Identification of a novel class of dithiolethiones that prevent hepatic insulin resistance via the adenosine monophosphate–activated protein kinase–p70 ribosomal S6 kinase-1 pathway†
Potential conflict of interest: Nothing to report.
Abstract
Several established liver diseases of various causes are highly associated with hepatic insulin resistance, which is characterized by the desensitization of target cells to insulin. Peripheral insulin resistance is observed in most patients who have cirrhosis. Conversely, insulin-resistant diabetic patients are at increased risk for developing liver disease. Current therapeutic interventions in insulin resistance are limited and therefore likely to be advanced by new tailor-made drugs. Oltipraz, a prototype dithiolthione, inhibits transforming growth factor β1 and has the ability to regenerate cirrhotic liver. We investigated the effects of oltipraz and synthetic dithiolthiones on hepatic insulin resistance and the molecular basis of action. Oltipraz and other dithiolethione compounds were tested on tumor necrosis factor α (TNF-α)–induced insulin resistance and glucose homeostasis in vitro and in vivo via immunoblotting, plasmid transfection, kinase analysis, and functional assays. Oltipraz treatment inhibited the ability of TNF-α to activate p70 ribosomal S6 kinase-1 (S6K1) downstream of mammalian target of rapamycin, thus preventing insulin receptor substrate-1 serine phosphorylation and protecting insulin signals. Moreover, oltipraz activated AMP-activated protein kinase (AMPK), whose inhibition by a dominant negative mutant abolished S6K1 inhibition and protected insulin signaling, indicating that AMPK activation leads to S6K1 inhibition. In hepatocyte-derived cell lines, oltipraz inhibited glucose production. Oltipraz prevented hepatic insulin resistance in C57BL/6 mice challenged with endotoxin (or TNF-α), leptin-deficient mice, and mice fed a high-fat diet. Synthetic dithiolethiones comparably inhibited insulin resistance. Conclusion: Our findings led to the identification of dithiolethione compounds that prevent insulin resistance through a mechanism involving AMPK-mediated S6K1 inhibition and thereby sensitize hepatic insulin response. (HEPATOLOGY 2007.)
Insulin resistance and altered insulin metabolism are involved in the pathogenesis of liver disease. Established liver disease of any cause thereby leads to glucose intolerance, which is supported by the observations that approximately 80% of patients with cirrhosis are glucose-intolerant and that approximately 25% become frankly diabetic.1 Peripheral insulin resistance is observed in most cirrhotic patients. Conversely, insulin-resistant diabetic patients are at increased risk for developing a variety of liver diseases, including steatosis, viral hepatitis, and cirrhosis.2
The states of inflammation, stress, infection, and obesity commonly cause the secretion of tumor necrosis factor α (TNF-α), a toxic cytokine that promotes apoptosis of hepatocytes, amplifies inflammation, and may lead to insulin resistance. Improvements of insulin sensitivity by TNF-α signaling ablation in mice fed a high-fat diet or in leptin-deficient (Lepob/ob) mice proved the critical role of TNF-α in insulin resistance.3 The activation of insulin receptor (IR) transmits signals to mammalian target of rapamycin (mTOR)–p70 ribosomal S6 kinase-1 (S6K1) via phosphatidylinositol 3-kinase (PI3K). S6K1, when activated, blunts insulin signaling in a negative feedback action, and in pathological states, TNF-α secreted by inflammatory cells or adipocytes also stimulates S6K1.4 The physiological importance of S6K1 in insulin resistance is supported by a study on S6K1-null mice5 that implies that S6K1 is an attractive target for treating insulin resistance.
The regulatory mechanism of S6K1 is highly complex, and the activation of adenosine monophosphate–activated protein kinase (AMPK), a metabolic energy sensor, has been shown to block the mTOR-S6K1 pathway.6 It has also been shown that the actions of metformin and rosiglitazone converge on AMPK, which may contribute to insulin sensitivity enhancement.7 We hypothesized that the inhibition of S6K1 is feasible in vivo via AMPK activation using a new class of compounds.
