Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways†
Potential conflict of interest: Nothing to report.
Abstract
Chronic hepatitis C virus (HCV) infection is associated with the production of serum cytokines, including transforming growth factor (TGF)-β2. Despite the occurrence of hepatic angiogenesis in liver conditions, the role of HCV proteins in this context is currently unknown. We demonstrated that the development of hepatic neoangiogenesis in patients infected with HCV is associated with the expression of TGF-β2 and vascular endothelial growth factor (VEGF) and with activation of endothelial cells, as evidenced by CD34 expression. The analysis of liver biopsies of HCV-positive and HCV-negative patients using immunostaining showed significant elevation of TGF-β2, VEGF, and CD34 expression in patients who were HCV-positive. Using an HCV established culture system, we confirmed further the production of both TGF-β2 and VEGF proteins, in the hepatoma cell lines HepG2 and Huh7 by transfection with full-length HCV RNA (JFH1) or by the regulated expression of core. In addition, regulated expression of core protein in HepG2 or Huh7 cells was found to induce expression and activation of the transcription factor E2F1 and apoptosis signal-regulating kinase 1 (ASK1), activation of c-jun-N-terminal kinase (JNK) and p38, and extracellular-regulated kinase (ERK), and transcription factors activator protein 1 (AP-1), activating transcription factor 2 (ATF-2), cyclic adenosine monophosphate response element binding (CREB), E2F1, hypoxia inducing factor 1 alpha (HIF-1α), and specificity protein 1. Furthermore, data obtained from inhibitor experiments revealed the importance of E2F1 and ASK1 in the modulation of core-induced activation of JNK and p38 pathways and suggested an essential role for JNK, p38, and ERK pathways in the regulation of core-induced production of TGF-β2 and VEGF proteins. Thus, our data provide insight into the molecular mechanisms whereby core protein mediates the development of hepatic angiogenesis in patients with chronic HCV infection. (HEPATOLOGY 2009.)
Persistent hepatitis C virus (HCV) infection often leads to chronic hepatitis,1, 2 which is characterized by the development of hepatocellular necrosis, inflammation, and fibrosis.3 Although the course of disease through the translation of fibrosis into hepatocellular carcinoma (HCC) is well known,4 the mechanisms underlying the development of hepatic angiogenesis in chronic HCV-infected patients, so far, are not fully understood. The occurrence of hepatic angiogenesis has been described in liver conditions such as viral hepatitis, cirrhosis, autoimmune hepatitis, primary biliary cirrhosis, and HCC.5, 6 HCC is a highly malignant tumor that is characterized by active neovascularization.7 Most studies carried out in this field reported variation at the expression level of factors regulating angiogenesis.8, 9 Thus far, there are limited data on the role of hepatitis C viral proteins in the regulation of angiogenic factors. Vascular endothelial growth factor (VEGF) is a potent angiogenic factor that plays a central role during the development of angiogenesis in various cancer types,10 including HCC.11, 12 VEGF is regulated by pathological conditions13, 14 and by tumor necrosis factor alpha15 and transforming growth factor beta (TGF-β).16
Transforming growth factor beta is one of the best characterized cytokines that affect cell growth, cell death, and morphogenesis.17 Physiologically, TGF-β is expressed in the nonparenchymal cells of the liver but not in the hepatocytes from normal or regenerating liver.18 In addition, elevated expression of TGF-β1-3 was observed in liver tissues of patients with chronic HCV infection.19
HCV genome has a long open reading frame, flanked with a 5′ and 3′ untranslated region, which encodes a polyprotein precursor of approximately 3010 to 3033 amino acid residues.20 This polyprotein is cleaved by both host and viral proteases to generate four structural proteins (C, E1, E2, and P7) and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B).21-24
Core protein is derived from the first 191 amino acids of the N-terminal of the precursor polyprotein.20 Besides its function as a component of viral nucleocapsids, core protein has been shown to target a wide spectrum of viral and cellular factors and different signaling pathways.21, 24-28
Here, we provide evidence for the contribution of HCV core protein in the development of hepatic angiogenesis, during chronic HCV infection, by the regulation of multiple pathways.
Abbreviations
ALT, alanine aminotransferase; AP-1, activator protein 1; ASK1, apoptosis signal-regulating kinase 1; ATF-2, activating transcription factor 2; cDNA, complementary DNA; CREB, cyclic adenosine monophosphate response element binding; ELISA, enzyme-linked immunosorbent assay; EMSA, electrophoretic mobility shift assay; ERK, extracellular-regulated kinase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIF-1α, hypoxia-inducing factor 1 alpha; JNK, c-Jun-N-terminal kinase; PKC, protein kinase C; siRNA, small interfering DNA; TGF-β2, transforming growth factor-beta2; VEGF, vascular endothelial growth factor.
Patients and Methods
For details of methods, please see Supporting Methods
Patients.
Thirty-two patients were (1) positive for anti-HCV core by second generation enzyme-linked immunosorbent assay (ELISA), (2) had at least four serial estimations of the serum alanine aminotransferase (ALT) (at least 4 weeks apart), and (3) had undergone percutaneous liver biopsy. All liver biopsy specimens were assessed by a single experienced liver pathologist blinded to the liver biochemistry and scored according to standard criteria29 and by Knodell's histological activity index.30 All available liver biopsy specimens as well as ALT values were obtained from naive patients with chronic HCV infection. The study was approved by the local ethical Committee, University of Alexandria, Alexandria, Egypt.
Immune Fluorescence Staining of Liver Biopsies.
