Bile salt–induced pro-oxidant liver damage promotes transplanted cell proliferation for correcting Wilson disease in the Long-Evans Cinnamon rat model†
Potential conflict of interest: Nothing to report.
Abstract
Insights into disease-specific mechanisms for liver repopulation are needed for cell therapy. To understand the efficacy of pro-oxidant hepatic perturbations in Wilson disease, we studied Long-Evans Cinnamon (LEC) rats with copper toxicosis under several conditions. Hepatocytes from healthy Long-Evans Agouti (LEA) rats were transplanted intrasplenically into the liver. A cure was defined as lowering of copper to below 250 μg/g liver, presence of ATPase, Cu++ transporting, beta polypeptide (atp7b) messenger RNA (mRNA) in the liver and improvement in liver histology. Treatment of animals with the hydrophobic bile salt, cholic acid, or liver radiation before cell transplantation produced cure rates of 14% and 33%, respectively; whereas liver radiation plus partial hepatectomy followed by cell transplantation proved more effective, with cure in 55%, P < 0.01; and liver radiation plus cholic acid followed by cell transplantation was most effective, with cure in 75%, P < 0.001. As a group, cell therapy cures in rats preconditioned with liver radiation plus cholic acid resulted in less hepatic copper, indicating greater extent of liver repopulation. We observed increased hepatic catalase and superoxide dismutase activities in LEC rats, suggesting chronic oxidative stress. After liver radiation or cholic acid, hepatic lipid peroxidation levels increased, indicating further oxidative injury, although we did not observe overt additional cytotoxicity. This contrasted with healthy animals in which liver radiation and cholic acid produced hepatic steatosis and loss of injured hepatocytes. We concluded that pro-oxidant perturbations were uniquely effective for cell therapy in Wilson disease because of the nature of preexisting hepatic damage. (HEPATOLOGY 2009.)
The potential of liver cell therapy has been studied in several animal models, although new mechanisms are required for achieving therapeutic levels of liver repopulation.1 Recent studies in rat and mouse models demonstrated that coupling of hepatic genotoxic damage and oxidative stress in the native liver benefited liver repopulation with cells.2-6 Similarly, hepatotoxicity induced by toxic transgenes or other genetic mechanisms has been effective in liver repopulation with cells.7, 8 Nonetheless, the responses of healthy and diseased native liver cells to conditioning regimens may be different, which may alter cell therapy outcomes.9, 10 Therefore, insights into mechanisms of transplanted cell proliferation under disease-specific conditions are needed.
Wilson's disease (WD) constitutes an appropriate condition for study and is amenable to cell and gene therapy.4, 9-13 This condition arises from mutations in the copper-transporting gene, ATP7B, with retention of copper in the liver, brain, and other organs.11 Excellent rat and mouse models of WD have been developed.14, 15 In the Long-Evans Cinnamon (LEC) rat, cell transplantation after hepatic preconditioning with DNA the adduct-forming alkaloid, retrorsine, produced liver repopulation with healthy hepatocytes,4 whereas without preconditioning, liver was either not repopulated or required much longer.9 Similarly, hepatic preconditioning with radiation (RT) and ischemia-reperfusion proved less effective in LEC rats.10
To develop further insights in mechanisms of hepatic conditioning for cell therapy in WD, we addressed the potential of hydrophobic bile salts, which produce liver injury in several ways, including oxidative stress.16-19 We were especially interested in defining whether hydrophobic bile salts will synergize with other forms of oxidative stress and promote proliferation of healthy hepatocytes in the liver. Here, we report cell transplantation studies in the LEC rat to define this conditioning mechanism in the context of WD.
Abbreviations
atp7b, Cu++ transporting, beta polypeptide; LEA, Long-Evans Agouti rat; LEC, Long-Evans Cinnamon rat; MAPK, mitogen-activated protein kinase; mRNA, messenger RNA; PH, partial hepatectomy; RT, radiation; RT-PCR, reverse transcription polymerase chain reaction; SOD, superoxide dismutase; WD, Wilson disease.
Materials and Methods
Animals.
LEC rats, syngeneic healthy Long-Evans Agouti (LEA) rats, and F344 rats were bred in the Special Animal Core of Marion Bessin Liver Research Center. Rats studied were 8 to 10 weeks old and were housed in 14-hour light and 10-hour dark cycles with unlimited access to water and chow containing 11.8 mg copper/kg (Ralston Purina, St. Louis, MO). Some rats were given chow with 0.1% cholic acid (Test Diets, Harlan Teklad, Madison, WI).
