Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis†
Potential conflict of interest: Drs. Monia, Bhanot, and Yu own stock in Isis Pharmaceuticals.
Abstract
Retinyl ester (RE) stores decrease during hepatic stellate cell (HSC) activation and liver fibrosis. Although retinol esterification is mostly catalyzed by lecithin:retinol acyltransferase (LRAT), diacylglycerol acyltransferase (DGAT)1 also does this. In previous reports, LRAT−/− mice had reduced hepatic RE but neither excessive HSC activation nor liver fibrosis, and DGAT1−/− mice had increased liver levels of RE and retinol. We sought to clarify the role of DGAT1 in liver fibrosis. Expression of DGAT1/2 was compared by real time PCR in freshly isolated, primary mouse HSCs and hepatocytes. To induce nonalcoholic steatohepatitis (NASH) and liver fibrosis, adult male db/db mice were fed methionine choline–deficient (MCD) diets. Half were treated with DGAT1 antisense oligonucleotide (ASO); the rest were injected with saline. Results were compared with chow-fed controls. Inhibition of DGAT1 in liver had no effect on hepatic triglyceride content or liver necroinflammation but reduced HSC activation and liver fibrosis in mice with NASH. To evaluate the role of DGAT1 in HSC activation, HSC were isolated from healthy rats treated with DGAT1 ASO or saline. DGAT1 was expressed at relatively high levels in HSCs. HSC isolated from DGAT1 ASO-treated rats had reduced DGAT1 expression and increased messenger RNA (mRNA) levels of LRAT and cellular retinol binding protein-1. During culture, they retained more vitamin A, had repressed collagen a2 (I) transcriptional activity, and expressed less collagen a1 (I) and a2 (I) mRNA. Conclusion: DGAT1 may be a therapeutic target in NASH because inhibiting DGAT1 favorably altered. HSC retinoid homeostasis and inhibited hepatic fibrosis in mice with NASH. (HEPATOLOGY 2007.)
Transition of vitamin A–storing quiescent hepatic stellate cells (HSC) to vitamin A–depleted myofibroblastic HSC represents a key event in the genesis of cirrhosis. Acquisition of a myofibroblastic phenotype, with consequent induction of type 1 collagen and alpha-smooth muscle actin (α-SMA) expression, is not merely accompanied by the loss of lipid droplets containing retinyl esters (RE),1, 2 but the transition process itself is thought to be regulated by vitamin A and its derivatives.3, 4 In quiescent HSC, lecithin:retinol acyl transferase (LRAT) converts retinol to RE, the predominant storage form of vitamin A.5 Hepatic LRAT messenger RNA (mRNA) levels are regulated by retinoic acid (RA), a metabolite of vitamin A, and parallel the accumulation of hepatic RE.6-8 Loss of both RE stores and RA signaling occurs during hepatic stellate cell (HSC) activation and liver fibrosis.9, 10 Reduced mRNA expression of the RA receptors RARβ and RXRα11, 12 in myofibroblastic HSCs contributes to decreased retinoid responsiveness.9, 10 Depletion of RA and its receptors promotes fibrogenesis partially because RA normally limits collagen accumulation by activating RARβ–RXRα heterodimers that interact with RA-responsive elements to repress transcriptional activation of the α2 (I) collagen promoter.13-15
Paradoxically, neither accumulation of myofibroblastic HSCs nor liver fibrosis was observed in LRAT−/− mice, despite reduced hepatic RE levels and retinol levels.8 These findings suggested that, in addition to LRAT, HSC might express other enzymes that modulate their vitamin A homeostasis. Consistent with this concept, RE accumulation in one HSC line required both LRAT and acyl coenzyme A retinol acyltransferase (ARAT) activity. Unlike LRAT activity, which depended on RA availability, ARAT activity was regulated by the availability of fatty acyl coenzyme A and, hence, overall lipid metabolism.16 Diacylglycerol acyltransferase (DGAT1), a well-known enzyme of triacylglycerol biosynthesis, can function as an ARAT.17 The livers of DGAT1−/− mice have increased RE and retinol levels,17, 18 suggesting that DGAT1 may interact with LRAT to regulate hepatic retinoid homeostasis.
In this study, we sought to clarify the role of DGAT1 in HSC activation and liver fibrosis. To evoke the latter responses, we used a model of progressive nonalcoholic steatohepatitis (NASH) in db/db mice.19 Db/db mice spontaneously develop obesity, diabetes, and fatty livers because of a functional defect in the long form of the leptin receptor. Feeding such mice a methionine choline deficient (MCD) diet induces NASH and liver fibrosis within 4 to 8 weeks, providing a useful model for progressive obesity-related nonalcoholic fatty liver disease.19 We treated MCD diet–fed db/db mice with DGAT1 antisense oligonucleotide (ASO) that was previously shown to exert no significant effect on diet-induced hepatic steatosis.20 Our objective was to determine whether inhibiting hepatic DGAT1 expression influenced the progression of liver fibrosis in this model of NASH. To evaluate the role of DGAT1 in HSC activation more directly, we also studied primary HSC that were isolated from healthy rats treated with DGAT1 ASO or vehicle.