Immune fluorescence staining of liver biopsies of HCV-negative and positive patients was performed using anti-TGF-β2 (SC-90) or anti-VEGF (SC-507) antibodies, all from Santa Cruz, as described.31 Pictures were taken on an Axiovert 135 Microscope (Zeiss, Germany) with an Apochromat x63 oil immersion lens using OpenLab software (Improvision, Tübingen, Germany).
Immunohistochemistry.
Immunohistochemical staining was performed for CD34 on 5-mm-thick, formalin-fixed, paraffin wax-embedded sections, using the streptavidin-biotin immunoperoxidase technique. A monoclonal anti-CD34 antibody (SC-19587, Santa Cruz Biotechnology) was used at a 1:100 dilution. Antigen retrieval consisted of microwave treatment with citrate buffer, pH 6.0, for 10 minutes. As a negative control for each case, the primary antibody was replaced with normal rabbit serum. We chose CD34 because it is more sensitive than other markers for liver endothelial cells.32
Cell Lines.
Human hepatoma cell lines HepG2 and Huh7 (ATCC, Rockville, MD), and the RetroPack pT67 cells (Clontech, Inc) were grown in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum.
In Vitro RNA Synthesis and Transfection.
Plasmids pJFH1 and pFJH1/GND,33 which encode the full-length JFH1 and JFH1/GND RNA, respectively, were used for in vitro transcription using the RiboMax Large Scale RNA Production System (Promega, Madison, WI). After DNaseI (RQ-1 RNase-free DNase, Promega) treatment, the transcribed RNAs were purified using SV Total RNA Isolation system (Promega, Madison, WI). For RNA transfection, Huh-7 cells were washed twice, and 2 × 106 cells resuspended in Nucleofactor buffer (Amaxa) containing 10 μg of either JFH1 or JFH1/GND were electroporated using the nucleofactor device as described.21-23, 34 The levels of HCV RNA and viral protein expression were detected by northern and western blot analysis, respectively. The expression and production of both TGF-β2 and VEGF were analyzed by both western blot and ELISA.
Infection of Naïve Huh7 Cells with Supernatant Harvested from JFH1 RNA-Transfected or JFH1/GND RNA-Transfected Cells.
Seventy-two hours after transfection, culture media of JFH1 RNA-or JFH1/GND RNA-transfected cells were collected and cleared as described.33 Huh7 cells were plated 24 hours before infection at a density of 4 × 105 cells/10-cm dish. Concentrated culture media (5 mL) were used for inoculation of cells for 3 hours with periodic rocking. At the end of inoculation, cells were washed three times with phosphate-buffered saline, followed by addition of 10 mL complete culture medium. Inoculated cultures were allowed to grow for another 24, 48, and 72 hours before the collection of culture media or the preparation of cell lysates. Culture media were used for the measurement of both TGF-β2 and VEGF, whereas cell lysates were used for western blot analysis.
Generation of HepG2-HCV and Huh7-HCV Clones Constitutively Expressing the Full-Length HCV Complementary DNA.
The construction of the full-length HCV complementary DNA (cDNA) into pcDNA.3.1(+) as well as transfection and selection of HepG2-HCV and Huh7-HCV colonies expressing the full-length HCV cDNA were performed as described recently21-23.
Construction of HCV Core Plasmid, Generation of Viruses, and Infection of Target Cells.
The construction of the HCV core plasmid encoding for the complete HCV core protein, HCV NS3 protein, the generation of viruses, and the infection of target cells were performed as described.21-23
Immune Fluorescence Staining of HCV Core Transfected Cells.
Immunofluorescence staining of Huh7-core transfectants was performed using anti-HCV core (Research Diagnostics, Inc), anti–TGF-β2 (SC-90; Santa Cruz), and anti-VEGF (SC-507; Santa Cruz Biotechnology) antibodies as described.31, 33 Pictures were taken on an Axiovert 135 Microscope (Zeiss, Germany) with an Apochromat ×63 oil immersion lens using OpenLab software (Improvision, Tübingen, Germany).
RNA Interference.
SMARTpool E2F1, apoptosis signal-regulating kinase 1 (ASK1) small interfering RNA (siRNA), and negative control siRNA (Dharmacon Research, Lafayette, Co) cells were transfected with lipofectamine 2000 as described.33
Immunoblot.
Immunoblot analysis was performed according to the standard procedures. The following antibodies were used at the indicated dilution: anti-HCV core, 1:1000; anti-HCV NS3, 1:1000, all from Research Diagnostic, Inc,); anti-HCV E1, 1:1000 (Austral Biological, San Ramon, CA), 1:1000; anti-HCV E2, 1:1000; anti-HCV NS4B, 1:1000: anti-NS5A, 1:1000 all from Biodesign international, Kennebunk, ME); anti-NS5B, 1:1000 (AXXORA, Germany); anti-ASK1 (Sc-7931), 1:1000; anti-TGF-β2 (SC-90), 1:1000; anti-VEGF (SC-507) (1:1000), 1:1000; anti–c-jun-N-terminal kinase (JNK) (Sc-474), 1:2,000; anti-p38 (Sc-535), 1:2,000; anti–extracellular-regulated kinase (ERK)1/2 (Sc-154-G), 1:2000; E2F1 (Sc-193), 1:1000; anti-actin (SC-1615), 1:5000; anti–activating transcription factor 2 (ATF-2) (SC-242), 1:1000; anti–phospho-ATF-2 (SC-8398), 1:1000; anti-Sp1 (SC-420); anti-c-jun (SC-1694), 1:1000; anti-phospho-c-jun (SC-7981) 1:1000, all from Santa Cruz Biotechnology, Inc; anti–phospho-Sp1(ab 59257, ABCAM),1:1000; anti–cyclic adenosine monophosphate response element binding 1 (CREB-1) (#9104), 1:1000; anti–phospho CREB (#9191), 1:1000, all from Cell Signaling Technologies.