Animals were anesthetized with inhaled ethyl ether or ketamine and xylazine. The Animal Care and Use Committee at Albert Einstein College of Medicine approved animal protocols according to National Institutes of Health guidelines.
Donor Hepatocytes and Cell Transplantation.
Hepatocytes were isolated from LEA donor rats by two-step collagenase perfusion. Cell viability was analyzed by trypan blue dye exclusion. For transplantation, 1 × 107 viable cells were suspended in 0.5 mL Roswell Park Memorial Institute 1640 medium and injected over 10 to 15 seconds in the splenic pulp within 2 hours after isolation. Hemostasis was secured with ligature around injection site at the lower pole of the spleen.
Liver RT.
Anterior liver lobes were radiated to 50 Gy with an orthovoltage X-ray unit after shielding adjacent abdominal organs with lead plates as described previously.3, 5, 10
Two-Thirds Partial Hepatectomy.
Anterior liver lobes were removed according to the Higgins and Anderson method described previously.20
Serum Ceruloplasmin.
Total oxidase activity was measured with 1 mg/mL dimethoxybenzidine dihydrochloride (o-dianisidine; all chemicals were from Sigma Chemical Co., St. Louis, MO) in 0.1 M sodium acetate buffer, pH 5.6. To 10 μL serum was added 20 μg o-dianisidine with sodium acetate buffer to 100 μL total volume. For negative controls, 0.1 mg sodium azide was added to an aliquot of each sample. After adding 100 μL of 9 M sulfuric acid, reactions were continued for 90 minutes at 37°C. Absorbance at 540 nm was compared with corresponding negative controls. Standard curves were obtained with ceruloplasmin, and oxidase activity was converted to ceruloplasmin in milligram per deciliter.
Liver and Bile Copper.
Liver samples were desiccated at 65°C under vacuum for 12 hours. Bile was collected by cannulating bile duct with P20 tubing at intervals before and after intrasplenic injection of copper-histidine as described previously.21 Bile and liver samples were stored at −20°C. Tissues were solubilized in nitric acid, and tissue and bile copper were measured by graphite furnace atomic absorption spectroscopy.
Reverse Transcription Polymerase Chain Reaction for atp7b mRNA.
RNA was extracted from frozen samples with Trizol Reagent (Life Technologies, Grand Island, NY). Atp7b and beta-actin mRNAs were co-amplified by reverse transcription polymerase chain reaction (RT-PCR) with a commercial kit (Access RT-PCR, Promega Corp., Madison, WI). PCR primers and conditions were as described.4 PCR products were resolved in 1.8% agarose containing ethidium bromide. Expected PCR products for atp7b and beta-actin were 380 and 200 base pairs, respectively.
Total Hepatic Glutathione.
Liver samples were homogenized in 5% sulfosalicylic acid (100 mg tissue per milliliter). Cell debris was removed by centrifugation at 10,000g for 5 minutes at 4°C. Glutathione assay was performed as described previously.22 Absorbance of reaction product was measured at 412 nm over 2 minutes, and data were plotted against curves using glutathione standards. All analyses were in triplicate. Total protein concentration in aliquots was measured by Bradford assay.
Hepatic Catalase.
Liver was homogenized in phosphate buffer (200 mg tissue per milliliter of 260 mM monobasic potassium phosphate and 40 mM dibasic sodium phosphate dehydrate). Cell debris was removed by centrifugation at 10,000g for 10 minutes at 4°C. Catalase activity was measured as described previously.22 Time (T) for change in optical density from 0.45 to 0.40 at 240 nm was measured at room temperature. Catalase activity was estimated from the formula 17/T = units/assay mixture. Protein content was determined in aliquots by Bradford assay. All assays were performed in triplicate.
Hepatic Superoxide Dismutase.
Cu/Zn−, Mn−, and Fe-superoxide dismutase (SOD) was measured with a commercial assay (kit 706002; Cayman Chemical Company, Ann Arbor, MI). Tissue was homogenized [100 mg per milliliter 20 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 1 mM ethylene glycol tetra-acetic acid, 210 mM mannitol, and 70 mM sucrose, pH 7.2]. Cell debris was pelleted by centrifugation at 1500g for 5 minutes at 4°C. SOD was assayed at room temperature according to the manufacturer's instructions. Absorbance of reaction product was measured at 450 nm and results plotted against SOD standards provided.