Abbreviations
ARAT, acyl coenzyme A: retinol acyltransferase; ASO, antisense oligonucleotides; CRBP-1, cellular retinol binding protein-1; DGAT, diacylglycerol acyltranferase; HSC, hepatic stellate cells; LRAT, lecithin:retinol acyltransferase; MCD, methionine choline deficient; mRNA, messenger RNA; NASH, nonalcoholic steatohepatitis; PCR, polymerase chain reaction; RA, retinoic acid; RAR, retinoic acid receptors; RE, retinyl ester; RXR, retinoid X receptors; SMA, smooth muscle actin; TGF-β-1, transforming growth factor beta-1; TUNEL, terminal deoxynucleotidyl transferase-mediated nick-end labeling.
Materials and Methods
Animals and Treatments.
Six-week old male db/db mice (BKS.Cg-m+/+Leprdb/J) were purchased from Jackson Laboratories (Bar Harbor, ME), maintained in a temperature-controlled and light-controlled facility, and permitted ad libitum consumption of water. Thirty-six mice were fed either control diet (cat no. 960441; ICN, Aurora, OH, n = 6) or MCD diet (cat no. 960439; ICN, n = 30) for 4 or 8 weeks. Half of the MCD diet–fed mice were injected intraperitoneally with 25 mg/kg mouse DGAT1 ASO dissolved in saline (Isis Pharmaceuticals, Inc., Carlsbad, CA) twice weekly; the remainder were injected with saline.20 All animal experiments fulfilled National Institutes of Health and Duke University requirements for humane animal care.
Isolation and Culture of Primary Hepatic Stellate Cells.
Chow-fed, healthy adult male Sprague-Dawley rats (Harlan, Indianapolis, ID) and lean mice (Jackson Laboratories) were maintained in temperature-controlled and light-controlled facilities. The rats were injected intraperitoneally with either 25 mg/kg rat DGAT1 ASO dissolved in saline or saline (n = 3 rats/group/experiment) once. Two days after treatment, primary HSCs were isolated and then cultured in Dulbecco's modified Eagle's Medium containing 10% fetal bovine serum.21 Primary HSC and hepatocytes were also isolated from healthy lean mice,22 and RNA was obtained for subsequent analysis.
Transient Transfection and Luciferase Assay.
Transient transfection experiments were conducted in HSCs 2 days after isolation. HSCs were grown in 6-well polystyrene dishes to 60% to 70% confluence. The luciferase construct of the α2 (I) collagen promoter (PGL-1009) (from Dr. Esteban Mezey, GI Division, Johns Hopkins University, Baltimore, MD15) and PRL-TK control vector (Promega, Madison, WI) were transfected using FuGENE transfection reagent (Roche Diagnostic, Indianapolis, IN). After 48 hours, cells were rinsed with phosphate-buffered saline, and luciferase activities were determined by chemiluminescent assays.
Two-Step Real-Time Reverse Transcription Polymerase Chain Reaction.
Total RNA was extracted from whole livers and primary cells with RNeasy kits (Qiagen, Valencia, CA), reverse-transcribed using random primer and Superscript RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA), and analyzed by real-time polymerase chain reaction (PCR).23 Primers were designed by Primer Express Software (PE Applied Biosciences) (Table 1). For all primer pairs, specificity was confirmed by sequencing PCR products. Target gene levels are presented as a ratio of levels in treated versus corresponding control groups, according to the ΔΔCt method.24 Fold changes were determined using point and interval estimates.
| Gene | Genbank | Direction | Sequence | Amplicon Size |
|---|---|---|---|---|
| Gus | NM_010368 | Forward | GCAGTTGTGTGGGTGAATGG | 142 |
| Reverse | GGGTCAGTGTGTTGTTGATGG | |||
| DGAT1 | NM_010046 | Forward | TCCGCCTCTGGGCATTC | 65 |
| Reverse | GAATCGGCCCACAATCCA | |||
| DGAT2 | NM_0010123 | Forward | CTGGCTGATAGCTGCTCTCTACTTC | 78 |
| Reverse | TGTGATCTCCTGCCACCTTTC | |||
| α-SMA | NM_007392 | Forward | AAACAGGAATACGACGAAG | 134 |
| Reverse | CAGGAATGATTTGGAAAGGA | |||
| LRAT | NM_023624 | Forward | CTGACCAATGACAAGGAACGCTCTC | 370 |
| Reverse | CTAATCCCAAGACAGCCGAAGCAAGA | |||
| TGFβ-1 | NM_011577 | Forward | TTGCCCTCTACAACCAACACAA | 103 |
| Reverse | GGCTTGCGACCCACGTAGTA | |||
| TNFα | NM_013693 | Forward | TCGTAGCAAACCACCAAGTG | 209 |
| Reverse | AGATAGCAAATCGGCTGACG | |||
| Procollagen lα1 | NM_007742 | Forward | GACATCCCTGAAGTCAGCTGC | 167 |
| Reverse | TCCCTTGGGTCCCTCGAC | |||
| Procollagen lα2 | NM_007743 | Forward | CTCCAAGGAAATGGCAACTCAG | 108 |
| Reverse | TCCTCATCCAGGTACGCAATG | |||
| CRBP-1 | NM_012733 | Forward | ATGATCATCCGCACGCTGA | 107 |
| Reverse | GTCATGCACTTGCGGTCATCT | |||
| RXRα | NM_012805 | Forward | ACCGCTCCATAGCTGTGAAAGA | 111 |
| Reverse | CCGTTAGCACCCTGTCAAAGAT | |||
| RARβ | XM_223843 | Forward | GCTTTTTCCGACGAAGCATTC | 124 |
| Reverse | ACTTCAAAGCACTTCTGCAGGC |
Immunohistochemistry and Analysis of Liver Architecture.