Northern Blot, Electrophoretic Mobility Shift Assay, and In Vitro Kinase Assays.
Northern blot analysis and electrophoretic mobility shift assays (EMSAs) have been performed using double-stranded synthetic oligonucleotides carrying binding sites for ATF-2, activator protein 1 (AP-1), CREB, E2F1, hypoxia inducing factor 1 alpha (HIF-1α), and Sp1 (Santa Cruz), and in vitro kinase assays were performed as described.21-23, 31, 32
Measurement of TGF-β2 Production in Culture Media.
The post-culture media of JFH1 RNA and JFH1/GND-transfected Huh7 cells as well as both HepG2-core and Huh7-core transfectants cultured in the presence (+Tc) or in the absence (−Tc) of 4 μg/mL tetracycline up to 48 hours were harvested and stored at −80°C. TGF-β2 concentration was determined by ELISA as described.35
Measurement of VEGF Production in Culture Media.
The postculture media of JFH1 RNA and JFH1/GND-transfected Huh7 cells as well as both HepG2-core and Huh7-core transfectants were seeded onto multiple-well culture plates at a density of 0.1 × 104 cells/well. Twenty-four hours later, the cells were washed with serum-free medium and allowed to grow further in serum-free medium. After 24 hours, the culture medium was collected and VEGF level was determined using RayBio Human VEGF ELISA kit (Ray Biotech, Inc.).
Construction of Luciferase Reporter Plasmids.
The DNA sequences of the TGF-β2 and VEGF promoters were amplified by polymerase chain reaction using genomic DNA from Huh7 cells as a template. The design of the specific primer pairs used for the amplification of the TGF-β2 promoter was made as described.33 The amplified DNA fragment was sequenced and inserted into the promoterless luciferase vector pGL3-Basic (Promega) as described24 to make the plasmid pGL3-TGF-β2 promoter. The design of the specific primer pairs used for the amplification of VEGF promoter was made as described.36 The amplified DNA fragment was sequenced and inserted into the promoterless luciferase vector pGL3-Basic (Promega) as described.23
Cell Transfection and Luciferase Assay.
The HepG2-core and Huh7-core transfectants were seeded onto 24-well culture plates at a density of 0.5 × 104 cells per well and cultured in medium with (+Tc) 4 μg/mL tetracycline. Twenty-four hours later, the cells were transfected using FuGENE 6 reagent (Roche) according to the protocol of the manufacturer. After the transfection of the HepG2-core and Huh7-core transfectants with either pGL3-TGF-β2 promoter or pGL3-VEGF promoter (0.5 μg/well) in combination with 0.25 μg of pβgal (Clontech Inc.), the cells were allowed to grow in medium with (+Tc) or without (−Tc) tetracycline and then pretreated or not treated with the specific inhibitors of JNK (SP600125), p38 (SB-203580), protein kinase C (PKC) (Calphostin) or HIF-1α (PX-478). Forty-eight hours later, the luciferase activity was measured and normalized to the transfection efficiency as described.23
Results
Expression of TGF-β2 and VEGF and Activation of Endothelial Cells in Liver Biopsy Specimens of Patients Who Were HCV-Positive.
We started with the quantitative analysis of the ALT pattern in both male and female patients with chronic HCV infection compared with control (patients who were HCV-negative). In control patients, the total mean of ALT activity in men (30 IU/L) and women (20 IU/L) was in the normal limit (Fig. 1A), whereas the total mean ALT activity in HCV-infected patients was 64 IU/L in male and 50 IU/L in female (Fig. 1A) patients, demonstrating a significant elevation of ALT activities during the course of HCV infection.
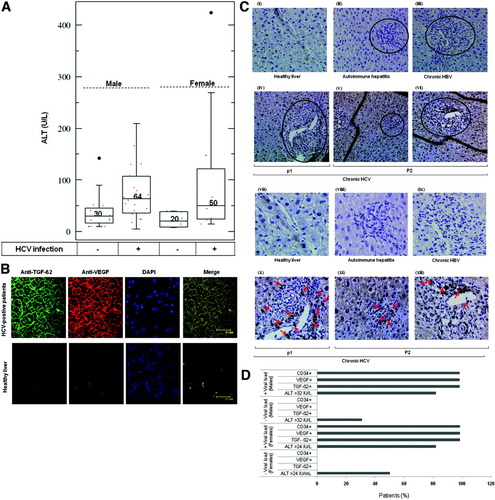
(A) ALT pattern in HCV-positive and negative (male and female) patients. (B) Immunofluorescence staining of TGF-β2 and VEGF and their merged image in liver biopsy specimens obtained from HCV-positive and HCV-negative patients. Both TGF-β2 and VEGF are predominantly expressed in HCV-positive patients. The merging of TGF-β2, VEGF, and 4′,6-diamidino-2-phenylindole stained nuclei showing a similar distribution. (C) Immunoperoxidase analysis of CD34 in liver biopsy specimens of patients who were HCV-negative and HCV-positive. Marked portal inflammation in autoimmune hepatitis (original magnifications: ii, 100× and viii, 400×) and in chronic hepatitis B virus (original magnifications: iii, 100× and ix, 400×) shows no evidence of angiogenesis. Evidence of angiogenesis (active endothelial cells stained with anti-CD34) in portal tract in HCV-positive patients (original magnifications: iv, v, vi, 100× and x, xi, xii, 400×). (D) ALT, TGF-β2, VEGF, and CD34 pattern in HCV-positive and HCV-negative (male and female) patients.
