Activation of peroxisome proliferator-activated receptor-α inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia–reperfusion†
Potential conflict of interest: Nothing to report.
Abstract
Hepatic steatosis is a major risk factor in ischemia–reperfusion (I/R). Adiponectin acts as an antiobesity and anti-inflammatory hormone. Adiponectin activates peroxisome proliferator-activated receptor-α (PPAR-α), a transcription factor that regulates inflammation in liver disease. Ischemic preconditioning (PC) based on brief periods of I/R protects steatotic livers against subsequent sustained I/R injury, but just how this is achieved is poorly understood. This study explains the role of PPAR-α and adiponectin in the vulnerability shown by steatotic livers to I/R and the benefits of PC in this situation. PPAR-α and adiponectin levels in nonsteatotic livers undergoing I/R were similar to those found in the sham group. However, reduced PPAR-α and increased adiponectin levels, particularly the high molecular weight isoform, were observed in steatotic livers as a consequence of I/R. Our results suggest that mitogen-activated protein kinases (MAPKs) may be positive regulators of adiponectin accumulation in steatotic livers. The addition of adiponectin small interfering RNA (siRNA) before I/R protected steatotic livers against oxidative stress and hepatic injury. The induction of PC before I/R increased PPAR-α and reduced adiponectin levels in steatotic livers. PC, which increased PPAR-α, as well as PPAR-α agonist pretreatment reduced MAPK expression, adiponectin, oxidative stress, and hepatic injury that follows I/R. In addition, the administration of a PPAR-α antagonist in preconditioned steatotic livers eliminated the beneficial effects of PC on MAPKs, adiponectin, oxidative stress, and hepatic injury. Conclusion: Steatotic livers are more predisposed to down-regulate PPAR-α and overexpress adiponectin when subjected to I/R. PPAR-α agonists and adiponectin siRNA are promising candidates to protect steatotic livers. PPAR-α agonists as well as PC, through PPAR-α, inhibited MAPK expression following I/R. This in turn inhibited adiponectin accumulation in steatotic livers and adiponectin-worsening effects on oxidative stress and hepatic injury. (HEPATOLOGY 2007.)
Minimizing the adverse effects of ischemia–reperfusion (I/R) injury in steatotic livers is an urgent need. The first step toward achieving this objective is a fuller understanding of the mechanisms involved in I/R injury in this type of liver.
Peroxisome proliferator-activated receptor-α (PPAR-α) regulates inflammation in a number of liver diseases.1 To the best of our knowledge, only 1 previous study has examined the benefits of PPAR-α agonists in hepatic I/R, and this was based solely on nonsteatotic livers.2 The role of PPAR-α in steatotic livers undergoing I/R remains to be identified.
Since the discovery of adiponectin in 1995,3 diverse clinical studies and a variety of animal models such as small-for-size fatty graft transplants have demonstrated that adiponectin mediates antiobesity and anti-inflammatory effects.3-6 Thus, the possibility that adiponectin could be beneficial in other surgical procedures—such as steatotic livers undergoing warm ischemia associated with hepatic resection or steatotic liver grafting from cadaveric donors—should be considered.
Adiponectin activates PPAR-α in LPS-induced hepatic injury and isolated hepatocytes.7, 8 This study evaluates the respective roles of PPAR-α and adiponectin in steatotic livers undergoing warm ischemia. In addition, we compare the effects of pharmacological treatments that modulate PPAR-α and adiponectin in hepatic I/R to those obtained after applying ischemic preconditioning (PC). PC is an endogenous protective procedure by which brief periods of I/R protect the liver against subsequent I/R injury. To date, despite intense research efforts, PC is the only surgical strategy that has been successfully applied in patients with steatotic livers undergoing warm ischemia.9 As far as we are concerned, the effects of PC on PPAR-α and adiponectin in hepatic I/R injury have not been determined previously. Only a full appraisal of the molecular basis underlying PC in both nonsteatotic and steatotic livers will facilitate the design of new clinical applications of PC in liver surgery, as well as new pharmacological strategies to simulate its effectiveness.