Malondialdehyde Content.
The assay measured thiobarbituric acid reactive substances (kit 10009055; Cayman). Livers were ultrasonicated at 40V for 15 seconds (100 mg liver per milliliter of radio immunoprecipitation assay buffer: 50 mM Tris HCl, 0.25% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, 1 mM ethylenediaminetetra-acetic acid, 1 mM phenylmethylsulphonyl fluoride, 1 mM sodium vanadate, 1 mM NaF, and 1 μg/mL each of aprotinin, leupeptin, and pepstatin). Cell debris was removed by centrifugation at under 1600g for 10 minutes at 4°C. Sample optical density (OD) was measured at 530 nm, and the concentration of malondialdehyde-thiobarbituric acid adducts was obtained from standard curves.
Histological Analysis.
Liver samples were fixed in 10% buffered formalin, embedded in paraffin, and sections were stained with hematoxylin-eosin. Macrovesicular and microvesicular steatosis, polyploidy, apoptosis, and mitosis were graded as described previously.23 The maximal possible score with advanced liver damage was 13, and the minimal possible score in healthy livers was 2.
Statistical Analysis.
Data are shown as means ± SD or as median and range, according to parametric or nonparametric distributions, respectively. Significances were analyzed by chi-squared test with Yates correction, Student t test, and the Kruskall-Wallis analysis of variance (ANOVA) with Dunn's test for multiple pairwise comparisons of group ranks (SigmaStat 3.1; Systat Software Inc., Point Richmond, CA). P < 0.05 was considered significant.
Experimental Design.
We established groups of LEC rats, including those without perturbations (group I), and rats subjected to manipulations (groups II-V) (Fig. 1A). In group II, rats received hepatic RT alone followed 2 weeks later by cell transplantation. In group III, rats received hepatic RT plus two-third PH 2 weeks before cell transplantation. In group IV, rats received 1% cholic acid for 3 weeks with cell transplantation 2 weeks after commencing the diet. In group V, rats received hepatic RT and 1% cholic acid before cell transplantation. As indicated in the study time-line (Fig. 1B), outcomes were analyzed after 6 months.
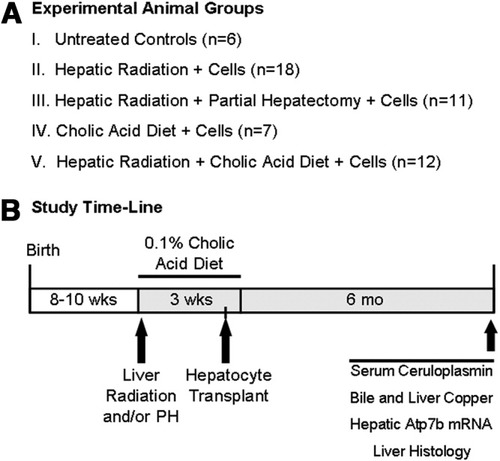
Experimental design. (A) Animal groups, including LEC rats subjected to no manipulation (group I). To determine efficacy of cell therapy, rats in groups II-V were subjected to preconditioning as indicated. All groups contained at least six rats for final analysis. (B) Timeline to indicate that animals were 8 to 10 weeks old at the start of studies. Hepatic preconditioning included radiation (RT) and partial hepatectomy (PH) in the same session followed by cholate-containing diet and cell transplantation via spleen. Various analyses were 6 months after cell transplantation.
A cure was defined as detectable hepatic atp7b mRNA, copper content less than 250 μg/g dry liver weight, and improved liver histology. Additional groups of LEC rats and F344 rats were administered cholic acid or hepatic RT without cell transplantation to determine the effects of these manipulations. These animals were grouped as follows: untreated rats as controls; rats given RT alone; rats given 1% cholic acid diet for 3 weeks; and rats given RT and then cholic acid diet for 3 weeks. Animals were sacrificed 1 week after treatments for tissue analysis. Healthy LEA rats and F344 rats served as controls for assays.