Serial sections were stained with hematoxylin-eosin. After deparaffinization, microwave antigen retrieval, and blocking endogenous peroxidase activity, other sections were incubated with terminal deoxynucleotidyl transferase–mediated deoxyuridine 5-triphosphate–digoxigenin nick end labeling (TUNEL) reaction mixture containing terminal deoxynucleotidyl transferase and fluorescein-deoxyuridine 5-triphosphate (Roche), anti–α-SMA (DakoCytomation, Carpinteria, CA). Antigens were demonstrated using secondary anti-fluorescein antibody peroxidase conjugate (Roche), anti-mouse or rabbit polymer horseradish peroxidase (Dako), and diaminobenzidine chromagen (Dako) and counterstaining with Gill's hematoxylin (Vector Laboratories). TUNEL-positive hepatocytes were quantitated in 3 randomly selected fields/section (20× magnification) with Meta Morph software (Molecular Devices Corporation, Downingtown, PA).25
Quantification of Hepatic Collagen Content.
Liver sections were stained with picrosirius red (Sigma-Aldrich, St. Louis, MO) and counterstained with fast green (Sigma-Aldrich). Sirius red staining was quantitated by Meta Morph software in 3 randomly selected fields/section (20× magnification). Hydroxyproline content was quantified colorimetrically as described.21
Tissue and Serum Biochemical Measurements.
Serum aspartate aminotransferase and alanine aminotransferase were assayed.20 Tissue triglyceride and thiobarbiturate acid-reactive substances were measured with a triglyceride detection kit (Sigma-Aldrich) and thiobarbiturate acid-reactive substances assay kit (ZeptoMetrix Co., Buffalo, NY).
Sample Preparation and Measurement for Total Vitamin A Analysis.
Freshly-isolated HSC (approximately 3 × 106 cells) and 4-day cultured HSC (approximately 3 × 106 cells) were subjected to retinoid extraction as described by Satre et al.26 Aliquots of the organic phase were taken to dryness, redissolved in absolute ethanol:dichloromethane (10:1), and analyzed for retinoid content.27, 28 Retinol autofluorescence intensity was measured at the maximum excitation (332 nm) and emission (474 nm) wavelengths in 0.01 M Brij (Sigma-Aldrich) within the linear range of calibration curves for vitamin A (Sigma-Aldrich) in 0.01 M Brij (in other words, between 28.6 μg/L and 3.5 mg/L).
Statistical Analysis.
Results are expressed as mean ± SEM. Significance was established using Student t test and analysis of variance. Differences were considered significant when P was less than 0.05.
Results
Localization of DGAT1 Expression in Healthy Liver.
In a previous study, DGAT1 ASO did not improve hepatic steatosis in mice with genetic or diet-induced obesity, whereas treatment with DGAT2 ASO markedly reduced hepatic fat accumulation.20 One explanation for the discrepant actions of DGAT1 ASO and DGAT2 ASO might be that different types of liver cells differentially express the 2 DGAT isoforms. To clarify this issue, DGAT1/2 expression was assessed by real-time PCR in freshly isolated primary mouse hepatocytes and HSCs. Hepatocytes expressed predominately DGAT2 mRNA, whereas DGAT1 expression was high in both HSC and hepatocytes (Fig. 1). Expression of the RE-synthesizing enzyme LRAT was restricted to HSC. These results suggest that DGAT1 may regulate triglyceride and retinoid metabolism in HSCs.
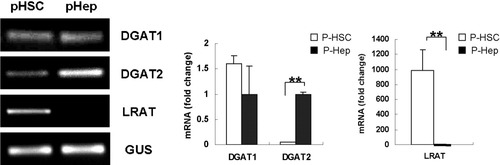
Differential expression of DGAT1, DGAT2, and LRAT in primary mouse HSCs and hepatocytes. DGAT1/2 and LRAT mRNAs were analyzed by quantitative real time PCR. Results were normalized to β-glucuronidase (GUS) expression. Mean ± standard error (SE) data from freshly isolated primary mouse hepatocytes (n = 3) are presented as fold change relative to freshly isolated primary mouse HSCs (n = 3) (**P < 0.01).
Treatment with DGAT1 ASO Selectively Reduced DGAT1 Expression in Mice and Primary Rat HSCs.