Abbreviations
ALT, alanine aminotransferase; HMW, high molecular weight; IL, interleukin; I/R, ischemia–reperfusion; Ln, lean; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; mRNA, messenger RNA; Ob, obese; PC, ischemic preconditioning; PPAR-α, peroxisome proliferator-activated receptor-α; siRNA, small interfering RNA; TNF-α, tumor necrosis factor α.
Material and Methods
Experimental Animals
Homozygous (Ob) and heterozygous (Ln) Zucker rats (Iffa-Credo, France) aged 16–18 weeks were used (60%–70% steatosis). The animals were anesthesized with ketamine and xylazine (100 and 8 mg/kg, respectively).5 The study followed European Union regulations (Directive 86/609 EEC) governing animal experiments.
Experimental Design
Protocol 1.
Protocol 1 comprises the role of PPAR-α and adiponectin in steatotic liver undergoing I/R.
Group 1: sham [n = 12 (6 Ln, 6 Ob)].
Hepatic helium vessels of Ln and Ob animals were dissected.
Group 2: I/R [n = 12 (6 Ln, 6 Ob)].
Ln and Ob animals were subjected to 60 minutes of partial (70%) ischemia followed by 24-hour reperfusion.10
Group 3: I/R + PPAR-α agonist (n = 12).
As in group 2, but treated with WY-14643, a PPAR-α agonist (10 mg/kg intravenously), 1 hour before ischemia.2
Group 4.
This group is divided into 3 subgroups. (4.1) I/R + adiponectin siRNA(1) (n = 12). As in group 2, but treated 24 hours before ischemia with adiponectin small interfering RNA (siRNA) (100 nmol/kg) in phosphate-buffered saline (100 mL/kg) via hydrodynamic injection.11 (4.2) siRNA control (n = 12). As in group 4.1, but administering cyclophilin B siRNA (100 nmol/kg) instead of adiponectin siRNA.11 (4.3) Blank control (n = 12). As in group 4.1, but administering only phosphate-buffered saline (100 mL/kg).11
Group 5: PC [n = 12 (6 Ln, 6 Ob)].
To induce PC, the hepatic inflow to the median and left lobes (70%) was occluded by an application of a microvascular clamp for 5 minutes followed by a reflow for 10 minutes.10 Livers were then subjected to I/R as in group 2.
Group 6: PC + PPAR-α antagonist (n = 12).
As in group 5, but treated with MK886, a PPAR-α antagonist (1 mg/kg orally) 30 minutes before PC.12
Group 7: PC + adiponectin(1) (n = 12).
As in group 5, but treated with recombinant adiponectin (1.5 mg/kg intravenously) 48 and 24 hours before PC.4
After reperfusion, we determined gene expression and protein levels of PPAR-α and adiponectin, oligomers of adiponectin [trimers, hexamers, and high molecular weight (HMW) complexes], messenger RNA (mRNA) expression and protein levels of both receptors (AdipoR1 and AdipoR2), interleukin (IL)-1, malondialdehyde (MDA), H2O2, triglyceride and fatty droplet content, as well as histological parameters of hepatic injury. In addition, hepatic injury markers and adiponectin protein levels were measured in plasma.
Protocol 2.
Protocol 2 comprises the role of tumor necrosis factor α (TNF-α) and mitogen-activated protein kinases (MAPKs) on adiponectin accumulation in steatotic livers following I/R. TNF-α was measured in steatotic livers of groups 1 and 2. To evaluate the effect of MAPKs on adiponectin, the following experimental groups were added.
Group 8: I/R + p38 inhibitor (n = 6 Ob).
Ob animals were subjected to I/R (as in group 2) but treated with SB20358, a p38 inhibitor (1 mg/kg intraperitoneally), 24 hours before ischemia.13
Group 9: I/R + JNK inhibitor (n = 6 Ob).
Ob animals were subjected to I/R (as in group 2) but treated with SP600125, a JNK inhibitor (6 mg/kg subcutaneously) 1 hour before ischemia.13
Adiponectin (mRNA and protein levels), MDA, and H2O2were measured in the livers and alanine aminotransferase (ALT) levels were measured in plasma. MAPKs (p38 and JNK) in the livers of groups 1, 2, 3, 5, and 6 (protocol 1) were analyzed.