The compound 4-methyl-5-(2-pyrazinyl)-1,2-dithiol-3-thione (oltipraz), a prototype dithiolthione, has been under investigation for the treatment of cirrhosis. The finding that oltipraz inhibits TGF-β1 and has the ability to regenerate cirrhotic liver8 provided the basis for its phase IIa trial for cirrhosis therapy. Its pharmacological effect is explained in part by PI3K-dependent gene regulation.9 The modulation of S6K1 by the PI3K pathway prompted us to consider whether oltipraz affects S6K1, and thereby inhibits insulin resistance, and if so, whether AMPK activation regulates S6K1-mediated insulin resistance. In this study, we investigated the effect of oltipraz on the TNF-α activation of S6K1 and found that oltipraz provides a novel means of treating hepatic insulin resistance. In addition, we synthesized a series of dithiolethione analogs and identified a new class of compounds with the ability to inhibit S6K1 via AMPK and thereby to prevent insulin resistance.
Abbreviations
AMPK, adenosine monophosphate–activated protein kinase; IR, insulin receptor; IRS1; insulin receptor substrate-1; Lepob/ob mice, leptin-deficient mice; LPS, lipopolysaccharide; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; S6K1, p70 ribosomal S6 kinase-1; TNF-α, tumor necrosis factor α.
Materials and Methods
Materials.
Cell lines were purchased from ATCC (Rockville, MD). Information on the expression constructs and antibodies are described in the Supplementary Methods section.
S6K1 and AMPK Activity.
S6K1 and AMPK kinase activities were assayed as described in the Supplementary Methods section.
Glucose Uptake and Production.
Glucose uptake was determined by 2-deoxy-D-[1-3H]glucose incorporation into the cells, whereas glucose production was assessed using the Amplex Red glucose/glucose oxidase assay kit (Invitrogen).
Chemical Synthesis of Oltipraz and Dithiolethione Analogs.
4-Methyl-5-(2-pyrazinyl)-1,2-dithiol-3-thione (oltipraz), 4-methyl-5-(2-pyrazinyl)-1,2-dithiol-3-one (M1), 5-pyrazinyl-1,2-dithiole-3-thione (CJ11764), 5-(6-methoxypyrzinyl)-4-methyl-1,2-dithiole-3-thione (CJ12064), 4-methyl-5-phenyl-1,2-dithiole-3-thione (CJ11842), 5-benzo[b]thiophene-3-yl-1,2-dithiole-3-thione (CJ11840), 4,5,6,7-tetrahydrobenzo-1,2-dithiole-3-thione (CJ11792), and 5,6-dihydro-4H-cyclopenta-1,2-dithiole-3-thione (CJ11788) were synthesized as described in the Supplementary Methods section.
Animal Treatments.
Animal experiments were conducted after the approval of the study protocol by the Animal Care and Use Committee at Seoul National University. The procedures are described in the Supplementary Methods section.
Results
Effects of Oltipraz on TNF-α–Activated Cell Signaling.
TNF-α is known to strongly induce IRS1 Ser307 phosphorylation. Because this phosphorylation is a molecular marker of insulin resistance,10 we investigated the effect of oltipraz on TNF-α–induced IRS1 phosphorylation at Ser307. Exposure of H4IIE cells to TNF-α resulted in IRS1 Ser307 phosphorylation, which was blocked by oltipraz (Fig. 1A). Similar inhibition by oltipraz of IRS1 serine phosphorylation was observed in C2C12 myotubes and 3T3-L1 adipocytes. Figure 1B shows that oltipraz pretreatment at ≥30 μM for 1-6 hours markedly inhibited IRS1 serine phosphorylation.
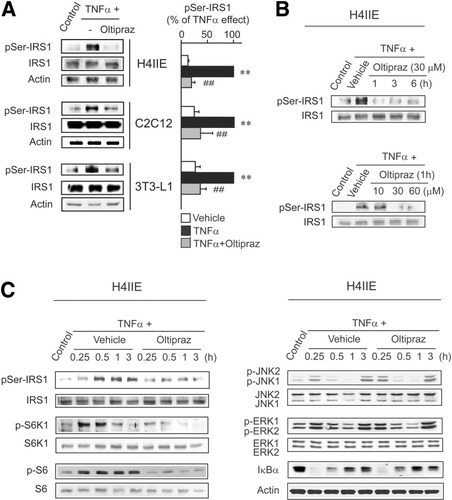
Oltipraz inhibits IRS1 serine phosphorylation and S6K1 activation. (A) IRS1 serine phosphorylation. Immunoblottings were performed in lysates of cells treated with 30 μM oltipraz for 1 hour and subsequently exposed to 10 ng/mL TNF-α for 1 hour. Data represent the mean ± SE for at least 3 separate experiments (significant compared with control [**P < 0.01] or TNF-α [##P < 0.01]). (B) Time and dose responses of oltipraz in H4IIE cells. (C) Oltipraz inhibition of S6K1 activation by TNF-α in H4IIE cells. Cells were treated with TNF-α for the indicated time periods with or without oltipraz pretreatment for 1 hour.