To evaluate the specificity and efficacy of DGAT1 ASO, MCD diet–fed mice were treated with either saline or DGAT1 ASO, and whole liver RNA was isolated for quantitative real-time PCR analysis. Results in both MCD-fed groups were compared with those in untreated db/db mice that were fed comparable methionine-sufficient diet without DGAT1 ASO treatment (control). Interestingly, MCD diets themselves influenced hepatic expression of DGAT1. In saline-treated MCD diet–fed mice, liver DGAT1 mRNA levels fell by 60% after 4 weeks but returned to baseline after 8 weeks (Fig. 2A). In contrast, DGAT2 mRNA levels remained relatively constant. Treatment of MCD diet–fed mice with DGAT1 ASO further reduced hepatic expression of DGAT1. At both 4 and 8 weeks, levels of DGAT 1 mRNA in the ASO-treated group were reduced by more than 90% compared with controls. DGAT1 ASO was specific for DGAT1, with no effect observed on DGAT2 mRNA levels (Fig. 2A).
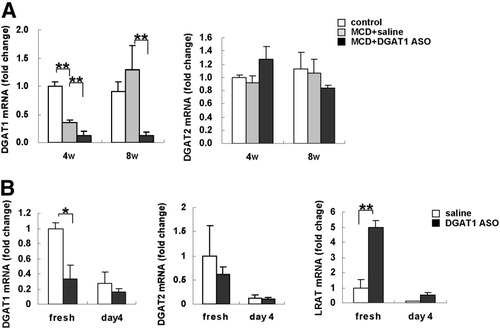
Effect of MCD diet and DGAT1 ASO on expression of DGAT1, DGAT2, and LRAT in db/db mice and primary rat HSCs. (A) DGAT1 and DGAT2 expression were evaluated by quantitative real time PCR analysis of total RNA from chow-fed db/db controls (n = 3) and both groups of MCD diet–fed mice (n = 7-8/group/time point) after 4 and 8 weeks of treatment with either saline or DGAT1 ASO. Results were normalized to GUS expression. Mean ± SE data are displayed as fold change relative to control db/db mice (**P < 0.01, *P < 0.05). (B) Healthy rats were injected intraperitoneally with saline (n = 3 rats) or DGAT1 ASO (n = 3 rats); 2 days later, HSC were isolated and cultured. Expression of DGAT1, DGAT2, and LRAT were evaluated by quantitative real-time PCR analysis of total RNA from freshly isolated and 4-day culture-activated cells. Results were normalized to GUS expression. Mean ± SE data from all groups are displayed as fold change relative to freshly isolated HSCs from saline-treated control rats (**P < 0.01, *P < 0.05).
To examine the effect of DGAT1 ASO treatment on HSC, we isolated primary rat HSCs 2 days after in vivo treatment with DGAT1 ASO and cultured them for 4 days to induce their activation. In vivo treatment with DGAT1 ASO selectively reduced DGAT1 expression in freshly isolated primary HSCs, and increased LRAT expression 5-fold compared with HSCs that were isolated from rats treated with saline (Fig. 2B). During culture, mRNA expression of all 3 enzymes decreased; however, LRAT mRNA expression in HSCs from DGAT1 ASO–treated rats remained higher than in HSC harvested from controls. These results showed that animals treated with DGAT1 ASO up-regulated HSC expression of LRAT when DGAT1 expression was inhibited.
DGAT1 ASO Inhibited HSC Activation in Mice with MCD Diet–Induced NASH and Blocked Culture Induced Activation of Rat HSC.
MCD diet increased markers of HSC activation, including hepatic expression of α1(I) and α2(I) collagen, α-SMA, and transforming growth factor beta (TGF-β1) mRNAs (Fig. 3A). Inhibiting expression of DGAT1 with ASO decreased MCD diet–induced HSC activation. Compared with livers of saline-treated MCD diet–fed mice, livers of MCD diet–fed mice treated with DGAT1 ASO exhibited decreased hepatic expression of collagen mRNA at both 4 and 8 weeks. Expression of α-SMA and TGF-β were reduced to control levels at the 4-week point (Fig. 3A). Likewise, immunohistochemistry demonstrated increased Sirius red–stained collagen fibrils and α-SMA(+) cells in livers of MCD diet–fed mice (Fig. 3B). Livers of MCD diet–fed mice treated with DGAT1 ASO had less Sirius Red and α-SMA staining than MCD diet–fed mice that were treated with saline (Fig. 3B). Reduced liver fibrosis was confirmed by biochemical quantitation of hepatic hydroxyproline content and morphometric assessment of Sirius red staining (Fig. 3C).