To examine whether the production of both TGF-β2 and VEGF and CD34 are associated with HCV infection, control liver biopsy specimens including healthy liver (n = 6), chronic hepatitis B virus (n = 10), and autoimmune hepatitis (n = 10), and liver biopsy specimens of chronic HCV-infected patients (n = 32) were subjected to either immunofluorescence staining using anti–TGF-β2 and VEGF or immunoperoxidase staining using anti-CD34 antibody. Almost no immunoreactivity was evident in control liver biopsy specimens, when probed with antibodies specific to either TGF-β2 or VEGF (Fig. 1B), or to CD34 (Fig. 1C). In contrast, liver biopsy specimens of HCV-infected patients clearly demonstrated hepatocytes that stained with anti–TGF-β2 or anti-VEGF (Fig. 1B) antibodies. Ultimately, nuclear staining with 4′,6-diamidino-2-phenylindole revealed that the expression of both TGF-β2 and VEGF is predominantly found either in the cytoplasm close to the cell membrane or extracellularly. The staining of endothelial cells by anti-CD34 was seen along the vascular channels, in groups of cells that apparently did not border vascular lumina, and in groups of cells located in portal tracts and septa (Fig. 1C).
Thirty-two of 32 (100%) patients who were HCV-positive were positive to immunostaining with anti–TGF-β2, VEGF, and CD34 antigens in comparison with control liver biopsies, as shown in Table 1. The expression of TGF-β2, VEGF, and CD34 was detectable but weak in six of 32 (18%), detectable but not strong in 11 of 32 (34%), and strong detectable in 15 of 32 (47%) HCV-positive patients, suggesting an association between HCV infection and the production of both TGF-β2 and VEGF and the activation of endothelial cells as evidenced by the expression of CD34.
| Viral status | (n) | TGF-β2, VEGF, CD34 | ||
|---|---|---|---|---|
| + | ++ | +++ | ||
| Control (healthy liver) | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HBV+ | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Autoimmune hepatitis | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HCV+ | 32 (100%) | 6 (18%) | 11 (32%) | 15 (47%) |
Although more than 50% (3/6) of control female and 30% (6/20) of control male showed an individual mean ALT activities of at least 24 IU/L and at least 32 IU/L, respectively, the immunostaining of TFG-β2, VEGF, or CD34 was either undectable for TGF-β2 and VEGF or for CD34 in both female and male (Fig. 1D). The quantitative analysis of HCV-positive patients indicated that 19% (3/11) with normal liver aminotransferase (ALT activities ≤ 24/IU/L) and 81% (8/11) with abnormal liver aminotransferase (ALT activities ≥ 24 IU/L) of female, and 16% (4/25) with normal liver aminotransferase (ALT activities ≤ 32/IU/L) and 84% (21/25) with abnormal liver aminotransferase (ALT activities ≥ 24 IU/L) of male were positive to immunostaining with anti–TGF-β2, VEGF, and CD34 antigens (Fig. 1D). In using antibodies against TGF-β2, VEGF, and CD34, a significant difference was also observed in the development of hepatic angiogenesis among liver biopsy specimens of the HCV-negative group and the HCV-positive group (Table 1).
Induction of TGF-β2 and VEGF by the Transfection of Full-Length JFH1 Genome.
To specify the role of HCV viral protein(s) in the development of hepatic angiogenesis, we transfected in vitro transcribed RNAs corresponding to the full-length JFH1 and JFH1/GND RNA, respectively, into Huh7 cells as described.33 As shown in northern blot (Fig. 1A), the full-length genome was noted in JFH1-transfected cells 24 hours after transfection, and remained detectable up to 72 hours. In addition, the detection of viral proteins core and NS3 at the indicated time points in JFH1 transfected cells, but not in JFH1/GND-transfected cells confirmed the replication of the full-length JFH1 RNA (Fig. 2B). Thus, the full-length JFH1 RNA could be properly replicated and translated to yield the complete polyprotein, which in turn could be processed to the individual proteins. Based on the clinical data, we therefore, set out to determine whether the transfection of Huh7 cells with the full-length JFH1 RNA would similarly induce the expression of TGF-β2 and VEGF. Using western blot analysis (Fig. 2B), we could show the up-regulation of both TGF-β2 and VEGF expression in Huh7 cells in response to transfection with full-length JFH1 RNA, when compared with control cells. In addition, we could detect the production of both TGF-β2 and VEGF in the culture media of JFH1 RNA-transfected cells as well as in culture media of core expressing cells, but not in those of NS3 expressing cells using ELISA (Fig. 2C). To examine whether the infection with supernatant collected from JFH1 RNA–transfected cells should also enhance the production of both TGF-β2 and VEGF, we inoculated naive Huh7 cells with concentrated supernatant harvested from JFH1 RNA–transfected cells for regulated time intervals up to 72 hours. Only cells inoculated with JFH1 medium were positive for both core and NS3 protein as evidenced by immune blotting (Fig. 2D). The induction of both TGF-β2 and VEGF expression was noted at 24 hours without any marked changes at the indicated time points (48 and 72 hours), as shown in Fig. 2D. Naive Huh7 cells inoculated with supernatant collected from JFH1/GND RNA–transfected cells were used as control.