The difference in the numbers of animals in various groups was attributable to variable availability of LEC rats. It should be noted that LEC rats are relatively difficult to breed. More animals were included in some groups to ensure availability of sufficient numbers for statistical comparisons. Also, we were restricted to RT-PCR for atp7b as an indicator of liver repopulation in LEC rats because reliable antibodies to rat atp7b protein are not available. Detection of atp7b mRNA by our assay required repopulation of greater than 10% of the LEC rat liver with healthy transplanted cells.4
Results
In group I, without any manipulations, LEC rats showed significant liver injury with serum ceruloplasmin of 0 to 5 mg/dL, undetectable atp7b mRNA, mean hepatic copper of 958 ± 168 μg/g, absence of bile copper excretion, and abnormal liver histology with extensive cholangiofibrosis, hepatic polyploidy, and interspersed mitotic activity or apoptosis. This agreed with anticipated liver injury in the setting of copper toxicosis caused by WD.
Cell Transplantation in LEC Rats After Hepatic Conditioning Improved Outcomes.
We examined outcomes 6 months after cell transplantation, because this time-frame was appropriate for analyzing liver repopulation in LEC rats treated with hepatic RT in previous studies.10
In groups II through V, cell therapy in several animals with hepatic preconditioning met our criteria for cure. After cell transplantation with hepatic RT alone in group II, 6 of 18 rats (33%) were cured, whereas in group III after preconditioning with RT plus partial hepatectomy (PH) and cell transplantation, six of 11 rats (55%) were cured (Fig. 2). In group IV, in which cell transplantation followed hepatic conditioning with cholic acid alone, only one of seven rats (14%) was cured. Finally, in group V, in which RT plus cholic acid constituted hepatic preconditioning, nine of 12 (75%) rats were cured, which was most effective, P < 0.05, analysis of variance—Dunn's test.
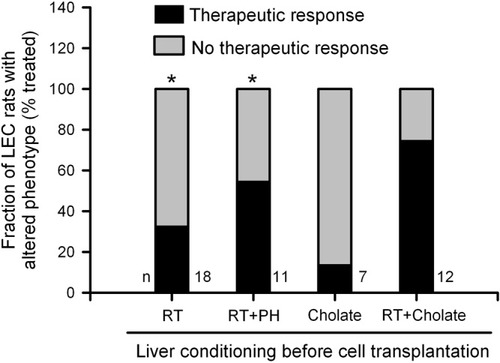
Cumulative therapeutic responses in LEC rats 6 months after cell therapy. In comparison with group I animals, therapeutic responses improved significantly in groups II, IV, and V with RT alone, RT plus PH, and RT plus cholic acid (asterisks indicate P < 0.05, analysis of variance with Dunn's test versus group I). Statistical comparison of therapeutic outcomes across groups II, IV, and V did not show significant differences, because sample sizes proved relatively small for this analysis.
WD-Specific Parameters Improved After Successful Cell Therapy.
Grouped data analysis indicated that LEC rats meeting our criteria for cure showed significant improvements (Table 1). Again, these parameters improved most in group V animals treated with RT plus cholic acid.
| Animal Groups | n | Serum Ceruloplasmin (mg/dL) | Hepatic Copper (μg/g) | Atp7b mRNA + | Basal Bile Copper (ng/min) | Stimulated Bile Copper (ng/min) |
|---|---|---|---|---|---|---|
| LEC group II RT alone + cells | 6/12 | 7* (5-11) | 148* (43-242) | 6 | 3 (2-5) | 18 (9-35)* |
| LEC group III RT + PH + cells | 6/11 | 13* (4-19) | 155* (90-216) | 6 | 3 (2-4) | 70 (21-115)* |
| LEC group IV Cholic acid + cells | 1/7 | 4 | 170 | 1 | 4 | 12 |
| LEC group V RT + cholic acid + cells | 9/12 | 22* (16-36) | 132* (45-190) | 9 | 4 (2-8) | 102 (48-175)* |
| Healthy LEA rats | 6 | 31* (25-41) | 25* (18-34) | 6 | 13 (10-21)* | 61 (28-104)* |
- Data are shown as median (range).
- * P < 0.05 versus group I, untreated LEC rats.
RT-PCR for hepatic atp7b mRNA as well as liver histology showing improvements were in agreement with other findings (Fig. 3). In particular, consistent with appearance of atp7b mRNA and decrease in hepatic copper content, liver histology improved after cell therapy in LEC rats with therapeutic cure. By contrast, untreated LEC rats in group I or LEC rats without therapeutic responses across various groups showed extensive liver injury (Fig. 3B-G).
