Characterization of ionotrophic purinergic receptors in hepatocytes†
Potential conflict of interest: Nothing to report.
Abstract
Ionotrophic purinergic (P2X) receptors function as receptor-gated cation channels, where agonist binding leads to opening of a nonselective cation pore permeable to both Na+ and Ca2+. Based on evidence that extracellular adenosine 5′-triphosphate (ATP) stimulates glucose release from liver, these studies evaluate whether P2X receptors are expressed by hepatocytes and contribute to ATP-dependent calcium signaling and glucose release. Studies were performed in isolated hepatocytes from rats and mice and hepatoma cells from humans and rats. Transcripts and protein for both P2X4 and P2X7 were detectable, and immunohistochemistry of intact liver revealed P2X4 in the basolateral and canalicular domains. In whole cell patch clamp studies, exposure to the P2X4/P2X7 receptor agonist 2′3′-O-(4-benzoyl-benzoyl)-adenosine 5′-triphosphate (BzATP; 10 μM) caused a rapid increase in membrane Na+ conductance. Similarly, with Fluo-3 fluorescence, BzATP induced an increase in intracellular [Ca2+]. P2X4 receptors are likely involved because the calcium response to BzATP was inhibited by Cu2+, and the P2X4 modulators Zn2+ and ivermectin (0.3-3 μM) each increased intracellular [Ca2+]. Exposure to BzATP decreased cellular glycogen content; and P2X4 receptor messenger RNA increased in glycogen-rich liver samples. Conclusion: These studies provide evidence that P2X4 receptors are functionally important in hepatocyte Na+ and Ca2+ transport, are regulated by extracellular ATP and divalent cation concentrations, and may constitute a mechanism for autocrine regulation of hepatic glycogen metabolism. (HEPATOLOGY 2007.)
Hepatocytes exhibit regulated release of adenosine 5′-triphosphate (ATP) into the extracellular space.1 Once outside the cell, these nucleotides function as potent autocrine/paracrine signaling molecules that modulate liver function through activation of purinergic receptors in the plasma membrane. Initially discovered in neurons, purinergic receptors are implicated in the control of a spectrum of essential physiologic mechanisms ranging from vascular tone to programmed cell death. In the liver, nanomolar concentrations of ATP, or its breakdown products, activate Cl− channels, decrease cell volume, and stimulate bile flow.2, 3 Moreover, exposure of perfused isolated livers to ATP stimulates a large release of glucose.4 Collectively, these coordinated pathways of ATP release, receptor binding, and degradation constitute a versatile mechanism for paracrine regulation of liver function.
These diverse effects of ATP are not readily explained by a single receptor type. Previous studies indicate that hepatocytes express several subtypes of G-protein coupled P2Y receptors, including P2Y1, P2Y2, P2Y4, and P2Y6. Selective stimulation of P2Y2 receptors activates plasma membrane chloride channels and stimulates ductular bile secretion.5 P2Y receptors also appear to play a role in the activation of glycogen phosphorylase, the rate-controlling enzyme in hepatic glycogenolysis,6 and regulate multiple signaling pathways with changes in cellular [Ca2+], eicosanoid production, and cyclic adenosine monophosphate levels.7-9 The diversity of responses suggests that additional receptors are involved.
Recently, a molecularly distinct family of ionotrophic P2X receptors has been identified in brain and epithelial tissues. P2X receptors, when activated by ATP, form cation-permeable channels that allow the influx of extracellular Na+ and Ca2+. Molecular cloning has revealed at least seven P2X receptor isoforms, denoted P2X1-P2X7.10 Previous studies have demonstrated that the P2X4 receptor is the dominant P2X isoform expressed by cholangiocytes.11, 12 Consequently, the purpose of these studies was to evaluate whether P2X4 receptors and other family members are expressed by hepatocytes and contribute to the cellular responses to purinergic stimulation, including glucose release.