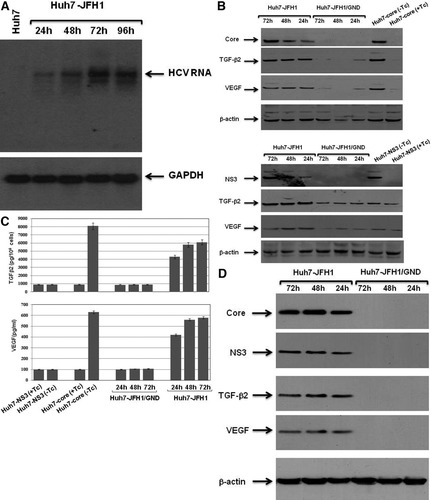
(A) Northern blot analysis of 20 μg total RNA extracted from Huh7 cells transfected with the full-length JFH1 RNA were electrophoresed in agarose gels and transferred onto nylon membrane. Blots were hybridized with cDNA probe spanning from nucleotides 350 to 3500 of the HCV genome. The equal loading of total RNA was judged by glyceraldehyde 3-phosphate dehydrogenase. (B) Western blot analysis of viral proteins core and NS3 as well as the induction of both TGF-β2 and VEGF in JFH1 RNA-transfected Huh7 cells at the indicated time points. Lysates of HCV core-transfected, HCV NS3-transfected Huh7 cells, and JFH1/GND RNA-transfected cells were used as positive and negative controls, respectively. (C) Measurement of both TGF-β2 and VEGF production in culture media of JFH1 RNA-transfected and JFH1/GND RNA-transfected cells using ELISA. Data are averages ± standard deviation of three independent experiments performed in duplicate. (D) Western blot analysis of viral proteins core and NS3 as well as the induction of both TGF-β2 and VEGF in Huh7 cells inoculated with media collected from JFH1 RNA-transfected and JFH1/GND RNA-transfected Huh7 cells. Lysates of Huh7-core and Huh7-NS3 transfectants induced to express core and NS3, respectively, were used as control. Beta-actin was used as internal control for loading and transfer. Data are representative of three independent experiments performed separately.
Induction of Both TGF-β2 and VEGF in Core-Expressing Cells.
We addressed the role of core in the development of hepatic angiogenesis during HCV infection. Using our established cell culture system,21-23 we could demonstrate the induction of both TGF-β2 and VEGF expression by regulated expression of core in HepG2-core and Huh7-core transfectants (Fig. 3A).
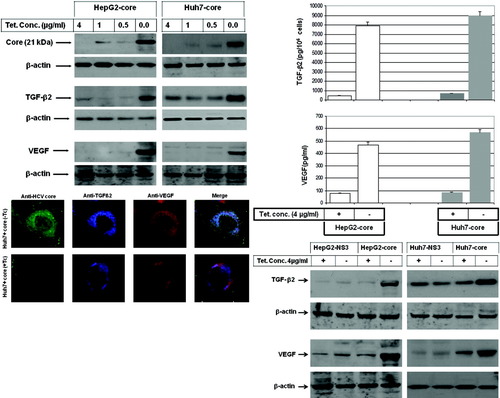
(A) Western blot analysis demonstrating the induction of TGF-β2 and VEGF expression by the regulated expression of core in hepatoma cells. Beta-actin was used as internal control for loading and transfer. Data are representative of three independent experiments. (B) ELISA demonstrating the production of TGF-β2 and VEGF in the culture media of hepatoma cells expressing core protein. Data are averages ± standard deviation of three independent experiments performed in duplicate. (C) Expression and subcellular localization of HCV core protein (green), TGF-β2 (blue), and VEGF (red) in Huh7-core transfectants cultured in the presence (+Tc) or in the absence (−Tc) of 4 μg/mL tetracycline for 48 hours. The three images were overlaid (HCV core, TGF-β2, and VEGF) by confocal laser microscopy. (D) Western blot analysis demonstrating the induction of both TGF-β2 and VEGF by the expression of core, but not by the expression of NS3. Data are representative of three independent experiments with identical results.
Next, we analyzed the effect of core expression on production of both TGF-β2 and VEGF using ELISA assay or immune fluorescence staining. Data obtained from ELISA assay (Fig. 3B) revealed the induction of TGF-β2 and VEGF production in culture media by the expression of core. To show whether the production of both TGF-β2 and VEGF during HCV infection is core dependent, we examined the production of TGF-β2 and VEGF proteins in culture media of both HepG2-NS3 and Huh7-NS3 transfectants after the induction of HCV nonstructural protein NS3, and compared this with core-induced production of TGF-β2 and VEGF in the same cell lines. After the induction of TGF-β2 and VEGF by the expression of core, the expression of NS3 was unable to influence the basal production of TGF-β2 or VEGF proteins (Fig. 3A, B), suggesting an important role for core in the induction of TGF-β2 and VEGF production, during HCV infection. Further analysis using immune fluorescence staining (Fig. 3C) demonstrated the expression and the localization of core (green), TGF-β2 (blue), and VEGF (red) in core-expressing cells.
Core-Induced Production of TGF-β2 and VEGF Is Mediated by JNK, p38, and ERK Pathways.
To address the role of mitogen-activated protein kinase pathways JNK, p38, and ERK in the regulation of core-induced production of VEGF and TGF-β2, 1 hour before withdrawal of tetracycline from the culture medium, core transfectants were treated with the specific inhibitors of JNK (SP600125), p38 (SB-203580), or ERK (PD98059) and subsequently subjected to western blot analysis and in vitro kinase assay. First, we confirmed our previously reported data30 demonstrating core-induced effects on JNK, p38, and ERK pathways (Fig. 4A). Interestingly, the suppression of core-induced expression of TGF-β2 by SP600125 and SB-203580, but not by PD98059 (Fig. 4B), revealed the involvement of the pathways JNK and p38, but not ERK pathway, in the regulation of core-induced production of TGF-β2, whereas the inhibition of core-induced production of VEGF by the inhibitors of JNK, p38, and ERK (Fig. 4B) confirmed the involvement of the JNK, p38, and ERK pathways in the regulation of core-induced production of VEGF.