Protocol 3.
Protocol 3 comprises the assessment of the predominant source of adiponectin in damaged steatotic livers. The following experimental groups were added.
Group 10: ischemia (n = 6 Ob).
Ob animals were subjected to 60 minute of partial (70%) ischemia (as in group 2) but without 24 hours of reperfusion.
Group 11.
This group is divided into 2 subgroups. (11.1) Isolated perfused liver [n = 12 (6 Ln, 6 Ob]. Ob and Ln animals were subjected to I/R using an experimental model of isolated perfused liver as described previously.14 Briefly, steatotic and nonsteatotic livers were preserved 24 hours in University of Wisconsin solution. Livers were then connected via the portal vein to a recirculating perfusion system for 120 minutes. (11.2) Control [n = 12 (6 Ln, 6 Ob). Livers from Ln and Ob rats were flushed and immediately perfused ex vivo without ischemic preservation. The isolated perfused liver model was used to evaluate adiponectin in the liver after I/R, isolated from the influence of other organs and plasma constituents.
Group 12: I/R + adiponectin siRNA(2) (n = 6 Ob).
Ob animals were subjected to I/R (as in group 2) but treated 48 hours before ischemia with siRNA (100 nmol/kg) in phosphate-buffered saline (100 mL/kg) via hydrodynamic injection.11
Group 13: I/R + adiponectin siRNA(2) + adiponectin(2) (n = 6 Ob).
Same as group 12, but treated with adiponectin (1 μg/g intravenously) 30 minutes before ischemia.15
At the end of the protocol, we evaluated the mRNA and protein levels of adiponectin in the liver samples of groups 10–13.
The pretreatment times of adiponectin siRNA in groups 12 and 4.1 were different. In group 12, the aim was to reach the minimal expression of adiponectin mRNA needed to clarify whether the systemic administration of adiponectin leads to an accumulation of adiponectin in steatotic livers. In group 4.1, the aim was to reduce adiponectin to sham levels to clarify the possible role of adiponectin in the I/R process. In group 13, to evaluate the liver's capacity to take up adiponectin from the circulation, we used an adiponectin treatment that has proved to work in this context.15 In group 7, to evaluate adiponectin's role in warm hepatic I/R, we used another adiponectin treatment that has also proved to work under ischemic conditions.4
Reverse Transcription and Quantitative Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction analysis was performed using premade Assays-on-Demand TaqMan probes (Rn00566193_m1 for PPAR-α, Rn00595250_m1 for adiponectin, Rn01114954_g1 for AdipoR1, Rn01463173_m1 for AdipoR2 and Rn00667869_m1 for β-actin) (Applied Biosystems) according to the manufacturer's protocol.
Western Blotting of PPAR-α, Adiponectin Oligomers, AdipoR1 and AdipoR2, p38, JNK, and β-Actin.
Western blotting was performed as described13, 16 using the following antibodies: PPAR-α (Abcam, UK), adiponectin (Alexis Biochemical, Germany), AdipoR1 (Alpha Diagnostic, San Antonio, TX), AdipoR2 (Santa Cruz Biotechnology, Santa Cruz, CA), total p38, phospho-p38, total JNK and phospho-JNK (Cell Signaling Technology Inc., Beverly, MA), and β-actin (Sigma, Spain).
Biochemical Determinations
Adiponectin protein levels, triglycerides, IL-1β, TNF-α, MDA, H2O2, and ALT levels were measured following a previously described protocol.4, 10, 17
Histology and Red-Oil Staining
Hematoxylin-eosin–stained sections were evaluated using a point-counting method on an ordinal scale.10 To appraise the hepatic fatty droplet content, red-oil staining was performed using standard procedures.
Statistics
Data are expressed as the mean ± standard error and were compared statistically via analysis of variance followed by Student-Newman-Keuls test. A P value of <0.05 was considered significant.
Results
Role of PPAR-α in Hepatic I/R Injury.
PPAR-α mRNA and protein levels in nonsteatotic livers of the I/R and PC groups were similar to those found in the sham group (Fig. 1A–B). Accordingly, in nonsteatotic livers, the I/R + PPAR-α agonist did not protect against hepatic injury induced by I/R (data not shown).