Abbreviations
ATP, adenosine 5′-triphosphate; BzATP, 2′3′-O-(4-benzoyl-benzoyl)-adenosine 5′-triphosphate; ChREBP, carbohydrate response element binding protein; CM, canalicular membrane; DMEM, Dulbecco's modified Eagle's medium; EBP50, Ezrin-radixin-moesin–binding phosphoprotein-50; EGTA, ethylene glycol tetraacetic acid; F, Fluo-3 fluorescence; Fmax, fluorescence at saturating [Ca2+]i levels; HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; HTC, hepatoma tissue culture; Huh7, human hepatoma cell line; mRNA, messenger RNA; MRP2, multidrug resistance protein 2; ob−/−, obesity spontaneous mutation; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RT-PCR, reverse-transcriptase polymerase chain reaction; SM, sinusoidal membrane; Tris, trishydroxymethylaminomethane.
Materials and Methods
Cell Culture.
Studies were performed in rat (Rattus norvegicus) and mouse (C57BL/J6 JAX, Jackson Laboratory, Bar Harbor, Maine) liver cells and in rat hepatoma tissue culture (HTC) and human Huh7 cells, model hepatoma cell lines that express ion channels and signaling pathways similar to those found in primary rat hepatocytes.13-15 Cells were maintained in HCO3−-containing minimal essential medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine (2 mM), penicillin (100 IU/mL), and streptomycin (100 μg/mL), as previously described.16 Whole liver samples from obesity spontaneous mutation (ob−/−), carbohydrate response element binding protein–deficient (ChREBP−/−), and ob−/− + ChREBP−/− mice were provided by Dr. Kosaku Uyeda of the University of Texas Southwestern Medical Center.
Reagents.
2′3′-O-(4-Benzoyl-benzoyl)-adenosine 5′-triphosphate (BzATP), copper chloride (CuCl2), zinc chloride (ZnCl2), and ivermectin were obtained from Sigma Chemical Company (St Louis, MO). BzATP (0.1-100 μM) was used as a P2X-selective agonist.17, 18 In heterologous expression studies, BzATP causes half-maximal activation of P2X4 and P2X7 receptors at concentrations of 1-10 μM10, 17, 19 and is a weak agonist for other purinergic receptors. For these studies, the threshold for BzATP responses was higher in whole cell patch clamp studies (∼10 μM), and this was likely related to intracellular dialysis with ethylene glycol tetraacetic acid (EGTA)–containing pipette solutions. For P2X4 receptors, Zn2+ potentiates the effects of ATP, Cu2+ blocks the P2X4 pore,20 and ivermectin is a specific pharmacologic modulator.21 For P2X7 receptors, both Zn2+ and Cu2+ block the P2X7 pore, and ivermectin has no effect.22, 23 D-[U-14C]glucose was obtained from Amersham Biosciences (Piscataway, NJ).
Detection of P2X Receptor RNAs by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR).
RNA extraction and RT-PCR were performed with standard techniques. Briefly, total RNA was extracted from purified hepatocytes and whole rat liver preparations by acid/chloroform/phenol extraction24 and reverse-transcribed with Superscript II (Invitrogen) with random hexamer primers. Previously published primer sequences for P2X1-7 were used for nonquantitative RT-PCR (Fig. 1A,B).25 Polymerase chain reaction (PCR) conditions were 94°C (3 minutes), 35 cycles [94°C (45 seconds), 60°C (45 seconds), and 72°C (45 seconds)], and 72°C (7 minutes). Amplification products were separated by gel electrophoresis (1.5% agarose) and visualized with ethidium bromide. In additional studies not shown, PCR products were extracted with a QIAEX II gel extraction kit (Qiagen, Valencia, CA) and sequenced with an automated sequencer to confirm their identities. For quantitative PCR, independent sense and antisense primers were designed from cloned receptor sequences, available in GenBank, for P2X4 and P2X7 receptors. Real-time PCR reactions were performed in a final volume of 10 μL containing complementary DNA from 5 or 20 ng of reverse-transcribed total RNA. Forward and reverse primers (150 nM) and SybrGreen universal PCR mix were added to 384-well plates with the ABI-Prism 7900HT sequence detection system (Applied Biosystems). Reactions were performed in triplicate. Relative messenger RNA (mRNA) levels were calculated by the comparative cycle threshold method. Levels of 18s RNA were used as a control.