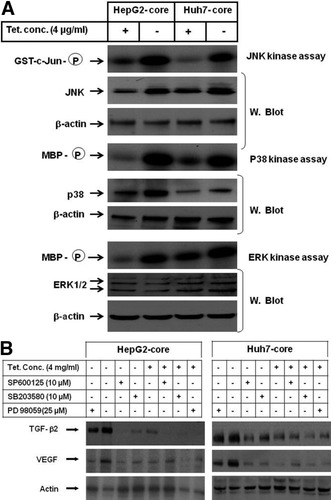
(A) In vitro kinase and western blot analysis of the mitogen-activated protein kinase signaling pathways JNK, p38, and ERK. The kinase activities of JNK, p38, and ERK were determined using GST-c-Jun as substrate for JNK, and MBP as substrate for both p38 and ERK. The expression of JNK, p38, and ERK were analyzed by immune blotting using anti-JNK, anti-p38, and anti-ERK antibodies, respectively. Beta-actin was used as internal control for loading and transfer. Data are representative of three independent experiments. (B) Western blot analysis demonstrating the inhibition of core-induced production TGF-β2 by the inhibitors of JNK (SP600125) and p38 (SB-203580) pathways, and inhibition of core-induced production of VEGF by the inhibitors of JNK (SP600125), p38 (SB-203580), and ERK (PD98059) pathways. Beta-actin was used as internal control. Western blots are representative of three independent experiments performed with identical results. GST, glutathione-S-transferase; MBP, myelin basic protein.
Core-Induced E2F-1 Is Involved in the Regulation of ASK1 and Its Downstream Pathways.
Based on our recent findings,21 we asked whether core-enhanced DNA-binding activities of E2F1 are involved in the regulation of ASK1. We examined the effect of core on E2F1 and ASK1 using western blot analysis, in vitro kinase assay, and EMSA. Figure 5A demonstrates the up-regulation of both E2F1 and ASK1 in core-expressing cells compared with control cells, whereas Fig. 5B demonstrates core-induced DNA-binding activity of E2F1 as transcription factor. To investigate whether core-induced activation of E2F1 is involved in the regulation of ASK1, we sought to inhibit core-induced effects on E2F1 by its specific siRNA. We found that the depletion of E2F1 by its specific siRNA not only prevented its own induction by core protein, but also impaired the induction of ASK1 expression and activity (Fig. 5C). These data suggest that core-induced E2F1 is involved in the regulation of ASK1.
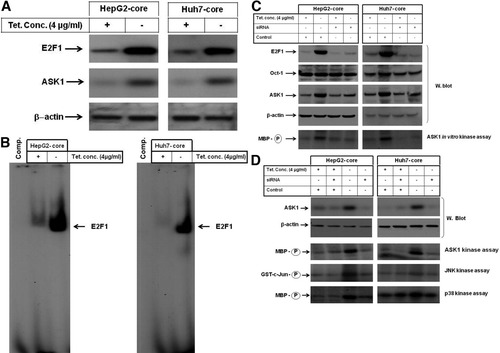
(A) Western blot analysis of E2F1 in cells expressing core protein. Beta-actin was used as internal control. (B) Analysis of DNA-binding activity of E2F1 by EMSA in hepatoma cells expressing core protein. (C) Inhibition of E2F1 by siRNA impaired core-inducted expression of ASK1. Western blot analysis demonstrated the suppression of E2F1 and subsequent ASK1 protein, whereas in vitro kinase assay using MBP as substrate for ASK1 demonstrated the inhibition of core-induced ASK1 activity by the knockdown of E2F1. Beta-actin was used as internal control for loading and transfer. Data are representative of three independent experiments. (D) In vitro kinase assay demonstrating the inhibition of core-induced activation of both JNK and p38 by the knockdown of ASK1 in cells expressing core protein. Data are representative of three independent experiments.
To determine the role of core-induced activation of ASK1 in the regulation of JNK and p38 pathways, we blocked ASK1 expression by siRNA. Western blot analysis (Fig. 5D) demonstrated the knockdown of ASK1 protein by siRNA. In vitro kinase assay (Fig. 5D) showed the inhibition of core-induced activation of ASK1, JNK, and p38 by the knockdown of ASK1, suggesting the involvement of core-induced activation of ASK1 in the regulation of JNK and p38 pathways.
Core-Induced Activation of the Transcription Factors AP-1, ATF-2, CREB, HIF-1α, and Sp1 Is Mediated by Multiple Pathways.