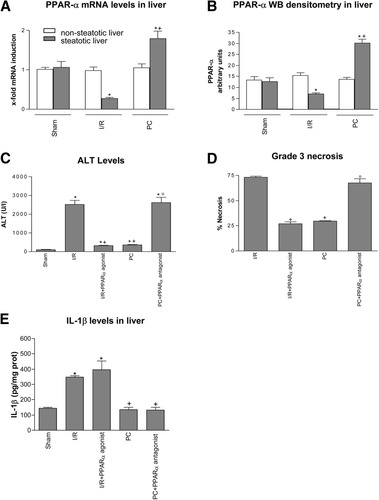
PPAR-α in steatotic liver. (A) Quantitative PPAR-α mRNA expression in nonsteatotic and steatotic liver. Polymerase chain reaction fluorescent signals for PPAR-α were normalized to polymerase chain reaction fluorescent signals obtained from an endogenous reference (β-actin). Comparative and relative quantifications on PPAR-α gene products normalized to β-actin and control sham group were calculated by 2-ΔΔCTmethod. Values are expressed as the mean ± standard error of 6 separate experiments. (B) PPAR-α Western blot densitometric analysis in nonsteatotic and steatotic liver. Values are expressed as the mean ± standard error of 6 separate experiments. (C) ALT, (D) percentage of grade 3 necrosis, and (E) IL-1β levels in steatotic livers. *P < 0.05 versus sham. +P < 0.05 versus I/R. °P < 0.05 versus PC.
In the presence of steatosis, a reduction in mRNA expression and protein levels of PPAR-α was observed in the I/R group compared with the results found in the sham group (Fig. 1A–B). PC increased mRNA expression and protein levels of PPAR-α in steatotic livers when compared with the I/R group. As shown in Fig. 1C, the I/R + PPAR-α agonist reduced ALT levels in steatotic livers with respect to those recorded in the I/R group. The PC + PPAR-α antagonist eliminated the beneficial effects of PC on hepatic injury resulting in aminotransferase levels similar to those obtained in the I/R group (Fig. 1C). The percentage of grade 3 necrosis (Fig. 1D) showed a similar pattern to that described for aminotransferases. Steatotic livers of the I/R group showed extensive and confluent areas of coagulative necrosis with neutrophil infiltration (Fig. 2A) that were reduced in number and extension in the I/R + PPAR-α agonist group (Fig. 2B). The hepatic lesions observed in steatotic livers of the PC (Fig. 2C) and PC + PPAR-α antagonist (Fig. 2D) groups were comparable to those observed in the I/R + PPAR-α agonist and I/R groups, respectively.

Histological lesions in steatotic liver. (A) I/R, (D) PC + PPAR-α antagonist, and (F) PC + adiponectin(1): widespread coagulative hepatic necrosis with hemorrhage and neutrophil infiltration (arrows). (B) I/R + PPAR-α agonist, (C) PC, and (E) I/R + adiponectin siRNA(1): small area of coagulative hepatic necrosis with neutrophil infiltration (arrows). (Hematoxylin-eosin staining.)
A previous study of our group revealed the injurious effects of IL-1β in steatotic livers undergoing I/R and the benefits of PC in reducing IL-1β generation.10 Although PPAR-α is likely to down-regulate IL-1 in gut I/R,18 under our conditions PPAR-α agonists and the activation of PPAR-α by PC seem to be unlikely to down-regulate IL-1β in steatotic livers. In fact, I/R + PPAR-α agonist and PC + PPAR-α antagonist groups had IL-1β levels similar to those observed in the I/R and PC groups, respectively (Fig. 1E).
Role of Adiponectin in Hepatic I/R Injury.
PPAR-α, adiponectin mRNA expression, and protein levels in nonsteatotic livers of the I/R and PC groups were similar to those found in the sham group (Fig. 3A–B). Thus, in nonsteatotic livers, I/R + adiponectin siRNA(1) did not protect against hepatic injury induced by I/R (data not shown).