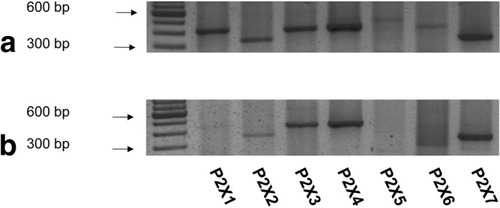
Identification of P2X receptor transcripts. P2X receptor transcripts were assessed in RNA (A) from rat liver and (B) in isolated rat hepatocytes. Comparison with a 100-bp nucleotide ladder was used to determine the approximate size of the various P2X PCR products detected by ethidium bromide staining. Direct sequencing confirmed the results.
Western Blot Analysis.
Cells and tissue homogenates were prepared in 10 mM trishydroxymethylaminomethane (Tris)-Cl (pH 7.8) buffer as previously described.26 Equal amounts of protein (20 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose in a pH 9.9 carbonate buffer.27 Immunodetection was performed with primary antibodies [P2X1, P2X2, P2X4, P2X6, and P2X7 from Alomone Labs (Jerusalem, Israel) and P2X3 from Chemicon International (Temecula, CA)] probed with horseradish peroxidase–conjugated anti–immunoglobulin IgG and developed with enhanced chemiluminescence (Amersham Biosciences). Benchmark protein standards (Gibco, Grand Island, NY) were used for molecular weight estimation.
Localization of P2X4 Receptors.
Sinusoidal and canalicular membrane fractions were isolated from rat livers as previously described.28, 29 The liver samples used were identical to those used in previous studies by McWilliams et al.28 For immunohistochemistry, rat liver sections were permeabilized with 0.1% Tween in phosphate-buffered saline (PBS) and blocked (10% fetal calf serum, 2.5% glycine in PBS) for 30 minutes. Blocked samples were incubated in primary antibody (1:100) for 60 minutes and in Alexa Flour 546 goat anti-rabbit immunoglobulin G (Molecular Probes, Eugene, OR) for 30 minutes, and this was followed by 4′,6-diamidino-2-phenylindole for nuclear staining. Sections were viewed with an inverted Olympus IX70 microscope. The background was set to black with nonimmune serum controls. Images were refined with Deltavision digital deconvolution.
Measurement of Na+ Currents.
Membrane Na+ currents were measured with whole cell patch clamp techniques.30 Cells on a cover slip were mounted in a chamber (volume ∼ 400 μL) and perfused at 4-5 mL/minute with a standard extracellular solution containing 140 mM NaCl, 4 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 1 mM KH2PO4, 10 mM glucose, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)/NaOH (pH ∼ 7.40). The standard intracellular (pipette) solution for whole cell recordings contained 130 mM KCl, 10 mM NaCl, 2 mM MgCl2, 10 mM HEPES/KOH, 0.5 mM CaCl2, and 1 mM EGTA (pH 7.3), corresponding to a free [Ca2+] of ∼100 nM.31 Recordings were made with an Axopatch ID amplifier (Axon Instruments, Foster City, CA) and were analyzed with pCLAMP version 6.0 (Axon Instruments).32, 33 Results are compared with control studies measured on the same day to minimize any effects of day-to-day variability and reported as the current density (pA/pF) to normalize for differences in cell size.16
Measurements of [Ca2+]i.
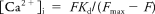
Measurement of Glycogen Content.
The measurement of glycogen using D-14C(U)-glucose was performed as previously described.36 Monolayers of Huh7 cells were incubated at 37°C in high-glucose Dulbecco's modified Eagle's medium (DMEM; 4500 mg/L D-glucose) until they were confluent. The medium was replaced with low-glucose DMEM (1000 mg/L D-glucose) supplemented with labeled glucose (0.1μCi/μmol) to allow incorporation into cellular glycogen. After 24 hours, cells were washed with PBS, and the medium was replaced with low-glucose DMEM. After 15 minutes, 2 μM BzATP was added to experimental plates. After 30 minutes of exposure, cells were washed four times with PBS containing 40 mM unlabeled glucose. Cells were homogenized by sonication in 0.33 N KOH. Fifty microliters of carrier glycogen was added to 200 μL of cell homogenate and brought to 35% KOH. Glycogen was extracted by incubation at 95°C for 30 minutes, which was followed by precipitation with ice-cold 95% ethanol overnight. The precipitate was centrifuged (13,000g, 6 minutes) and washed twice with ethanol. The final precipitate was solubilized in 1 mL of liquid scintillation counter solution (Research Products International, Mount Prospect, IL). Cellular glycogen content was measured with a Beckman (Fullerton, CA) LS1800 liquid scintillation counter.