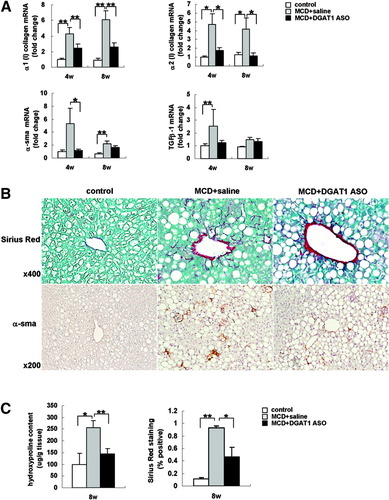
Effect of MCD diets and DGAT1 ASO treatment on markers of HSC activation and liver fibrosis in db/db mice. (A) mRNA levels of α1/2 (I) procollagen, α-SMA, and TGF-β1 were determined by quantitative real time PCR analysis of total liver RNA obtained after 4 and 8 weeks of treatment. Results were normalized to GUS expression and then expressed as fold change relative to gene expression in control db/db mice. Mean ± SE data from controls (n = 3 mice) and each MCD diet–fed group (n = 7-8 mice/group) (*P < 0.05, **P < 0.01). (B) Liver sections from all mice were stained with Sirius red and α-SMA antibody after 8 weeks of treatment. Photomicrographs from representative mice are shown. (C) Liver hydroxyproline content was assessed in all mice at 8 weeks. Results are expressed per g/tissue. Mean ± SE data from controls (n = 3) and each MCD diet–fed group (n = 7/group) (*P < 0.05, **P < 0.01). Morphometric analysis of Sirius red–stained sections from controls (n = 3) and each MCD diet–fed group (n = 7/group) at 8 weeks. Results are expressed as a percentage of section staining (+) for Sirius red (*P < 0.01, **P < 0.01).
To evaluate the effects of DGAT1 ASO on HSC activation more directly, HSC were isolated from healthy rats that had been treated acutely with DGAT1 ASO or saline. Although initial expression of collagen and α-SMA mRNAs was comparably low in the 2 groups of HSC, freshly isolated HSC from DGAT1 ASO–treated rats expressed less TGF-β mRNA than HSC from control rats. During culture, HSC from control rats up-regulated typical markers of HSC activation, increasing mRNA expression of α1(I) and α2(I) collagen, α-SMA, and TGF-β (Fig. 4A -D). The activation process was attenuated in HSC from DGAT1 ASO-treated rats. After culture, HSC from DGAT1 ASO-treated rats expressed significantly lower levels of α1(I) collagen and α2(I) collagen than controls (Fig. 4A, B). Thus, inhibiting DGAT1 increased HSC expression of LRAT (Fig. 2B), suppressed HSC activation (Figs. 3A, 4), and inhibited MCD diet–related hepatic fibrosis (Fig. 3 A-C).
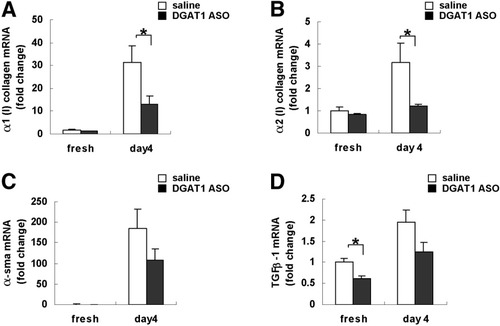
Effect of DGAT1 ASO treatment on culture-related activation of primary rat HSC. Healthy adult rats were injected intraperitoneally with saline (n = 3) or DGAT1 ASO (n = 3); 2 days later, primary HSC were isolated and cultured. Messenger RNA was obtained from freshly isolated HSC or day 4 culture activated HSC and analyzed by quantitative real time PCR. mRNA levels of (A) α1 (I) collagen, (B) α2 (I) collagen, (C) α-SMA, and (D) TGF-β-1 (n = 3 plates/group/time point). Results were normalized to GUS expression. Mean ± SE data are displayed as fold change relative to freshly isolated HSCs from saline-treated control rats (*P < 0.05).
DGAT1 ASO Had Little Effect on Hepatic Steatosis in Mice with NASH.
To determine to what extent reduced HSC activation in DGAT1 ASO-treated mice might be explained by improvements in MCD diet–induced NASH, hepatic steatosis was evaluated. We confirmed that DGAT1 ASO did not protect MCD diet-fed db/db mice from hepatic steatosis. Steatosis scores29 and liver triglyceride content were similarly increased (2-3 fold > chow-fed controls) in saline-treated and DGAT1 ASO-treated db/db mice after 4 weeks of MCD diet consumption, and higher in the DGAT1 ASO-treated group than in saline-treated controls after 8 weeks of MCD diet feeding (Fig. 5A, B).
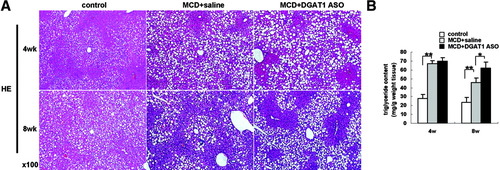
Effect of MCD diets and DGAT1 ASO on liver histology and triglyceride content of db/db mice. (A) Hematoxylin-eosin of liver sections from representative mice from each treatment group. (B) Liver triglycerides were measured with commercial kits. Results are expressed per gram tissue. Mean ± SE data from controls (n = 3/time point) and each MCD diet–fed group (n = 7-8/group/time point) at 4 and 8 weeks are graphed (*P < 0.01, **P < 0.01).
