ConA injection. Thus, modulation of specific pathways preventing the formation of the Fas-associated protein with death domain–containing complex II in TNF-R1 signaling1 could have a clinical impact and be more selective in preventing hepatocyte death. In this regard, the use of inhibitors of S6K1 could have clinical benefits.
As stated, in liver cells, trophic factors promote survival mainly through the activation of tyrosine kinase receptor signaling, which, among other pathways, results in the activation of mTOR/S6K1.20 When we investigated the impact of S6K1 deficiency on the susceptibility to apoptosis triggered by growth factor withdrawal, we unexpectedly found that S6K1−/− hepatocytes, either immortalized or primary cultured cells, were refractory to apoptosis induced by serum deprivation. At the molecular level, the BclxL/Bim ration was significantly higher in serum-deprived S6K1−/− hepatocytes than in controls. These results clearly indicate that S6K1 levels modulate the balance between the expression of these anti-apoptotic (BclxL) and pro-apoptotic (Bim) proteins in response to serum withdrawal, which may influence the subsequent cellular events, leading to mitochondrial membrane permeabilization, cytochrome C release, caspase-3 activation, DNA fragmentation, and cell death. These results, combined with those discussed later, strongly suggest that S6K1 expression must be tightly controlled during liver development, because a defect in this protein affects hepatocytes' susceptibility to apoptotic signals.
Previous investigations in immortalized hepatocytes cultured under serum-free conditions showed the gradual dephosphorylation of Akt in parallel with FOXO1 entry into the nucleus.14 Nuclear FOXO1 binds to promoters of target genes that induce cell death, including Bim25 and BCL-6, a transcriptional repressor of BclxL,14 indicating that nuclear FOXO1 controls the BclxL/Bim ratio. Surprisingly, in serum-deprived S6K1−/− hepatocytes, the phosphorylation of Akt remained high and FOXO1 was not detected in the nucleus. Altogether our data indicate that under serum-free conditions, S6K1 deficiency in hepatocytes maintains activated survival signaling pathways, resulting in protection against cell death.
As stated, the possibility that S6K1 might be the crucial molecule responsible for the negative feedback mechanism that reaches the cell membrane and switches off receptor tyrosine kinase growth factor signaling has been proposed in previous studies performed with rapamycin-treated cells.26 However, the current study provides the first evidence demonstrating that S6K1 directly modulates this mechanism in hepatocytes. In this regard, our results clearly show a remnant activation of S6K1 in serum-deprived wild-type hepatocytes that probably abrogates the activation of Akt and ERK. Thus, S6K1 deficiency releases the negative feedback inhibition of Akt and ERK activities, antagonizing apoptosis by promoting the phosphorylation of both survival pathways. This molecular mechanism might be critical in the liver, where it could have impact in serious pathological states such as hepatitis, HCC, and acute liver failure.18
IRS-1 is the common upstream mediator of Akt and ERK activation.17 In our study, high levels of phosphorylated IRS-1 in Ser-307 were observed in wild-type hepatocytes in parallel with low levels of its tyrosine phosphorylation under apoptosis-inducing serum-free conditions. The result was the inhibition of the downstream signaling pathways mediated by PI3K/Akt and Grb-2/Ras/ERK that trigger hepatocyte survival.5, 6 By contrast, IRS-1 Ser-307 phosphorylation was virtually absent in S6K1 null hepatocytes or in wild-type cells with reduced levels of S6K1 induced by siRNA, resulting in its sustained tyrosine phosphorylation and, subsequently, in the activation of PI3K/Akt and ERK. Importantly, the pharmacological inhibition of either PI3K/Akt or ERK resulted in a modest induction of apoptosis in S6K1−/− hepatocytes. However, the combination of both compounds significantly abrogated the survival effect of S6K1 deficiency. These results indicate that, at least in hepatocytes, both Akt and ERK signaling needs to be disrupted to release the survival effect elicited by S6K1 inhibition. Thus, our data have important pharmacological implications. First, the specific inhibition of S6K1 by rapamycin and its derivatives augments Akt and ERK signaling, which might promote tumor survival. Second, from a therapeutic point of view, our findings suggest that the combination of rapamycin-like compounds with inhibitors of Akt and ERK could bypass the feedback mechanisms that confer protection against survival.
In summary, our results demonstrated that specific inhibition of S6K1 in hepatocytes confers protection against apoptosis induced by the extrinsic or the intrinsic apoptotic pathways. A common molecular event is the maintenance of BclxL expression in S6K1−/− hepatocytes under apoptotic stimuli, resulting in protection against acute liver failure and enhancement of survival against death receptor ligands or withdrawal of trophic factors. This mechanism might explain the resistance to mTOR inhibitors in cancer treatments and strongly suggests that S6K1 inhibition could be beneficial in preventing acute liver failure and, in combination with inhibitors that abrogate sustained activation of Akt and ERK, could improve the efficacy of HCC treatment.
Acknowledgements
The authors thank Javier Naval (University of Zaragoza, Spain) for the supply of Jo2 and Maryellen Daston for manuscript editing.




