Hepatic Niemann-Pick C1–like 1: The canalicular side of the coin†
Potential conflict of interest: Nothing to report.
Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, et al. Hepatic Niemann-Pick C1–like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest 2007;117:1968–1978. (Reprinted with permission.)
Abstract
Niemann-Pick C1-like 1 (NPC1L1) is required for cholesterol absorption. Intestinal NPC1L1 appears to be a target of ezetimibe, a cholesterol absorption inhibitor that effectively lowers plasma LDL-cholesterol in humans. However, human liver also expresses NPC1L1. Hepatic function of NPC1L1 was previously unknown, but we recently discovered that NPC1L1 localizes to the canalicular membrane of primate hepatocytes and that NPC1L1 facilitates cholesterol uptake in hepatoma cells. Based upon these findings, we hypothesized that hepatic NPC1L1 allows the retention of biliary cholesterol by hepatocytes and that ezetimibe disrupts hepatic function of NPC1L1. To test this hypothesis, transgenic mice expressing human NPC1L1 in hepatocytes (L1-Tg mice) were created. Hepatic overexpression of NPC1L1 resulted in a 10- to 20-fold decrease in biliary cholesterol concentration, but not phospholipid and bile acid concentrations. This decrease was associated with a 30%–60% increase in plasma cholesterol, mainly because of the accumulation of apoE-rich HDL. Biliary and plasma cholesterol concentrations in these animals were virtually returned to normal with ezetimibe treatment. These findings suggest that in humans, ezetimibe may reduce plasma cholesterol by inhibiting NPC1L1 function in both intestine and liver, and hepatic NPC1L1 may have evolved to protect the body from excessive biliary loss of cholesterol.
Comments
Systemic cholesterol homeostasis is maintained by balancing endogenous synthesis and dietary intake with fecal disposal and hepatic conversion into bile acids. Biliary secretion of cholesterol, either unmodified or after catabolism into bile acids, is the primary route for cholesterol elimination from the body. Given the high cardiovascular risk associated with elevated serum cholesterol levels, several laboratories are focusing on the metabolic programs that regulate cholesterol acquisition and removal from the body. Cholesterol is a highly insoluble molecule in a watery environment. Within the biliary tract and the intestinal lumen, the sterol is carried by mixed micelles and vesicles, which are composed of different proportions of bile acids and phospholipids, mainly phosphatidylcholine.1 Multiple energy-dependent transporters localized at the canalicular membrane of the hepatocyte, termed ATP-Binding Cassette (ABC) transporters, act in concert to secrete the correct amount of lipids into bile. The bile salt export pump ABC-B112 is responsible for the secretion of bile acids, whereas the multidrug resistance P-glycoprotein ABC-B43 “flips” phosphatidylcholine molecules from the inner to the outer hemileaflet of the hepatocyte canalicular membrane, thus facilitating its biliary extraction. Lastly, the heterodimer ABC-G5/ABC-G84 is responsible for biliary secretion of cholesterol (Fig. 1).
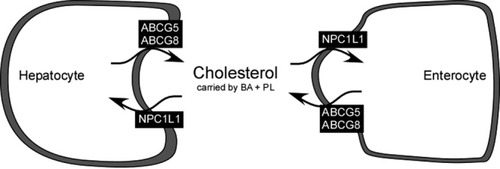
Molecular transporters responsible for biliary secretion and intestinal absorption of cholesterol. The functional heterodimer ABC-G5/ABC-G8 pumps cholesterol from hepatocyte (right panel) into the canalicular lumen and from the enterocyte (left panel) into the intestinal lumen. On the contrary, cholesterol can be reabsorbed in the hepatocyte and enterocyte by NPC1L1. Once in the biliary and intestinal lumen, cholesterol is carried by bile acids (BA) and phospholipids (PL) mostly in mixed micellar structures. Abbreviations: NPC1L1, hepatic Niemann-Pick C1–like 1; ABC, ATP-binding cassette transporters.
Stored in the gallbladder in between meals, bile is mainly released in the intestine following food intake. Bile acid plus phospholipid mixed micelles are essential for proper handling of intestinal cholesterol.5 An increase in the hydrophobicity index of the bile acid pool has been associated with increased efficiency of intestinal cholesterol absorption.6 On the contrary, bile acid depletion by sequestrants, such as cholestyramine, drastically reduces intestinal cholesterol absorption.7 Similarly to the canalicular membrane of the hepatocyte, the intestinal ABC-G5/ABC-G8 heterodimer is responsible for pumping cholesterol in the intestinal lumen, thereby increasing the disposal of fecal cholesterol. The heterodimer ABC-G5/ABC-G8 is localized mainly in the upper part of the small intestine, and it is transcriptionally regulated by the oxysterol liver X receptors (LXRα and LXRβ), which sense increased levels of intracellular sterols and activate a transcriptional program that results in up-regulation of genes, including sterol effluxers.8 Bile acids are absorbed by the energy-dependent intestinal bile acid transporter (IBAT or ASBT [apical sodium-dependent bile acid transporter]) in the distal ileum, after having secured the proper solubilization of cholesterol and other fat-soluble nutrients and vitamins throughout the entire length of the small intestine.