DGAT1 ASO Had Little Effect on Liver Injury in Mice with NASH.
Because inflammatory and oxidative stresses drive progression from steatosis to NASH, we evaluated the effect of DGAT1 ASO treatment on various markers of hepatic necroinflammation. Hepatic TNF-α mRNA levels (Fig. 6A), serum aspartate aminotransferase and alanine aminotransferase (Fig. 6B), lobular necroinflammation (Fig. 6C), the number of hepatic TUNEL-positive cells (Fig. 6D), and hepatic thiobarbiturate acid-reactive substance levels (Fig. 6E) increased significantly when db/db mice were fed MCD diets. Treatment with DGAT1 ASO did not lessen any of these responses. Given that inhibiting DGAT1 did not protect mice from MCD diet–related hepatic steatosis or necroinflammation (Fig. 6A-E), and culture-induced activation was suppressed in HSC from healthy, DGAT1 ASO-treated rats (Fig. 4), it seems likely that DGAT1 ASO reduced hepatic fibrosis in mice with NASH via direct effects on HSC.
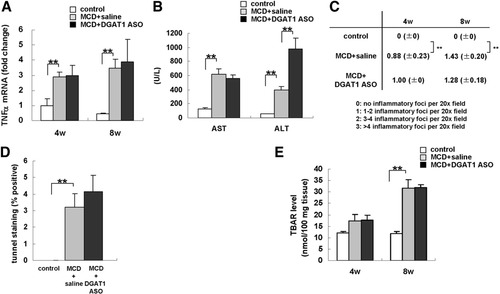
Effect of MCD diets and DGAT1 ASO on injury-related and lipotoxicity/oxidant stress parameters in db/db mice. (A) Tumor necrosis factor alpha expression was evaluated by quantitative real time PCR analysis of total RNA from controls (n = 3/time point) and both groups of MCD diet–fed mice (n = 7-8/group/time point) at 4 and 8 weeks. Results were normalized to GUS expression. Mean ± SE data are displayed as fold change relative to control db/db mice (**P < 0.01). (B) Serum aspartate aminotransferase and alanine aminotransferase was measured after 8 weeks of treatment. Mean ± SE results from control (n = 3) and each MCD diet–fed group (n = 8/group) (**P < 0.01). (C) A lobular inflammatory grade was determined for each mouse at the time of sacrifice following 4 to 8 weeks of treatment. The number of inflammatory foci per 20× field was counted on 2 sections from every animal. Mean ± SE data from controls (n = 3/time point) and each MCD diet–fed group (n = 7-8/group/timepoint) (*P < 0.05, **P < 0.01). (D) Hepatocyte apoptosis was evaluated by TUNEL staining of liver sections after 8 weeks of treatment. Mean ± SE data from controls (n = 3) and each MCD diet–fed group (n = 7-8/group) (**P < 0.01). (E) TBAR levels were evaluated at 4 and 8 weeks. Results were normalized per 100 mg tissue. Mean ± SE data from controls (n = 3/time point) and each MCD diet–fed group (n = 7-8/group/time point) (**P < 0.01).
DGAT1 ASO Inhibited the Loss of Total Vitamin A Content in 4-Day Culture Primary HSCs, But Had No Effect on HSC Triglyceride Content.
Because DGAT1 catalyzes synthesis of both triglyceride and RE, DGAT1 ASO might reduce the content of one or both of these lipids in HSC. To clarify this issue, healthy rats were treated with DGAT1 ASO or saline; primary HSCs were isolated 2 days later and then cultured for 4 days to induce spontaneous HSC activation. Oil red O staining showed that lipid droplets gradually disappeared during culture in both groups (Fig. 7A). Consistent with the staining results, triglyceride content declined during culture in both groups of HSCs but was similar in the control and DGAT1 ASO-treated groups at each time point (Fig. 7B). Although vitamin A content also declined during culture, HSC from DGAT1 ASO-treated rats retained more vitamin A than control HSCs after culture (Fig. 7C).
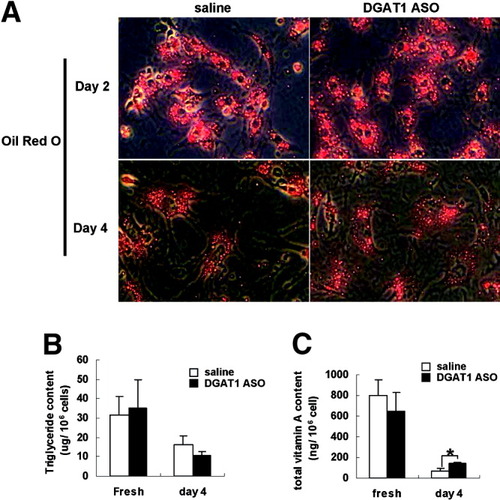
Effect of DGAT1 ASO on triglyceride and total vitamin A content in primary rat HSCs. Healthy adult rats were injected intraperitoneally with saline (n = 3) or DGAT1 ASO (n = 3); 2 days later, primary HSC were isolated. (A) Triglyceride and (B) vitamin A content were determined biochemically in freshly isolated HSC and in HSC that had been activated by 4 days in culture. Results (n = 3 plates/group/time point) are expressed per 106 cells (*P < 0.05).