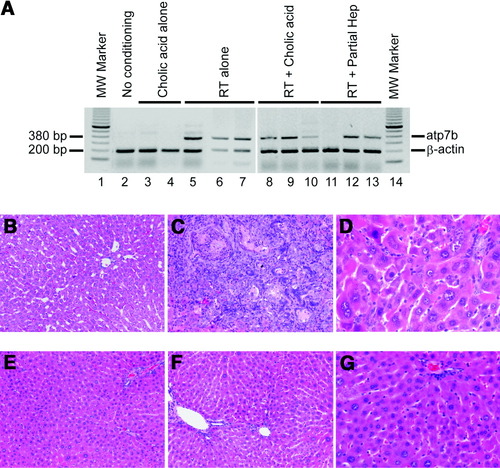
Changes after cell therapy. (A) Hepatic atp7b mRNA expression. Lanes 1 and 14, molecular size marker; lane 2, LEC rat without any conditioning or cell transplantation showing no atp7b mRNA; lanes 3, 4, two LEC rats conditioned with cholic acid alone showing only beta-actin band; lanes 5-7, three LEC rats conditioned with RT alone, showing atp7b mRNA in all samples; lanes 8-10, LEC rats conditioned with RT plus cholic acid showing atp7b mRNA expression in all samples; and lanes 11-13, LEC rats conditioned with RT and PH showing atp7b mRNA in two of three samples. (B-G) Liver histology. (B) Healthy liver from donor LEA rat. (C and D) Untreated LEC rat with extensive cholangiofibrosis in C and hepatic polyploidy in D. (E-G) LEC rats 6 months after cell transplantation with RT plus PH (E) or RT plus cholic acid (F, G), where liver morphology was normal. (Original magnifications: A, B, D, E, ×100; C, F, ×400, hematoxylin-eosin stain.
The contrast in histology grading between untreated LEC rats in group I and animals in group V meeting the definition of cure after preconditioning with RT plus cholic acid is given in Table 2.
Differences in Pro-oxidant Hepatic Conditioning Mechanisms in Healthy Rats and LEC Rats.
We considered that intergroup and even intragroup responses to cell therapy were attributable to differences in transplanted cell engraftment or proliferation under the experimental conditions, arising from perturbations in the hepatic microenvironment. It should be noteworthy that in healthy F344 rats subjected to cell transplantation after RT-based preconditioning, animal-to-animal variability in proliferation of transplanted cells was infrequent or not encountered.5 Therefore, we compared changes in F344 rats and LEC rats.
In the first instance, we examined responses of healthy F344 rats and LEC rats to conditioning with RT or cholic acid 4 weeks after these treatments without cell transplantation. Liver histology in untreated healthy F344 rats was normal, whereas LEC rats showed significant baseline morphological abnormalities, including hepatic polyploidy and nuclear changes, as before (Fig. 4). In F344 rats, morphological changes after RT or cholic acid alone were restricted to enlargement of hepatocyte nuclei. After RT plus cholic acid, F344 rats showed further alterations with fatty change and losses of hepatocytes, especially in perivenous areas. By contrast, in LEC rats, no additional morphological changes were observed after RT, cholic acid, or RT plus cholic acid, although occasional apoptotic hepatocytes were present.
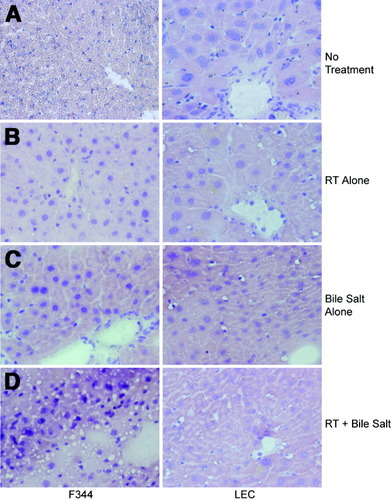
Effects of treatments on liver morphology in healthy rats and LEC rats. Shown are livers 4 weeks after manipulations in healthy rats (left) and LEC rats (right). (A) Without conditioning, LEC rats showed hepatic polyploidy and enlarged hepatocyte nuclei compared with healthy rat. (B) After RT alone, hepatocyte nuclei enlarged in healthy animals although less than in LEC rats. (C) Rats treated with cholic acid alone showing enlarged hepatocyte nuclei in healthy rats. Occasional hepatocytes in LEC rats were apoptotic. (D) Animals treated with RT plus cholic acid with steatosis, nuclear abnormalities, and areas of cell dropout in healthy rats, whereas LEC rat liver was unchanged. (Original magnification ×400, hematoxylin-eosin stain.