Based on the roles of Sp1 and CREB37 and ATF-238 in the regulation of TGF-β2, and the role of AP-1 and HIF-1α39 in the regulation of VEGF, we examined the activation of Sp1, CREB, ATF-2, AP-1, and HIF-1α in core-expressing cells by EMSA or western blot analysis using the corresponding phospho-specific antibodies. EMSA demonstrates the enhancement of DNA-binding activity (Fig. 6A) and specificity (Fig. 6B) of HIF-1α by the expression of core. Moreover, we demonstrated the ability of PX-478, the inhibitor of HIF-1α, to abrogate core-induced activity of HIF-1α in HepG2 and Huh7 (Fig. 6B). Western blot analysis (Fig. 6C-F) demonstrates the phosphorylation of Sp1, CREB, ATF-2, and c-jun/AP-1 proteins by the expression of core in Huh7-core transfectants. Similar results were noted by the expression of core in HepG2-core transfectants (data not shown). To determine the pathways involved in the regulation of core-induced activation of Sp1, CREB, ATF-2, AP-1, or HIF-α, we sought to block JNK, p38, and ERK pathways, and the protein kinase C (PKC) pathways by their specific inhibitors SP600125, SB203580, PD98059, and calphostin C, respectively. The pretreatment of core-expressing cells with calphostin C was found to suppress core-induced phosphorylation of Sp1, CREB, and ATF-2 (Fig. 6C), whereas treatment with SP600125 inhibited the phosphorylation of AP-1/c-jun and ATF-2 (Fig. 6D), and pretreatment with SB-203580 inhibited the phosphorylation of AP-1/c-jun, ATF-2, and CREB (Fig. 6E), and pretreatment with PD98059 inhibited the phosphorylation of AP-1/c-jun and the DNA-binding activity of HIF-1α (Fig. 6F), suggesting the involvement of multiple pathways in the modulation of core-induced activation of Sp1, CREB, ATF-2, AP-1/c-jun, and HIF-1α in liver cells.</P>
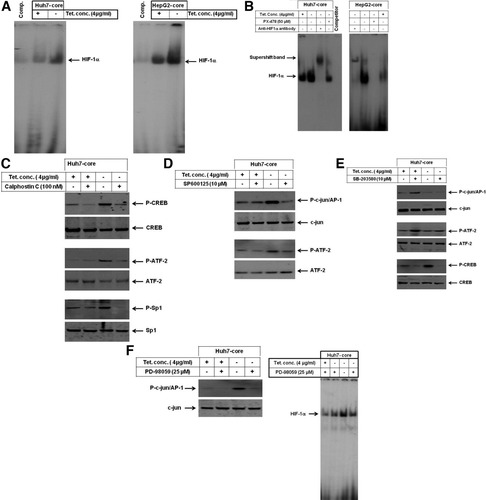
(A) Induction of DNA-binding activity of the transcription factor HIF-1α by core in hepatoma cells. (B) Super shift analysis demonstrated the specificity of core-induced HIF-1α in hepatoma cells, whereas the pretreatment with the inhibitor of HIF-1α, PX-478, blocked core-induced activity of HIF-1α. (C) Inhibition of core-induced phosphorylation of Sp1, CREB, and ATF-2 by the inhibitor of PKC (calphostin C). (D) Inhibition of core-induced phosphorylation of AP-1/c-jun and ATF-2 by the inhibitor of JNK (SP600125). (E) Inhibition of core-induced phosphorylation of CREB and ATF-2 by the inhibitor of p38 (SB-203580). (F) Inhibition of core-induced activation of HIF-1α, and the phospohorylation of AP-1/c-jun by the inhibitor of ERK (PD98059). Western blot analysis was performed using antibodies specific to p-c-jun/AP-1, p-ATF-2, p-CREB, and p-Sp-1 or to total c-jun, ATF-2, CREB, and Sp-1 proteins. Data are representative of three independent experiments with similar results.
Sp1, CREB, and ATF-2 Proteins Are Involved in the Transcriptional Regulation of TGF-β2 Promoter in Core-Expressing Cells.
To show whether core-induced production of TGF-β2, in liver cells is mediated by Sp1, CREB, or ATF-2 proteins, HepG2-core and Huh7-core transfectants were transiently transfected with TGF-β2 promoter-derived construct and pβgal, and allowed to grow in culture medium with or without tetracycline in the presence of the inhibitors of JNK (SP600125), p38 (SB-203580), or PKC (calphostin C) for 48 hours and then subjected to luciferase assay. Figure 7 demonstrates the enhancement of the transcriptional activity of TGF-β2 promoter in HepG2-core (>eightfold) and Huh7-core (>ninefold) transfectants by expression of core. The inhibition of core-induced effects on TGF-β2 promoter by SP600125, SB-203580, or PKC (Fig. 7A, B) provides evidence for the involvement of ATF-2, CREB, and specificity protein 1 in the regulation of core-induced production of TGF-β2 in liver cells.
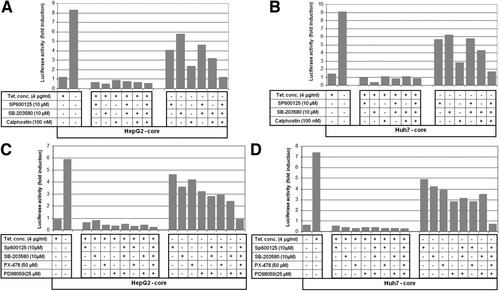
HCV core protein transactivates both TGF-β2 and VEGF in the liver cells. (A and B) Luciferase reporter assay of TGF-β2 promotor, after the pretreatment of the transiently transfected cells with the specific inhibitors of JNK (SP600125), p38 (SB-203580), and PKC (calphostin C) in the presence (+Tc) and absence (−Tc) of 4 μg/mL tetracycline. The TGF-β2 promoter activity was assessed in HepG2-core and Huh7-core cells after normalization to the transfection efficiency using luciferase reporter assay. The luciferase activities are represented as fold induction over the values obtained from control cells. (C and D) Luciferase reporter assay of VEGF promoter, after the pretreatment of the transiently transfected cells with the specific inhibitors of JNK (SP600125), p38 (SB-203580), ERK (PD98059), and HIF-1α (PX-478) cultured in the presence (+Tc) and absence (−Tc) of 4 μg/mL tetracycline. Luciferase activity was assessed in both HepG2-core (A) and Huh7-core (B) cells after normalization to transfection efficiency. The luciferase activities are represented as fold induction over the values obtained from HepG2 and Huh7 control cells.
AP-1 and HIF-1α Are Involved in the Transcriptional Regulation of Core-Induced Production of VEGF.
To investigate whether core-induced production of VEGF in liver cells is mediated by AP-1 or HIF-1α proteins, HepG2-core and Huh7-core transfectants were transiently transfected with VEGF promoter-derived construct and pβGal vector and allowed to grow in culture medium with or without tetracycline in the presence of the inhibitors of JNK (SP600125), p38 (SB-203580), or HIF-1α (PX-478). Forty-eight hours later, the cells were harvested and luciferase assay was performed. Data obtained from luciferase assay (Fig. 7) demonstrated the activation of VEGF promoter in HepG2-core (>5.8-fold) and Huh7-core (>7.4-fold) transfectants by the expression of core, when compared with control cells. The inhibition of core-induced activation of VEGF promoter by SP600125, SB-203580, or PX-478 (Fig. 7C, D) indicates the involvement of core-induced activation of AP-1 and HIF-1α in the regulation of core-induced production of VEGF in liver cells.