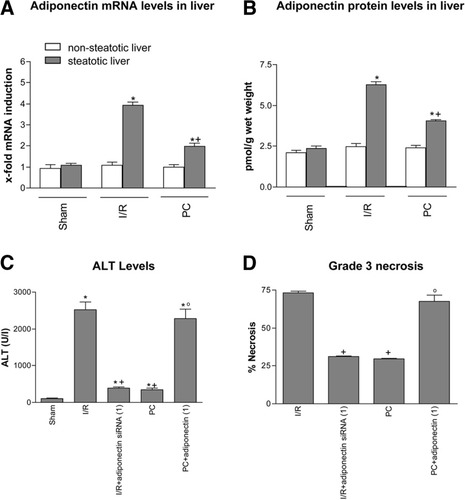
Adiponectin in steatotic liver. (A) Quantitative adiponectin mRNA expression in nonsteatotic and steatotic livers as described in Fig. 1A. (B) Adiponectin protein levels in nonsteatotic and steatotic liver. (C) ALT levels and (D) percentage of grade 3 necrosis in steatotic livers. *P < 0.05 versus sham. +P < 0.05 versus I/R. °P < 0.05 versus PC.
In the presence of steatosis, a marked increase in mRNA expression and protein levels of adiponectin was observed in the I/R group with respect to the sham group (Fig. 3A–B). PC reduced the mRNA expression and protein levels of adiponectin in the presence of steatosis when compared with the I/R group (Fig. 3A–B). Under the conditions evaluated herein, the increased adiponectin levels observed in steatotic livers in the I/R group were not associated with changes in adiponectin receptor expression (data not shown), a result similar to that described in white adipose tissue following β3-adrenoceptor agonist treatment.19 In the presence of steatosis, the results of hepatic injury revealed that I/R + adiponectin siRNA(1) resulted in lower biochemical and histological parameters of hepatic injury than those observed in the I/R group (Fig. 3C–D). PC + adiponectin(1) eliminated the benefits of PC on hepatic injury resulting in aminotransferase levels and grade 3 necrosis in steatotic livers similar to those obtained in the I/R group. The histological findings revealed that I/R + adiponectin siRNA(1) (Fig. 2E) reduced the extent and number of necrosis areas in steatotic livers compared with the I/R group (Fig. 2A). The hepatic lesions observed in the PC + adiponectin(1) group (Fig. 2F) were comparable to those observed in the I/R group (Fig. 2A). The potential of siRNA to inhibit adiponectin in steatotic livers of the I/R group was confirmed via real-time polymerase chain reaction (Fig. 4A–B). siRNA control and blank control groups showed adiponectin levels (Fig. 4A–B) and parameters of hepatic injury (Fig. 4C) similar to those observed in the I/R group, indicating that control siRNA failed to induce any significant suppressive activity.
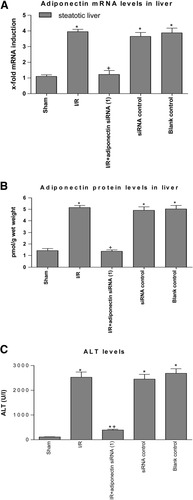
Adiponectin in Control experiments. (A) Quantitative adiponectin mRNA expression in steatotic livers as described in Fig. 1A. (B) Adiponectin protein levels and (C) ALT levels in steatotic livers. *P < 0.05 versus sham. +P < 0.05 versus I/R.
Analyses of triglyceride content and fatty droplet accumulation in steatotic livers revealed that, under our conditions, PPAR-α and adiponectin did not induce changes in liver steatosis (data not shown).
Role of TNF-α and MAPKs on Adiponectin Accumulation in Steatotic Livers Following I/R.