Statistics.
Results are presented as the mean ± standard error, with n representing the number of culture plates or repetitions for each assay as indicated. A Student paired or unpaired t test was used to assess statistical significance, and P values <0.05 were considered statistically significant.
Results
Hepatocytes Express Multiple P2X Receptor Transcripts.
To evaluate whether P2X receptors are expressed in liver, RNA from whole rat liver and isolated hepatocytes was probed by RT-PCR with primers specific for P2X1-P2X7. In whole liver, which is composed primarily of hepatocytes but also includes multiple additional cell types, transcripts for P2X1, P2X2, P2X3, P2X4, and P2X7 were detected by RT-PCR (Fig. 1A). In isolated hepatocytes, P2X3, P2X4, and P2X7 always produced robust RT-PCR bands (Fig. 1B), whereas P2X1 and P2X2 products were variable and P2X5 and P2X6 were never detected (n = 4). When P2X4 and P2X7 mRNA expression in hepatocytes in comparison to whole liver was quantified by quantitative PCR, normalized P2X4 mRNA transcript levels were essentially unaltered in hepatocytes in comparison to whole liver, but P2X7 transcripts were reduced by ∼50%.
Expression of P2X4 and P2X7 receptor protein in rat hepatocytes was evaluated by western blotting with isoform-specific antibodies, and they were detected at 72 and 42 kDa, respectively (Fig. 2). Rat brain lysates served as a positive control (not shown). The predicted molecular mass of P2X4 is ∼46 kDa, but it is generally detected near 60-70 kDa because of glycosylation.18 P2X4 and P2X7 were also present in mouse hepatocytes and rat HTC cells. P2X4 also was abundant in Huh7 cells, but P2X7 was present only in small amounts (<10% of P2X4 band density). Although P2X3 transcript was detected by RT-PCR, the antibody for P2X3 was uninformative (failure to detect protein in positive controls) over a range of conditions. Collectively, these findings indicate that P2X4 and P2X7 receptors are expressed on hepatocytes as well as other cell types in liver. In additional studies, a full-length P2X4 complementary DNA was cloned from HTC cells. The sequence was identical to the P2X4 receptor originally cloned from rat brain, where heterologous expression revealed an ATP-activated cation-selective channel highly permeable to Ca2+.11
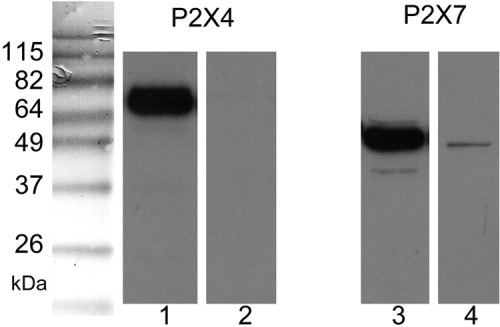
Western blot of P2X4 and P2X7 receptor proteins from isolated rat hepatocytes. Bands of appropriate size representing P2X4 and P2X7 are shown. Equal amounts of protein (10 μg) were loaded in each lane and incubated with either primary antibody (lanes 1 and 3) or the same antibody with corresponding peptide antigens (lanes 2 and 4). Rat brain lysates served as a positive control (not shown).
P2X4 Receptor Distribution.
To determine the cellular location of P2X4 protein, isolated hepatocyte membranes were separated into fractions enriched in sinusoidal versus canalicular domains (Fig. 3). On the basis of the distribution of CE9 as a marker for the sinusoidal membrane and multidrug resistance protein 2 (MRP2) and Ezrin-radixin-moesin–binding phosphoprotein-50 (EBP50) as markers for the canalicular membrane, P2X4 protein was detected in both membranes, but the intensity was greater in the canalicular membrane fraction. These findings were confirmed in intact rat liver sections by the use of immunohistochemical staining with the same P2X4 antibody. P2X4 was observed throughout the liver parenchyma but within hepatocytes was more pronounced in the canalicular domain.