The intestine indeed plays a key role in cholesterol homeostasis. It represents the exclusive site for absorption of dietary and biliary cholesterol, but also the obligate way for definitive disposal of cholesterol in the feces. In addition, recent evidence points to a direct secretory role of the intestine, which would actively participate in the process of fecal cholesterol excretion, even in the absence of ABC-G8.9 The hierarchical supremacy of the intestine is exploited therapeutically by inhibitors of intestinal cholesterol absorption, which represent an effective way to reduce serum cholesterol levels. A few years ago, Niemann-Pick C1–like 1 protein (NPC1L1) was identified as the likely, long-sought intestinal cholesterol acceptor, responsible for its uptake at the brush border of the enterocyte.10 NPC1L1 knockout mice displayed a dramatic reduction in intestinal cholesterol absorption, even when bile acids are dietarily supplemented. It is interesting to note the cholesterol absorption inhibitor ezetimibe was not able to further reduce intestinal cholesterol absorption in knockout mice, suggesting NPC1L1 as the putative therapeutic target.10 However, although the targeting of NPC1L1 by ezetimibe has been confirmed by direct binding assays,11 uncertainty still holds regarding both the exact cellular localization and precise functional role of intestinal NPC1L1. Indeed, using brush border membrane vesicles from NPC1L1 knockout mice, it has been recently demonstrated that NPC1L1 is mostly localized in intracellular membranes and that ezetimibe is also able to reduce cholesterol uptake in the absence of NPC1L1.12
Apart from its intestinal localization, in humans NPC1L1 is significantly expressed in the liver.10, 13 On the contrary, mice do not express NPC1L1 in the liver. Therefore, the data on NPC1L1 regulation as well as pharmacological manipulation via ezetimibe in the murine system are lacking important information for their translational value. Temel et al.14 recently generated transgenic mice with hepatic specific overexpression of NPC1L1, thus offering an exceptional tool to unveil the functional role of NPC1L1 in the liver. By generating a hepatoma cell line with stable expression of human NPC1L1,15 the same group previously suggested a direct role for NPC1L1 in hepatic uptake of cholesterol. They now take a step forward and investigate the role of hepatic NPC1L1 in vivo. First, the authors demonstrate the canalicular localization of the protein in the transgenic mice. They also confirm the translational value of the finding by verifying that in humans NPC1L1 localizes at the canalicular membrane of the hepatocyte. Interestingly, the phenotypic characterization of hepatic NPC1L1 transgenic mice reveals a drastic reduction in biliary cholesterol output, with no concomitant modification of biliary bile acid and phospholipid levels. These results underline the potential role of canalicular NPC1L1 in absorbing cholesterol from the biliary lumen into the hepatocyte. Remarkably, whereas in normal mice increased dietary intake of cholesterol results in augmented biliary cholesterol secretion, this homeostatic mechanism is absent in NPC1L1 transgenic mice. Therefore, when challenged with a high-cholesterol diet, hepatic NPC1L1 transgenic mice are not able to increase biliary cholesterol secretion. On the other hand, they present normal levels of hepatic free and esterified cholesterol, but significantly increased circulating cholesterol levels, probably due to alteration in the process of ABCA1 mediated high-density lipoprotein formation. In principle, a reduction in the expression level of cholesterol transporters ABC-G5/ABC-G8 could also justify the observed reduction of biliary cholesterol secretion. The authors address this issue by demonstrating that the expression of the 2 transporters is not reduced in transgenic mice, but is even slightly increased. Therefore, similar to the process in the intestine, the canalicular scenario is that the ABC-G5/ABC-G8 heterodimer is pumping cholesterol into bile, whereas NPC1L1 is regulating its reabsorption into the hepatocyte (Fig. 1).
In the second part of their study, Temel et al.14 treated the hepatic NPC1L1 transgenic mice with ezetimibe in order to better understand the role of NPC1L1 inhibition in both liver and intestine. As expected, inhibition of NPC1L1 in the liver “rescues” biliary cholesterol secretion without affecting the secretion of bile acids and phospholipids, and lowers plasma cholesterol levels. Indeed, the biliary cholesterol content of hepatic NPC1L1 transgenic mice treated with ezetimibe is similar to that of a normal mouse. The functional identity of hepatic and intestinal NPC1L1, similarly engaged in accepting cholesterol molecules inside the cell, depict a clear physiological role for NPC1L1. By contemporarily modulating biliary cholesterol secretion while enhancing intestinal cholesterol absorption, NPC1L1 effectively prevents excessive fecal elimination of cholesterol. In this respect, we agree with Temel et al. when they say that their findings support the clinical use of ezetimibe for the treatment of hypercholesterolemia, by demonstrating that it successfully eliminates cholesterol by acting both on the liver and the intestine.
This elegant report by Temel et al. clarifies the role of hepatic NPC1L1 function, thus filling an important gap in knowledge of the physiology and pharmacology of cholesterol metabolism. Now we know where NPC1L1 is and what it is doing in the liver. Of course, we now want to know more. Several questions remain to be answered. Given the importance of NPC1L1 for systemic cholesterol metabolism, it is easily predictable that its function is highly regulated. Besides the putative repressive role of LXR and peroxisome proliferator-activated receptor alpha (PPARα) on intestinal NPC1L1 transcription,16, 17 very little is known at the present time on the regulation of NPC1L1 expression and activity. Because NPC1L1 restricts cholesterol disposal both in the liver and in the intestine, it is reasonable to assume that the same sensor might be responsible for NPC1L1 regulation in the 2 anatomical districts. Alternatively, hormonal signals carried in the bloodstream or in bile could synchronize the regulation of NPC1L1 in the gut-liver axis. A last question would be on the long-term effect of blocking cholesterol “absorption” in the human canaliculus via ezetimibe with the subsequent increase in biliary cholesterol concentration. Would ezetimibe increase the cholesterol saturation index in bile, thus predisposing a patient to cholesterol gallstone formation and atherosclerosis of the bile ducts? Studies in this direction are urgently needed.