We also investigated the mechanisms that lead to increased adiponectin levels in steatotic livers. TNF-α increases adiponectin production in cultured cells.20 However, it appears unlikely that adiponectin expression observed in steatotic livers in the I/R group could be dependent on TNF-α. No significant increases in TNF-α levels were observed in steatotic livers of the I/R group compared with the sham group (data not shown). The minor role of TNF-α under the conditions evaluated in the present study has been reported previously.17 As shown in Fig. 5A, both p38 and JNK were notably phosphorylated in steatotic livers of the I/R group. Total p38 and JNK were unchanged in all groups (data not shown). Our results indicate that both I/R + p38 inhibitor and I/R + JNK inhibitor reduced adiponectin mRNA in steatotic livers compared with the results found in the I/R group (Fig. 5B). This effect was associated with a protection against oxidative stress and hepatic injury (Fig. 5C–D). The harmful effects of adiponectin on oxidative stress was confirmed by the reduction in MDA levels obtained in the I/R + adiponectin siRNA(1) group compared with the I/R group (Fig. 5C). H2O2 levels showed a similar pattern to that described for MDA (data not shown).
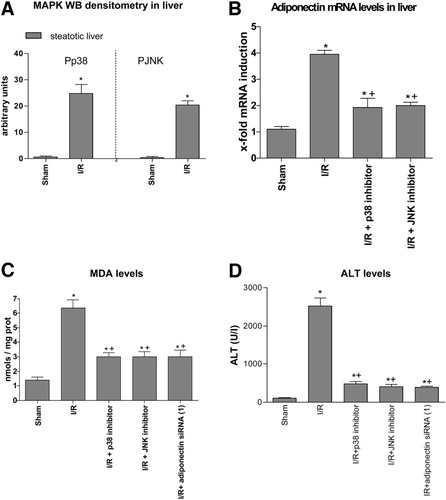
Effect of MAPKs on adiponectin. (A) Phosphorylated p38 and JNK Western blot densitometric analysis in steatotic liver. Values are expressed as the mean ± standard error of 6 separate experiments. (B) Quantitative adiponectin mRNA expression in steatotic livers as described in Fig. 1A. (C) MDA and (D) ALT levels in steatotic livers. *P < 0.05 versus sham. +P < 0.05 versus I/R.
As shown above, MAPKs (p38 and JNK) increased adiponectin mRNA expression (Fig. 5B), and adiponectin was responsible for reactive oxygen species generation in steatotic livers (Fig. 5C). Thus, strategies that inhibit MAPK expression would be able to reduce adiponectin accumulation in steatotic livers and its worsening effect on oxidative stress. Our results indicate that I/R + PPAR-α agonist and PC both reduced MAPK (p38 and JNK) expression in steatotic livers when compared with the I/R group (Fig. 6A). These effects on MAPKs were associated with a reduction in adiponectin mRNA and protein levels (Figs. 6B–C) and MDA levels (Fig. 6D). Conversely, the PC + PPAR-α antagonist increased MAPK expression in steatotic livers when compared with the PC group (Fig. 6A). This in turn was associated with an increase in adiponectin (Figs. 6B–C) and MDA levels (Fig. 6D). H2O2 levels showed a similar pattern to that described for MDA levels (data not shown).
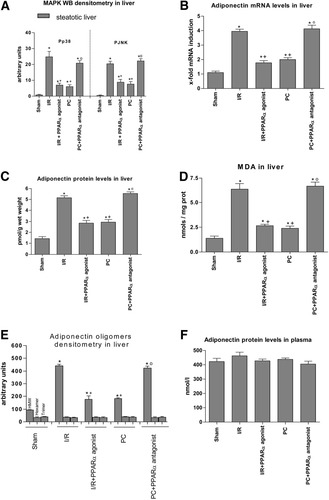
Effect of PPAR-α on MAPKs. (A) Phosphorylated p38 and JNK Western blot densitometric analysis. Values are expressed as the mean ± standard error of 6 separate experiments. (B) Quantitative adiponectin mRNA expression in steatotic livers as described in Fig. 1A. (C) Adiponectin protein levels, (D) MDA, and (E) densitometric analysis of adiponectin oligomers. Values are expressed as the mean ± standard error of 6 separate experiments. (F) Adiponectin protein levels in plasma of Ob rats. *P < 0.05 versus sham. +P < 0.05 versus I/R. °P < 0.05 versus PC.
Protocol 3: Assessment of the Predominant Source of Adiponectin in Damaged Steatotic Livers.