DGAT1 ASO Repressed α2 (1) Collagen Promoter Activities and Induced CRBP-1 Expression in HSC.
More than 98% of the retinoids in HSC are RE, but retinol, RA, and RA metabolites also contribute to net vitamin A content.30 Evidence that DGAT1 ASO induced HSC expression of LRAT (Fig. 2B) suggested that RA pools might expand during DGAT1 deficiency because RA accumulation is the major stimulus for LRAT transcription.6, 31, 32 Therefore, we compared expression of other RA-regulated genes in HSC from DGAT1 ASO-treated and vehicle-treated rats.
In HSC, RA differentially regulates expression of cellular retinol-binding protein-1 (CRBP-1) and α2 (I) collagen, stimulating the former while repressing the latter.6, 15 Freshly isolated HSC from DGAT1 ASO-treated rats expressed higher levels of CRBP-1 mRNA than controls (Fig. 8A), despite having similar expression of retinoid X receptor alpha (RXRα) and retinoic acid receptor beta (RARβ) mRNAs. Coupled with the demonstration of increased LRAT mRNA in DGAT1-deficient HSC, these findings suggest that DGAT1 ASO increased HSC content of RA. However, the effects of RA on gene transcription are difficult to assess, because they depend on the relative availability of various RA-binding proteins, RA receptors (RARs), RAR dimerization partners (such as RXR), and other orphan nuclear receptors, as well as cellular RA content.33 During HSC culture, for example, steady-state levels of CRBP-1 mRNA declined in cells from both DGAT1 ASO-treated and saline-treated rats as expression of RXRα and RARβ fell (Fig. 8A). Therefore, to complement the RNA analysis and evaluate RA transcriptional activity more directly, HSC from both groups of rats were transfected with RA-responsive elements containing α2(I) reporter constructs.15 Consistent with acknowledged retinoid repression of collagen gene transcription,15 α2 (I) collagen promoter activity was suppressed by more than 70% in HSCs from rats treated with DGAT1 ASO (Fig. 8B). Thus, inhibiting DGAT1 apparently enhanced RA signaling in HSC, leading to increased expression of LRAT (Fig. 2B) and CRBP-1 (Fig. 8A), but repression of α2(I) collagen gene transcription (Fig. 8B).
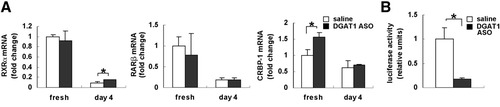
Effect of DGAT1 ASO on RXRα, RARβ, and CRBP-1 expression and α2 (I) collagen promoter activity in primary rat HSC. Healthy adult rats were injected intraperitoneally with saline or DGAT1 ASO (n = 3 rats/group); 2 days later, primary HSC were isolated. RNA was isolated from freshly isolated HSC (n= 3 plates/group) or from HSC that had been activated by 4 days in culture (n = 3 plates/group) and analyzed by quantitative real time PCR. (A) RXRα, RARβ, and CRBP-1 expression. Results were normalized to GUS expression. Mean ± SE data from each sample are displayed as fold change relative to freshly isolated HSC from saline-treated control rats (*P < 0.05). (B) Relative luciferase activities of the α2 (I) collagen promoter were measured in HSCs from saline-treated controls (n = 3/group) and HSC from DGAT1 ASO-treated rats after 4 days in culture (n = 3 plates/group) Results of firefly luciferase activities were normalized to Renilla activity as an internal control. Mean ± SE data are presented as fold change relative to control.
Discussion
This study demonstrates that HSC express DGAT1, and shows that inhibiting DGAT1 expression in HSC influences their retinoid homeostasis. Inhibiting DGAT1 activity in HSC increased their expression of LRAT, an enzyme that catalyzes conversion of retinol to RE, and also induced HSC expression of CRBP-1, a protein that facilitates LRAT-mediated conversion of retinol to RE.34, 35 The expression of both LRAT and CRBP-1 are stimulated by accumulation of RA, the product of retinol oxidation. Thus, it has been suggested that a major function of LRAT is to control cellular RA content by diverting retinol into RE for storage.6 Evidence that mRNA levels of 2 RA-inducible genes were increased in HSC that were freshly isolated from DGAT1 ASO-treated animals supports the concept that DGAT1 deficiency increased HSC RA content. This would be expected if DGAT1 normally cooperates with LRAT to convert retinol to RE in HSC, and hence, the loss of DGAT1 increased the pool of retinol available for RA synthesis.