Next, we examined oxidative stress by measuring hepatic catalase, total glutathione, SOD, and malondialdehyde. Typically, depletion of catalase, glutathione, or SOD indicates exposure of cells to acute oxidative stress, whereas increased malondialdehyde content represents lipid peroxidation attributable to acute or chronic oxidative stress.22 In comparison with healthy F344 rat livers, catalase and SOD activities increased in LEC rat livers by an average of threefold and 3.8-fold, respectively, P < 0.001, t tests; whereas total glutathione activity was 2.8-fold less, P < 0.001, t test; and malondialdehyde content was unchanged, P = NS (Fig. 5). This suggested significant differences in oxidative stress in the two situations.
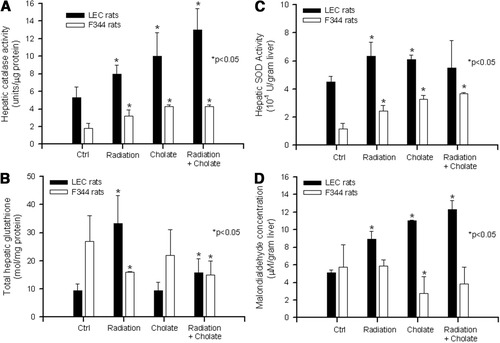
Biochemical assays of hepatic catalase, SOD, total glutathione, and malondialdehyde content. Data are from animals in Fig. 4, with analysis of multiple samples from each liver. Shown are control rats with no liver conditioning and rats treated with RT alone, cholic acid alone, and RT plus cholic acid. All assays were in triplicate and were repeated twice. Asterisks indicate P < 0.05, t tests, compared with controls in the first set of grouped bars.
Also, after exposure to RT, cholic acid, or RT plus cholic acid, we observed increases in hepatic catalase and SOD activities in F344 rats, as well as LEC rats, suggesting an appropriate directionality of the biochemical change. By contrast, total hepatic glutathione content decreased in F344 rats in response to all these treatments, whereas in LEC rats, exposure to RT increased hepatic glutathione levels, although cholic acid did not change hepatic glutathione levels. Finally, we observed divergent responses with respect to hepatic malondialdehyde levels, which increased in LEC rats after RT, cholic acid, or RT plus cholic acid, whereas malondialdehyde levels declined in all conditions in F344 rats.
Discussion
These studies demonstrated significant differences in transplanted cell proliferation and liver repopulation in healthy liver versus liver with copper toxicosis, oxidative injury, and other perturbations. The preconditioning regimens in our studies did not produce mortalities attributable to hepatic changes. Similarly, presence of acute or chronic liver damage does not prevent engraftment of transplanted cells in the liver, as indicated by cell transplantation studies in animals with toxin-induced acute liver failure or chronic liver fibrosis.24, 25 However, because preconditioning regimens exerted different effects on the kinetics of transplanted cell proliferation in normal animals and animals with preexisting liver damage, it should be appropriate to emphasize the role of disease-specific mechanisms in optimizing hepatic preconditioning for liver repopulation with cells. This consideration was particularly applicable to cell therapy after induction of additional oxidative stress in WD, in which RT and cholic acid proved most effective, RT alone was less effective, and cholic acid alone was least effective in producing therapeutic liver repopulation. Overall, these findings should be helpful in guiding clinical strategies for liver-directed cell therapies in WD and other relevant disorders.