Discussion
The occurrence of hepatic angiogenesis has been described in liver conditions including viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, cirrhosis, and hepatocellular carcinoma.5, 6 Although several studies have been carried out in the field of angiogenesis, the authenticity of the molecular mechanisms leading to the development of hepatic angiogenesis in HCV-infected patients is currently unknown.
We demonstrated that the development of hepatic neoangiogenesis in HCV-infected patients is associated with TGF-β2 and VEGF production and CD34 expression, a marker for hepatic angiogenesis.40 In addition, we specified the mechanistic role of HCV core in the course of the hepatic angiogenesis, during HCV infection.
Angiogenesis is implicated in cancer development by favoring progression, growth, and metastasis of cancer41, 42 and is regulated by growth factors, including transforming growth factor-beta (TGF-β), basic fibroblast growth factor, or platelet-derived growth factor in tumor cells.43-45
In this study, we demonstrated the expression of TGF-β2, VEGF, and CD34 protein in liver biopsy specimens of HCV-infected patients. Thus, in agreement with several reports,6, 46 our data provide evidence for the induction of hepatic angiogenesis during HCV and suggest that the occurrence of hepatic angiogenesis may be an essential step in the earlier phases of HCV-related oncogenesis.
However, the strategy whereby HCV chronic infections cause hepatocellular carcinoma seems to be different from that of chronic hepatitis B virus. HCV is a positive-strand RNA virus that seems incapable of integration into the host's genome; in addition, it does not seem to carry oncogenesis in its genome, suggesting that HCV causes HCC through an indirect pathway by causing chronic inflammation, cell death, proliferation, and cirrhosis. Accordingly, HCV-related HCCs have been reported almost exclusively in patients with cirrhosis. Conversely, the development of HCCs without cirrhosis in HCV-infected patients has been reported.47 Thus, the involvement of other pathways in the modulation of HCV-induced HCC is possible. In agreement with other reports,48 our findings suggest that induction of hepatic angiogenesis during chronic HCV infection may contribute to the early development of cancer cells and may explain the high incidence of HCC in HCV-infected patients without cirrhosis.
TGF-β2 is one of three mammalian TGF-β genes that are involved in the regulation of cell growth and differentiation.49 In addition, TGF-β2 has been shown to play an essential role in the development of tumors by regulation of key mechanisms including immunosuppression,50-52 metastasis,52 angiogenesis,51 and cell growth and differentiation46 and is thought to function as an important mediator in the development of liver fibroses.53 The expression of TGF-βs has been reported, in liver tissues of patients infected with HCV,19 and we confirmed this also in the current study. Thus, the contribution of TGF-β2 in the modulation of HCV-associated hepatic angiogenesis is considered. However, the detection of VEGF expression, the target gene of TGF-β2,54 in the liver of patients infected with HCV, supports further the role of TGF-β2 as mediator in the regulation of HCV-induced hepatic angiogenesis.
VEGF is one of the first isolated angiogenic factors, and is the most well studied factor. It has a specific angiogenic effect on endothelial cells,55 and its stimulation by HCV infection through a mechanism including the stabilization of HIF-1α has been recently reported.55
Besides their major role in the regulation of a diverse array of cellular functions, including cell growth56 and apoptosis,57 the E2F1 can also regulate upstream signal transduction pathways such as the AKT pathway.57, 58 The activation of the p38 pathway by E2F156 provides another example for the effect of E2F1 on signal transduction pathways. Further analysis of E2F1-mediated effects on p38 demonstrated that the activation of the p38 pathway is mediated by E2F1-induced ASK1 transcription.59
In the current study, we addressed the mechanistic role of HCV core protein in the regulation of hepatic angiogenesis during the course of HCV infection and demonstrated its ability to trigger the production of both TGF-β2 and VEGF proteins by multiple pathways including PKC, RB/E2F1, ASK1-JNK/p38, and ERK.
In conclusion, our findings may help to elucidate the mechanistic role of chronic HCV infection in the development of HCC in patients without cirrhosis and may form the basis for future novel therapeutic strategies for HCV-related HCC. 8
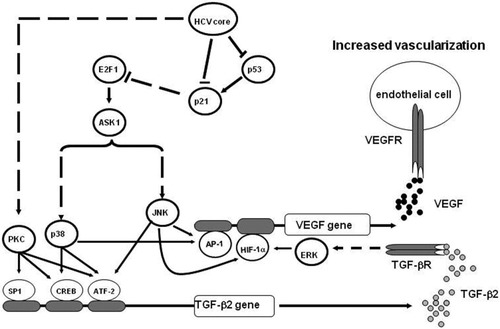
Model showing the possible contribution of HCV core protein in the development of hepatic angiogenesis, in patients with chronic HCV infection. The interference of HCV core protein with intracellular signalling pathways in liver cells results in the production of TGF-β2, which subsequently triggers the production of VEGF. Quiescent endothelial cells become sensitive to VEGF (or other angiogenic factors), proliferate, and migrate to form new vessels.
Acknowledgements
The authors thank Dr. Takaji Wakita, National Institute of Infectious Diseases, Tokyo, Japan, for providing the JFH1 replicon construct. We thank Dr. Sandeep Nambiar, Department of Dermatology, University Hospital of Duesseldorf, for his technical assistance.