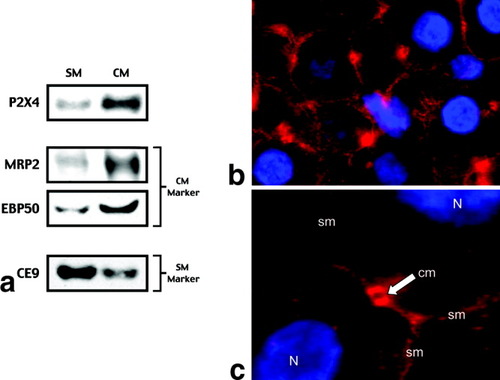
Cellular localization of P2X4. (A) Hepatocyte membranes were separated into sinusoidal membranes (SM) and canalicular membranes (CM), with MRP2 and EBP50 used as markers for CM and CE9 used as a marker for SM (samples in Fig. 7 of McWilliams et al.28 were used). P2X4 protein was detected in both fractions, but the intensity was greater in the canalicular membrane. (B) Low-power and (C) high-power immunohistochemical stains of rat liver sections are shown. Staining demonstrates that P2X4 receptors (red) are present at both the SM and CM. Nuclei (N) are stained blue.
P2X4 Channels.
Among the different P2X receptors detected in hepatocytes, BzATP is a weak agonist for P2X2 and P2X3 but a potent agonist for P2X4 and P2X7.10, 19 In isolated HTC cells, whole cell recordings were performed to evaluate the biophysical response to BzATP. Under basal conditions, the resting current density was −1.3 ± 0.1 pA/pF at a holding potential of −40 mV (n = 20). Exposure to BzATP (10 μM) stimulated an inward current that activated nearly instantaneously, peaked at −26.6 ± 2.5 pA/pF (n = 7, P < 0.01), and then decreased within seconds to a lower, more sustained plateau (Fig. 4). The amplitude of the peak response to BzATP increased to −60.0 ± 10.5 pA/pF (n = 7) in a low divalent cation solution. Furthermore, partial substitution of extracellular Na+ with the impermeable cation Tris+ (final extracellular [Na+], 20 mM) decreased the current response to −8.4 ± 2.5 pA/pF (n = 6, P < 0.01). These biophysical properties are consistent with the opening of a cation permeable pore with properties similar to those of the P2X4 or P2X7 receptor-channel complex.
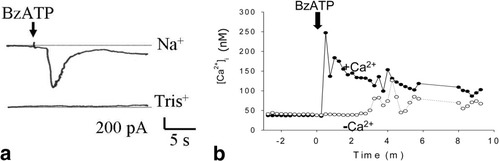
Biophysical properties of endogenous P2X4 as assessed by response to BzATP. (A) Representative whole cell patch clamp recording–activation by BzATP (10 μM) of inward currents (holding potential, −40 mV). Partial substitution of extracellular Na+ with the impermeable cation Tris+ decreased the current response. (B) Representative [Ca2+]i measurements after exposure to BzATP are shown. Exposure to BzATP (100 μM) resulted in a rapid increase of [Ca2+]i. The initial response was inhibited in the absence of extracellular Ca2+ (open circles).
P2X4 Dependent Increases in [Ca2+]i.
P2X receptor pores show significant permeability to Ca2+ ions.12 To assess the potential role of P2X receptors in calcium signaling, the effect of BzATP on [Ca2+]i was assessed in the presence (2 mM) and absence (0 mM, 5 mM EGTA) of extracellular [Ca2+] (Fig. 4B). In the presence of Ca2+, BzATP increased [Ca2+]i with a threshold response near 5 μM and a maximal response near 300 μM. The effects of exposure to BzATP (100 μM) are shown, with a rapid initial increase in [Ca2+]i to 153 ± 17 nM (n = 8) above basal levels, followed by a sustained increase that persisted for up to 10 minutes. In the absence of Ca2+, the response to BzATP was inhibited to 37 ± 1 nM (n = 5, P < 0.01); this suggests that the P2X receptor pore is permeable to Ca2+ and mediates the influx of Ca2+.