Previous studies established that RT by itself was insufficient for promoting transplanted cell proliferation in the healthy liver, although in combination with other perturbations, such as PH, ischemia–reperfusion, hormone-induced or carbon tetrachloride–induced hepatotoxicity, RT produced extensive liver repopulation in rats or mice over approximately 3 months.3, 5, 26 These manipulations share the common threads of hepatic oxidative stress and DNA damage with adverse manifestations, including mechanisms of cell ploidy, cell cycling, and cell death.27 In previous studies, PH was shown to produce oxidative stress in the liver, which resulted in decreased capacity for hepatocellular proliferation under growth factor stimulation conditions in vitro, as well as during liver repopulation conditions in vivo to explain how PH would synergize with RT and create favorable conditions for liver repopulation.20, 22 Conversely, in LEC rats with significant ongoing hepatic oxidative stress, RT by itself was beneficial for proliferation of transplanted cells, and induction of additional oxidative stress through RT plus cholic acid exerted further synergistic benefits on proliferation of transplanted cells. This synergistic effect of RT and the hydrophobic bile salt, cholic acid, was in agreement with previous studies of bile salt–induced hepatotoxicity, for example, short-term infusion of hydrophobic bile salt in rats, which produced acute perivenous liver injury,16 as well as addition to the cell culture medium of cultured hepatocytes, establishing induction of apoptotic cell death through subcellular mechanisms, such as endoplasmic reticulum stress-mediated or ceramide and protein kinase–dependent pathways.17-19 By contrast, hepatic preconditioning with RT-based strategies was less effective in LEC rats with copper toxicosis compared with healthy rats because fewer animals demonstrated successful correction of WD phenotype,10 whereas near-total liver repopulation was produced in the latter within 3 months after hepatic conditioning with either RT plus PH or RT plus ischemia–reperfusion.3, 5
Accumulation of copper produces multiple hepatic changes, including oxidative stress and lipid peroxidation, as demonstrated here, and previously,28 with likely impact on mechanisms regulating hepatic preconditioning through further oxidative stress, DNA damage, and other injuries. For instance, we observed different manifestations of RT and cholic acid–induced liver injury in healthy animals, whereas fatty change and necrosis were evident, whereas these changes were absent in LEC rats even though hepatic metabolism of lipids was abnormally regulated in these animals.29 However, over the long term, exposure of LEC rat hepatocytes to RT and cholic acid did produce advantages for transplanted healthy cells. At present, we do not know whether administration of cholic acid over longer durations would have offered further advantages to transplanted hepatocytes. In previous studies, after transplantation of healthy hepatocytes in mice with impaired biliary phospholipid excretion attributable to P-glycoprotein deficiency, which causes accumulation of toxic bile salts and hepatocellular injury, continued administration of toxic bile salts over several months produced significant liver repopulation without clearance of transplanted cells,30 suggesting that longer use of cholic acid should be effective.
Other reasons for slower kinetics of liver repopulation in LEC rats should include direct effects of copper on transplanted cell proliferation, including through increased intracellular shunting of copper during its removal, as well as oxidation of extracellular matrix components, which was found to perturb survival of cultured hepatocytes with altered outside-in cell signaling involving nuclear factor kappa B (NF-κB) and other transcriptional factors.31
The intracellular nature of signals that might regulate hepatic conditioning in the setting of chronic oxidative stress, such as in WD, are unknown and are likely to be highly complex. For instance, we could speculate that hepatic preconditioning in the setting of copper toxicosis may benefit from the activation of inflammatory cytokines through Kupffer cell–mediated responses,32 although substantiation of this mechanism will require further studies. Similarly, onset of oxidative stress in the liver activated changes, such as increased hepatic expression of gamma-glutamyltranspeptidase, which was associated with greater survival of cells, including resistance to further oxidative stress.33 How intracellular events may be altered during chronic oxidative stress is under active investigation with consideration of cell signaling mechanisms involving mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase 1/2, c-Jun N-terminal kinase and p38 MAPK, regulation of nuclear factor kappa B, or other processes capable of regulating cell cycling or cell death.34 Dissecting intracellular signaling pathways in the context of chronic copper toxicosis will likely be complex because of the possibility of redundancies and opposing subcellular responses in cellular pathways. Nonetheless, in short-term cell culture studies, copper has been demonstrated to increase expression of activating protein 1, cell cycle–regulated genes, such as c-Fos, c-Jun, and c-Myc, as well as c-Jun N-terminal kinase and p38 MAPK, within the settings of oxidative stress.35, 36 Also, oxidative preconditioning was found to protect against further metal toxicity, again through possible involvement of MAPK pathways,37 although development of these insights in the context of WD or other disorders will require studies in appropriate models, such as LEC rats. Such studies should help establish applications of differences in the responses to preconditioning regimens for cell therapy under disease-specific situations.
Acknowledgements
The authors thank Alan F. Hofmann for valuable discussions.