HSC have long been known to have at least two discrete enzymes that catalyze RE formation, LRAT and ARAT.16 LRAT and ARAT use different substrates as fatty acyl donors for retinol esterification. LRAT function also requires retinol to associate with CRBP, whereas ARAT does not.6 Within HSC, LRAT has a much lower Km for retinol than ARAT, and hence, LRAT plays the major role in HSC RE generation.36 LRAT activity is also the main factor that regulates HSC uptake of exogenous retinol.37 Thus, it is not surprising that the hepatic content of retinol and RE were both significantly reduced in LRAT knockout mice.8 However, these mice did not exhibit HSC activation, suggesting that ARAT was able to compensate for the loss of LRAT. Pertinent to this point, RE stores were noted to be increased in adipose tissues of LRAT knockout mice.8
Recent studies in other cell types suggest that DGAT1 can function as an ARAT.17 DGAT1 is strongly expressed in adipocytes.17, 18 Interestingly, hepatic levels of retinol and RE were increased in DGAT1 knockout mice.17 Together with the findings in LRAT-deficient mice, those observations suggest that retinoid homeostasis involves cross-talk between the liver and adipose tissues: DGAT1 appears to control RE storage in fat because loss of DGAT1 in adipose tissue places an increased demand for RE storage in liver. Conversely, RE storage in liver appears to rely mainly on LRAT and, hence, loss of hepatic LRAT results in redistribution of RE stores to fat.
Our results complement and extend this work by demonstrating that DGAT1 is the major contributor to the ARAT activity that was previously demonstrated in HSC.16 In addition, because intraperitoneal delivery of ASO targeted DGAT1 gene manipulation to the liver,38 our studies revealed that DGAT1–LRAT interactions modulate retinoid homeostasis within HSC themselves. These changes in retinoid homeostasis apparently helped the cells to maintain a more quiescent phenotype, as evidenced by reduced expression of the profibrogenic cytokines, TGF-β1, and decreased culture-related induction of collagen gene expression. Down-regulation of collagen gene expression in HSC with reduced DGAT1 activity was likely mediated by increased RA signaling because such cells exhibited transcriptional repression of α2(I) collagen promoter elements that are known to be inhibited via RARα–RXRβ/RA-responsive element interactions.13-15 Given these anti-fibrogenic effects of DGAT1 inhibition in HSC, it is not surprising that we observed reduced liver fibrosis in MCD diet-fed db/db mice that were treated with DGAT1 ASO.
Nevertheless, because HSC activation and liver fibrosis are believed to result from liver injury that occurs during earlier stages of nonalcoholic fatty liver/NASH,39 it was important for us to examine the effects of DGAT1 ASO on steatosis, hepatic necroinflammation, and mediators of those processes. Indeed, pharmacological inhibition of DGAT1 has emerged as a potential strategy for the therapy of obesity, and DGAT1 deficiency protects mice from hepatic steatosis induced by high-fat diets.17, 18 However, DGAT1 ASO treatment improved neither steatosis nor necroinflammation in our animal model of NASH. We also found no effect of DGAT1 ASO on body weight, epididymal fat mass, serum insulin, glucose, or adiponectin levels (data not shown). Therefore, the protective effects of DGAT1 ASO treatment on MCD diet–induced hepatic fibrosis cannot be attributed to changes in systemic insulin sensitivity or serum levels of antifibrogenic adipocytokines.
Thoughtful review of the findings in mouse models of DGAT1 deficiency and overexpression suggest an explanation for this apparent paradox. DGAT1 is strongly expressed in adipocytes, where it plays a major role in triglyceride synthesis.40 In DGAT1−/− mice, decreased adipose triglyceride synthesis stimulates compensatory induction of alternative mechanisms for fatty acid disposal, including increased fatty acid oxidation. Thus, adult DGAT1−/− mice have less adipose mass (<50%) and smaller adipocytes than wild-type mice.18, 40 Reduced adiposity in DGAT1−/− mice is accompanied by increased adiponectin production and enhanced insulin sensitivity.17, 18 Because hepatocytes normally express DGAT1, these cells are also deficient in DGAT1 activity in DGAT1−/− mice. A role for DGAT1 in hepatocyte triglyceride synthesis was further suggested by a report that adenoviral-mediated transfer of DGAT1 increased hepatic triglyceride content.41 However, in this and our earlier study,20 treatment with DGAT1 ASO did not reduce hepatic triglyceride accumulation in mice with MCD diet–induced hepatic steatosis. DGAT1 ASO treatment also failed to protect mice from high fat diet–induced hepatic steatosis.38 DGAT1 ASO was administered via intraperitoneal injection, and the latter study was unable to demonstrate effects on expression of DGAT1 in extrahepatic tissues. Together, these data suggest that extrahepatic factors (such as reduced adiposity, increased adiponectin, improved insulin sensitivity), rather than direct inhibition of hepatocyte DGAT1, protected DGAT1−/− mice from diet-induced hepatic steatosis. This concept is supported by growing evidence that DGAT2 is more important than DGAT1 in regulating hepatocyte triglyceride accumulation.20, 38, 42 Hence, in the current study, the major outcomes in mice treated with DGAT1 ASO likely reflected the consequences of inhibiting DGAT1 activity in HSC. The ability of DGAT1 ASO to inhibit liver fibrosis in mice with active NASH has potentially exciting therapeutic implications, because inhibiting DGAT1 with DGAT1 ASO provides a relatively selective approach to enhance retinoid signaling and block induction of collagen gene expression in HSC.