To assess the relative contributions of P2X4 and P2X7 receptors, the effects of extracellular cations and ivermectin were assessed. Exposure to Zn2+ (300 μM) increased [Ca2+]iby 216 ± 43 nM (n = 8) in the absence of BzATP. Similar results were obtained with a zinc concentration of 20 μM, which increased intracellular calcium by 137.2 ± 9.6 nM (n = 5). The effects of Zn2+ were eliminated in the absence of extracellular Ca2+, as demonstrated in Fig. 5 (peak increase of 8 ± 5 nM above basal levels, n = 8). To assess whether zinc potentiates the Ca2+ response to endogenous ATP present in the bath as a result of constitutive cellular release, additional studies were performed with apyrase (1 U/mL) to eliminate extracellular ATP. In the presence of apyrase, Zn2+ (300 μM) produced a small, steady decrease in [Ca2+]i (51 ± 3 nM, n = 8, P < 0.001).
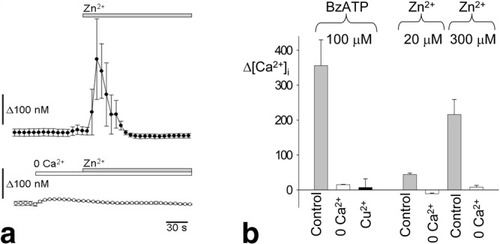
Effects of divalent cation concentrations on [Ca2+]i. (A) Representative changes in [Ca2+]i demonstrating that Zn2+, which potentiates the effects of ATP on P2X4 receptors, increases [Ca2+]i. The response to Zn2+ is eliminated in the absence of extracellular Ca2+. (B) The maximal change from the baseline Ca2+ levels (Δ[Ca2+]i) was measured during exposure to BzATP and Zn2+. Note that Cu2+ inhibits the Ca2+ influx caused by P2X4 receptor activation.
In the presence of Cu2+ (100 μM), the peak change from basal [Ca2+]istimulated by BzATP (100 μM) decreased from 356 ± 74 nM in control cells (n = 11) to 7 ± 25 nM (n = 30, P < 0.01). Inhibition by Cu2+ and stimulation by Zn2+ suggest a more prominent contribution from P2X4 receptors in hepatocytes. Although Cu2+ inhibits both P2X4 and P2X7 receptors, Zn2+ potentiates the effect of ATP on P2X4 receptors but blocks the P2X7 pore (half-maximal inhibitory concentration, 78 μM).23
In additional studies (Fig. 6), cells were exposed to ivermectin, a specific pharmacologic modulator of P2X4 but not P2X7.21 Ivermectin alone (0.1-3 μM) in the absence of exogenous ATP was sufficient to increase [Ca2+]i. On the basis of the inhibitory effects of apyrase on the response to zinc and the relatively high level of constitutive release of ATP by hepatocytes,37 the addition of ivermectin could enhance the response to constitutive ATP already present. To test this possibility, the response to ivermectin (3 μM) in control cells was compared to that in cells exposed to apyrase (1 U/mL). Under control conditions, ivermectin increased [Ca2+]iby 213 ± 25 nM (n = 10), and the presence of apyrase inhibited the response to ivermectin to 5 ± 1 nM (n = 8, P < 0.001). Collectively, these features of activation by BzATP, positive modulation by Zn2+ and ivermectin, and inhibition by Cu2+ support a role for P2X4 receptors in the [Ca2+]i response to extracellular ATP.
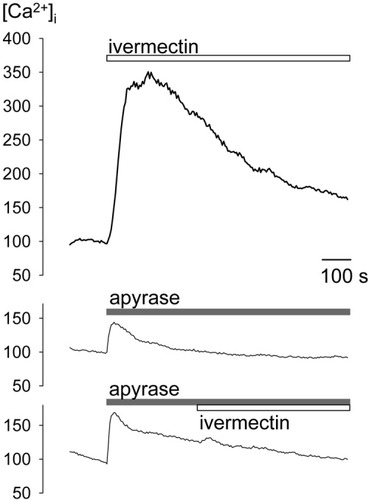
Effects of ivermectin on [Ca2+]i. Ivermectin is a specific modulator of P2X4, but not P2X7.21 Representative changes in [Ca2+]i associated with ivermectin exposure (0.3-3 μM) are shown. Apyrase (1 U/mL) was used to remove extracellular ATP. Under control conditions, ivermectin increased [Ca2+]i, and the response was inhibited in the presence of apyrase. Apyrase alone causes a small increase in [Ca2+]i. These findings suggest that ivermectin increases [Ca2+]i by potentiation of the effects of constitutive ATP release on P2X4 receptors.