In addition, because IRS1 Ser307 phosphorylation is catalyzed by multiple kinases (e.g., S6K1, JNK, ERK, and IKK),11-14 we determined the activation statuses of the kinase signals. TNF-α induced S6K1 phosphorylation at Thr389, which preceded IRS1 serine phosphorylation (Fig. 1C). Phosphorylation of endogenous S6 at Ser235 partly supported S6K1 activation. Prolonged phosphorylation of S6 might be partly due to an overlapping specificity of S6K1 with other kinases such as S6K2 or RSK1. Oltipraz treatment essentially eliminated the ability of TNF-α to induce the phosphorylations of S6K1 and S6 (Fig. 1C), which concurred with the inhibition of IRS1 Ser307 phosphorylation. Oltipraz did not affect TNF-α–induced JNK or ERK phosphorylations or IκBα degradation. JNK is involved in TNF-α–dependent IRS1 serine phosphorylation.12 JNK inhibitor treatment (JNKI, 20 μM) inhibited it, as did oltipraz, confirming that JNK basally regulates TNF-α–induced IRS1 Ser307 phosphorylation (Supplementary Fig. 1).
Role of S6K1 Inhibition by Oltipraz on Insulin Signaling.
Having identified inhibition of S6K1 by oltipraz, we studied the functional role of S6K1 in the serine phosphorylation of IRS1 by TNF-α. Expression of a dominant negative mutant of S6K1 (DN-S6K1) significantly inhibited the TNF-α–induced Ser307 phosphorylation of IRS1, but not JNK1/2 phosphorylation or IκBα degradation (Fig. 2A). When a constitutively active mutant of S6K1 (CA-S6K1) was expressed, IRS1 serine phosphorylation was enhanced irrespective of the presence of TNF-α (Fig. 2B). S6 phosphorylation verified good transfection efficiency in this assay. Rapamycin inhibits mTOR-S6K1 activity by inducing the dissociation of the mTOR–raptor complex by binding FKBP12, and thus, IRS1 Ser307 phosphorylation is rapamycin-sensitive.15 Figure 2C confirms the complete inhibition of TNF-α–induced IRS1 serine phosphorylation by rapamycin.
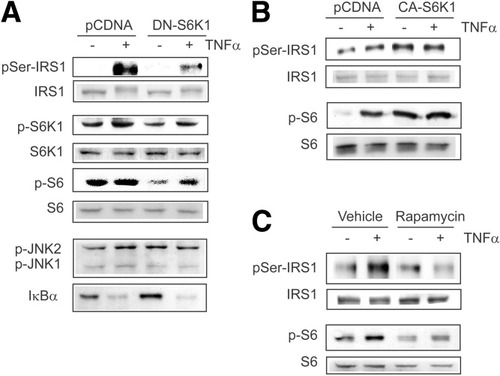
S6K1 regulates serine phosphorylation of IRS1. (A) DN-S6K1 inhibition of serine phosphorylation of IRS1. H4IIE cells transfected with pCDNA or a plasmid encoding DN-S6K1 were treated with TNF-α for 1 hour. (B) Increase in IRS1 serine phosphorylation by CA-S6K1. (C) Inhibition of IRS1 serine phosphorylation by rapamycin. Cells treated with 10 nM rapamycin for 1 hour were exposed to TNF-α for 1 hour.