The results of this study reveal a close correlation between adiponectin mRNA levels and adiponectin protein levels in steatotic livers (Fig. 6B–C). This was in agreement with the results corresponding to adiponectin oligomers (Fig. 6E). Hepatic I/R increased the HMW isoform in steatotic livers without affecting trimeric and hexameric complexes (Fig. 6E). In addition, the changes in adiponectin mRNA and protein levels induced by the different treatments (Fig. 6B–C) were reflected in differences in HMW oligomers (Fig. 6E). Given the possibility that adiponectin may be taken up by the hepatic cells from the circulation21 and other studies indicating that adiponectin accumulates in the damaged heart primarily as the result of leakage from the vascular compartment,15 we also measured the adiponectin levels in plasma. However, no correlation between circulating adiponectin levels (Fig. 6F) and hepatic adiponectin levels (Fig. 6C) was observed. Our findings in livers undergoing 60 minutes of ischemia without reperfusion (Fig. 7A) and the isolated perfused liver model (Fig. 7B) indicated an increase in adiponectin mRNA and protein levels in steatotic livers, whereas no changes in adiponectin levels were observed in nonsteatotic livers. In addition, studies based on the systemic delivery of adiponectin (Fig. 7C) revealed that I/R + siRNA(2) + adiponectin(2) leads to the accumulation of adiponectin in steatotic livers comparable to that observed in the I/R + siRNA(2) group.
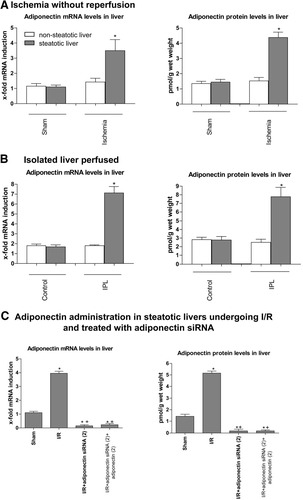
Adiponectin accumulation in steatotic liver in different experimental models of I/R. Quantitative adiponectin mRNA expression as described in Fig. 1A and adiponectin protein levels in steatotic livers in the following models of I/R are shown. (A) Ischemia without reperfusion. (B) Isolated liver-perfused and (C) adiponectin administration in steatotic livers undergoing I/R and treated with adiponectin siRNA. *P < 0.05 versus sham. +P < 0.05 versus I/R.
Discussion
Our results indicate that PPAR-α does not play a crucial role in I/R injury in nonsteatotic livers. This contrasts with a study published by Okaya and Lentsch,2 in which the authors reported the benefits of PPAR-α agonists on postischemic liver injury. Although the dose and pretreatment time of the PPAR-α agonist WY-14643 were similar in both studies, Okaya and Lentsch reported an ischemic period of 90 minutes; ours was 60 minutes, which is the ischemic period currently used in liver surgery.22 Thus, 60 minutes of ischemia seems to be insufficient to induce changes in PPAR-α in nonsteatotic livers. However, this was not the case in the presence of steatosis. We have shown that in contrast to nonsteatotic livers, the short-term administration of PPAR-α agonists (1 hour before ischemia) can limit the damage induced by I/R in steatotic livers. Although this was not the aim of the present study, research aimed at evaluating whether the long-term administration of these PPAR-α agonists might alleviate hepatic steatosis in steatotic livers before they undergo I/R would be of interest for clinical practice, because it could improve liver surgery outcomes. In fact, in clinical practice, long-term PPAR-α agonist administration (ie, 1 month) has been mainly applied as a hypolipidemic drug in liver disease.23 However, there are obvious difficulties concerning the feasibility of long-term PPAR-α agonist administration in some I/R processes—in particular liver transplantation from cadaveric donors, because this is an emergency procedure in which there is very little time to pretreat the donor with PPAR-α agonists.