Purinergic Regulation of Glycogen Content.
Exposure to ATP stimulates glucose release from intact liver.4, 38 To evaluate whether P2X receptors contribute to this response, glycogen content was measured with D-14C(U)-glucose in Huh7 cells in the presence or absence of BzATP. These cells were selected because they express P2X4 but very little P2X7 (<10% of P2X4 western blot band density). Exposure to 2 μM BzATP for 30 minutes resulted in a 14.6 ± 7.0% decrease in glycogen content in comparison with untreated controls (n = 6, P < 0.01). Thus, activation of P2X receptors results in a substantial decrease in glycogen content, and this suggests a role for these receptors in the glycogenolytic response to ATP.
P2X4 Receptor Transcripts Increase with Higher Hepatic Glycogen Stores.
To assess whether receptor expression varies with the cellular glycogen content, whole liver samples were obtained from mouse models with genetic mutations that result in increased hepatic glycogen, as described by Iizuka et al.39 Increased glycogen stores were associated with increased P2X4 mRNA transcripts (Fig. 7). The mRNA levels with respect to 18S ribosomal RNA were 0.99 in wild type, 1.1 in ob−/−, 1.5 in ChREPB−/−, and 1.6 in ob−/− ChREPB−/− double mutants. Although the mechanisms involved are unknown, the findings suggest that receptor expression may vary in response to physiological demands, providing an additional mechanism for modulation of the cellular response to ATP.
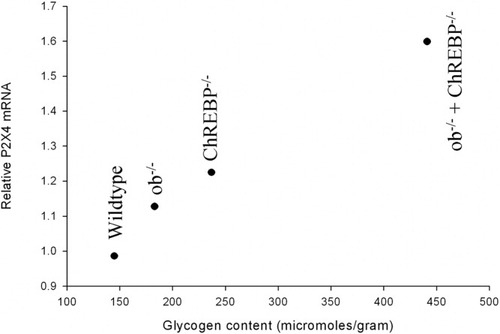
Correlation between P2X4 mRNA transcripts and hepatic glycogen content. P2X4 mRNA transcripts are presented with respect to 18s ribosomal RNA. These findings suggest that P2X4 receptor expression may correlate with physiologic cell demands. Hepatic glycogen content was previously performed in identical samples.39
Discussion
ATP is released by hepatocytes, and once outside the cell, it acts as a potent autocrine/paracrine factor that regulates hepatocyte glycogen metabolism, cell volume, bile formation, and other cell functions. These effects are mediated by activation of purinergic receptors, but the complex pharmacology and diverse physiologic roles imply that more than one receptor is involved. The principle findings of these studies are that (1) P2X4 and P2X7 receptors are expressed by hepatocytes and may be accompanied by other P2X isoforms as well; (2) P2X4 receptors are localized to both the apical and basolateral domains of hepatocytes; and (3) when activated, P2X receptors mediate Na+ and Ca2+ influx. The effects of divalent cations and ivermectin support a role for P2X4 receptors in this response. Collectively, these findings provide molecular and physiologic support for a previously unrecognized pathway involved in the hepatocyte response to ATP.