We also examined the effects of oltipraz on the tyrosine phosphorylation impairment of IR and IRS1/2 by TNF-α (Fig. 3A). When H4IIE cells were pretreated with oltipraz prior to TNF-α exposure, a recovery of the insulin-stimulated tyrosine phosphorylations of IRβ and IRS1/2 was observed versus vehicle-treated controls. TNF-α treatment decreased the ratio of tyrosine/serine phosphorylations in IRS1, which was prevented by oltipraz pretreatment. Because tyrosine phosphorylations of IRβ and IRS1/2 transmit a positive signal to PI3K-Akt, insulin-stimulated Akt phosphorylation became defective after TNF-α treatment (Fig. 3B). Consistent with this finding, oltipraz restored the phosphorylations of Akt and GSK3β, a downstream effector of Akt, in cells challenged with TNF-α. Moreover, transfection of cells with CA-S6K1 completely eliminated the protective effects of oltipraz on IRβ and IRS1/2 tyrosine phosphorylations as well as Akt/GSK3β phosphorylations (Fig. 3C). DN-S6K1 transfection did not affect the protective effects of oltipraz (Supplementary Fig. 2). These results in conjunction with no increase in PI3K activity by oltipraz9 suggest that oltipraz protects insulin responses against TNF-α by inhibiting S6K1 activation.
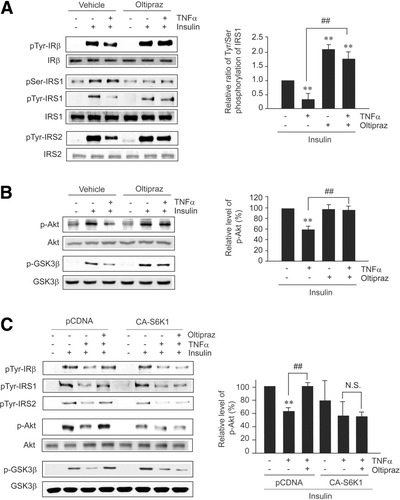
Oltipraz abrogates insulin resistance via S6K1 inhibition. (A) Phosphorylations of IRβ, IRS1/2, and IRS1. H4IIE cells pretreated with 30 μM oltipraz for 1 hour were incubated with TNF-α for 6 hours, and subsequently exposed to insulin for 10 minutes. Proteins in lysates were immunoprecipitated with specific antibodies and subjected to immunoblottings for phosphotyrosine or phosphoserine. (B) Akt and GSK3β phosphorylations. (C) Abrogation by CA-S6K1 of oltipraz's protection of insulin signals. Data represent the mean ± SE for at least 3 separate experiments (significant compared with vehicle [**P < 0.01] or TNF-α [##P < 0.01]).
Inhibition of S6K1 activity by oltipraz was also verified using S6K1 kinase assays. H4IIE cells treated with oltipraz showed significantly decreased S6K1 activity (Fig. 4A), which was comparable to that produced by rapamycin. Furthermore, we investigated whether oltipraz inhibits S6K1 in vitro. Incubation of S6K1 immunoprecipitates prepared from untreated cell lysates with oltipraz resulted in no change in S6K1 activity, thus supporting the notion that oltipraz does not directly affect S6K1. The inhibitory effect of oltipraz on S6K1 was also confirmed in HepG2 cells (data not shown). Next, we charted the dose–response curves of the phosphorylations of S6 (a major substrate of S6K1) and 4E-BP (mTOR substrate). Treatment of H4IIE cells with either oltipraz or rapamycin resulted in large decreases in S6 and 4E-BP phosphorylations (Fig. 4B), and their maximal decreases appeared to be comparable. Moreover, the combined inhibitory effect of oltipraz and rapamycin at minimal concentrations was greater than the sum of the inhibitory effects of each agent, thus supporting a notion of synergistic inhibition (Fig. 4C). Synergism was also observed in terms of insulin-induced Akt phosphorylation in cells treated concomitantly with oltipraz and rapamycin (Fig. 4D). In addition, oltipraz treatment did not affect the expression levels of FKBP12 and, unlike rapamycin, did not increase mTOR–FKBP12 complex formation (Fig. 4E), suggesting that oltipraz and rapamycin act through distinct pathways. Moreover, oltipraz pretreatment inhibited the phosphorylations of mTOR and S6K1 enhanced by insulin (Fig. 4F). These results, in conjunction with the lack of a direct inhibitory effect of oltipraz on S6K1 (Fig. 4A), suggest that the action site of oltipraz lies above mTOR. In H4IIE cells exposed to insulin for 8 hours, oltipraz was capable of inhibiting both IRS1/2 degradation and S6K1 phosphorylation (Fig. 4G). Our findings concerning the inhibitions of IRS1/2 degradation and of IRS1 serine phosphorylation by oltipraz corroborate its pharmacological intervention of insulin resistance.