To date, adipose tissue has been considered the major site for endogenous adiponectin production, although there are other potential sources, including the liver.3, 5 In contrast to nonsteatotic livers, increased adiponectin was detected in steatotic livers following I/R. Our results indicate that the predominant oligomeric form in steatotic livers was the HMW oligomer. This oligomer has been described as the most active form in the liver.24
Two hypotheses might explain why adiponectin is detected in the liver. The first hypothesis suggests that adiponectin may be taken up by the cells from the circulation.3, 21 Data obtained in fatty liver disease and liver cirrhosis21 support this hypothesis because they show that adiponectin mRNA was not detected in the liver. As previously reported,15 exhaustive investigations demonstrated that adiponectin accumulates in damaged tissues primarily as the result of leakage from the vascular compartment. The second hypothesis suggests that the liver might generate adiponectin.6, 25 Data obtained in models of concavalin A–mediated acute liver failure support this second hypothesis because they show increased expression of adiponectin mRNA in damaged livers when compared with healthy livers.6 Furthermore, other studies in nonalcoholic steatohepatitis found no correlation between circulating adiponectin levels and liver adiponectin expression.25 Our results concerning the interrelation of mRNA and protein adiponectin levels, as well as the lack of correlation between circulating and liver adiponectin levels, seem to be more in line with this second hypothesis. In addition, the results based on warm hepatic ischemia without reperfusion, the isolated perfused liver model, and the systemic delivery of adiponectin in livers treated with adiponectin siRNA suggest that steatotic livers by themselves can generate adiponectin as a consequence of I/R. Nevertheless, the possibility that steatotic liver can take up adiponectin from the circulation should not be ruled out. Further studies will be required to clarify this question.
In contrast with many studies that describe adiponectin as a promising candidate for the treatment of liver diseases, including endotoxin-induced liver injury, concavalin A–induced hepatotoxicity, and transplantation with small-for-size fatty grafts,4, 6, 7 the present study reports evidence of the injurious effects of adiponectin in stetatotic livers under warm ischemic conditions. The use of siRNA-based therapies to regulate the biological actions of mediators involved in a variety of diseases26 has attracted great interest in recent years. To the best of our knowledge, drugs that are able to inhibit adiponectin effects have not been reported. Our results suggest the clinical potential of gene therapy for I/R damage in steatotic livers by siRNA-mediated adiponectin gene silencing. RNA interference–based therapies meet all the criteria for therapeutic use: bioavailability, lack of toxicity and specificity of silencing effects, and efficacy in vivo.26
Data obtained in adipocytes treated with chronic growth hormone suggest that the signalling pathways involved in adiponectin production might include MAPKs.27 In agreement with this work, our results indicate that MAPKs (p38 and JNK) might be positive regulators of adiponectin gene expression in steatotic livers undergoing I/R. Previous data indicate that adiponectin increases oxidants in cultured hepatocytes.28 Our results suggest that reactive oxygen species production by adiponectin might be a possible mechanism to explain the negative effects of adiponectin on I/R in steatotic livers. In fact, it is well known that reactive oxygen species contribute to the vulnerability of steatotic livers to I/R injury.17 Thus, taking the results of the present study into account, the activation of MAPKs (p38 and JNK) in steatotic livers following I/R increased adiponectin levels, particularly HMW oligomers, thus inducing reactive oxygen species generation and hepatic injury.
PPAR-α may inhibit p38 in cardiomyocytes and JNK expression in macrophages.29, 30 Our results reveal that PPAR-α agonists as well as PC, through PPAR-α activation, reduced p38 and JNK expression. This would reduce adiponectin levels, particularly HMW, in steatotic livers, thus protecting against the injurious effects of adiponectin on oxidative stress and hepatic injury. Thus, in contrast with previous studies in the liver indicating that adiponectin is responsible for PPAR-α activation,7, 8 under the conditions reported here, strategies that modulated PPAR-α were associated with changes in adiponectin levels.
In conclusion, steatotic livers were found to be more predisposed to the down-regulation of PPAR-α and the overexpression of adiponectin, specifically the HMW isoform, when subjected to I/R. Pharmacological strategies aimed at modulating PPAR-α and adiponectin were irrelevant in nonsteatotic livers undergoing warm ischemia, whereas PPAR-α agonist pretreatment and adiponectin siRNA-based therapies protected steatotic livers. Our results suggest a potential pathway based on the relationship between MAPKs, adiponectin, and oxidative stress to explain the vulnerability of steatotic livers to I/R injury and the benefits of PPAR-α agonists and PC in this type of liver.
Acknowledgements
We are grateful to Robin Rycroft at the Language Advisory Service of the University of Barcelona for revising the English text.