Both P2X4 and P2X7 receptors were present consistently in all RT-PCR and western blot studies across a broad range of liver model systems. One exception was Huh7 cells, in which P2X7 expression was decreased. Both of these receptors are activated by ATP and BzATP, and in the absence of highly specific agonists and antagonists, it is difficult to assign distinct functions to the isoforms present. Although each functions as a ligand-gated, calcium-permeable cation channel, there are differences in regulation that support a specific role for P2X4. First, the effects of divalent cations on calcium responses are most compatible with a P2X4 response. Although Cu2+ inhibits both P2X4 and P2X7 receptors, Zn2+ has different effects. Zn2+ enhances the P2X4 response by potentiating the effects of ATP but in higher concentrations blocks the P2X7 pore.23 Second, ivermectin potentiates P2X4 but not P2X7 receptors through direct interaction with high-affinity sites on the receptor.21 It is emphasized, however, that P2X7 receptors are readily detectable as well, and further characterization of their distinct roles will require selective inhibition using small interfering RNA or related approaches. For example, Liang et al.40 used this approach successfully to demonstrate that transfection-mediated delivery of small interfering RNA specific to P2X4 inhibits zinc-induced calcium entry in airway epithelial cells. Although this is compatible with observations in liver cells, we have not been able to achieve sufficient inhibition to allow further comment.
Previous studies have shown that rat liver, when perfused with ATP, releases glucose.38 The observation that BzATP rapidly decreases glycogen content supports the hypothesis that P2X4 receptors mediate glucose release via glycogenolysis. Notably, these studies were performed in Huh7 cells that express comparatively little P2X7 receptor protein. Additionally, early experiments demonstrate no change in the transcription of phosphoenolpyruvate carboxykinase, an enzyme that catalyzes a rate-limiting step of gluconeogensis. The increase in P2X4 receptor mRNA transcripts in mice with abundant glycogen stores is equally intriguing. Further studies are required to determine which specific enzymes and pathways are activated with P2X4 stimulation, and additional characterization of the potential role of these receptors in the regulation of glycogenolysis is warranted.
Assuming that these findings are relevant to whole liver, several questions merit emphasis. First, it is unclear why P2X4 receptor protein is expressed on both sinusoidal and canalicular membrane domains. The hypothesis regarding stimulation of glycogenolysis is anatomically reasonable for sinusoidal receptors, which are exposed to portal blood, but a similar function is unlikely for receptors exposed to the canalicular lumen. Notably, hepatocytes appear capable of releasing ATP into bile and express canalicular ATPase activity.41 Furthermore, ATP in bile targets cholangiocytes downstream.42 Thus, although the specific function is not yet known, the findings are consistent with an emerging picture in which ATP represents an important signal coordinating hepatocyte-cholangiocyte signaling along the apical (luminal) axis.
Second, although these results support a role for P2X4 receptors, it is clear that P2X7 and possibly other isoforms are also present. P2X4 proteins have also been shown to form hetero-oligomeric receptors with other isoform subunits, resulting in changes in receptor function.43 Additional studies will be required to delineate the P2X receptor pattern of expression under various conditions. It is reasonable to assume that the various P2X isoforms are capable of modifying the P2X4 response to ATP under different conditions.
Finally, taking into account the potent effects exerted by Zn2+ and Cu2+ on P2X4 receptors, we find it interesting to speculate on the potential role of these receptors in human liver disease. Of particular interest are copper and its ability to block the P2X receptor pore. Wilson's disease is an autosomal recessive disorder characterized by abnormal intrahepatic copper metabolism and deposition of excess copper in the liver, brain, cornea, and other organs, thereby exposing P2X4 receptors to higher levels that might exert physiologic effects. Similarly, zinc, an activator of P2X4 ion channels, is an effective therapeutic agent in the treatment of Wilson's disease. These questions are intriguing as they pertain to the hepatic pathology of Wilson's disease, but they may also be relevant to the brain and other organs that express P2X4 receptors.
In summary, these findings indicate that P2X4 and P2X7 receptors are abundant and represent a novel class of purinergic receptors that are functionally important in the influx of extracellular Na+ and Ca2+. The link between P2X4 activation and glycogenolysis is less defined but merits further investigation because it constitutes an autocrine/paracrine system in which ATP release functions within the liver to stimulate glycogenolysis and glucose release. Thus, increases in cellular ATP would be expected to oppose the effects of insulin on systemic glucose metabolism. The presence of these receptors adds a new level of complexity and versatility contributing to the diverse and sophisticated roles of ATP but also accentuates the need to define the underlying cellular strategies for modifying receptor expression (and hence cellular responses) in response to rapidly changing physiologic demands.