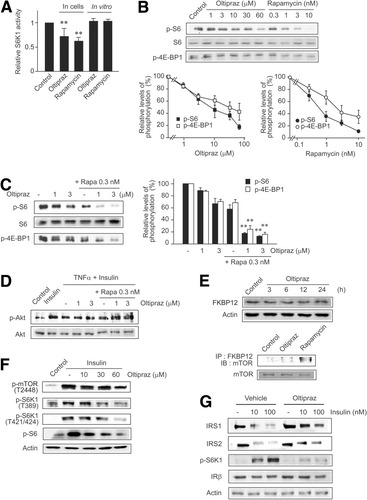
The effects of oltipraz on the mTOR–S6K1 pathway. (A) S6K1 kinase assays. H4IIE cells were treated with 30 μM oltipraz or 10 nM rapamycin for 1 hour (in cells). For in vitro assays (cell-free), S6K1 immunoprecipitates were incubated with oltipraz or rapamycin for 30 minutes and subjected to the kinase assays, as described in the Supplementary Methods section. Data represent the mean ± SE for at least 3 separate experiments (significant compared with control [**P < 0.01]). (B) Inhibition of S6 or 4E-BP1 phosphorylations by oltipraz or rapamycin. H4IIE cells were treated with oltipraz or rapamycin for 1 hour. (C) Combined effects of oltipraz and rapamycin on S6 and 4E-BP1 phosphorylations in H4IIE cells (significant compared with rapamycin alone [**P < 0.01]). (D) Combined effects of oltipraz and rapamycin on Akt phosphorylation in H4IIE cells. (E) Oltipraz's effect on the expression levels of FKBP12 in HepG2 cells. In addition, mTOR was immunoblotted in FKBP12 immunoprecipitates prepared from oltipraz- or rapamycin-treated cells (1 hour). (F) Oltipraz's inhibition of mTOR and S6K1 activation by insulin. H4IIE cells were treated with oltipraz for 1 hour and exposed to 10 nM insulin for 30 minutes in the continuing presence of oltipraz. (G) Inhibition of insulin-induced IRS1/2 degradation by oltipraz. H4IIE cells were treated with oltipraz for 1 hour and exposed to insulin for 8 hours in the continuing presence of oltipraz.
Activation of AMPK by Oltipraz Leads to S6K1 Inhibition.
AMPK inhibits the mTOR–S6K1 pathway.6 Having identified S6K1 as a regulatory target of oltipraz and considering the putative link between AMPK activation and mTOR-mediated S6K1 inhibition, we explored whether oltipraz activates AMPK. In H4IIE cells, oltipraz treatment increased ACC and AMPKα phosphorylations at 1-6 hours, and this was accompanied by commensurate decreases in S6 phosphorylation (Fig. 5A). This finding was also confirmed in other types of cells (e.g., HepG2 cells and 3T3-L1 preadipocytes). Oltipraz from a concentration of 10 μM induced the phosphorylations of ACC and AMPKα in a concentration-dependent manner, suggesting the role of AMPK activation in S6K1 inhibition by oltipraz. Furthermore, we measured cellular and in vitro AMPK activities. Immunocomplex kinase assays verified that oltipraz significantly increased cellular AMPK activities, as did metformin (Fig. 5B). However, oltipraz failed to activate purified AMPK, indicating that the agent is not a direct AMPK activator. Inhibition of AMPK activities by compound C validated the assay conditions.
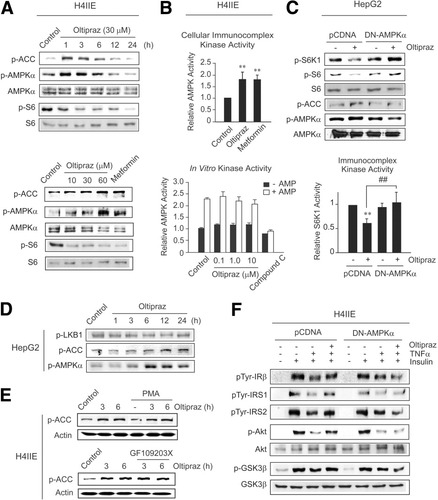
Oltipraz activates cellular AMPK that leads to S6K1 inhibition. (A) AMPK immunoblot analyses. Metformin (1 mM) was used as a positive control. (B) AMPK kinase assays. Cells were incubated with 30 μM oltipraz or 1 mM metformin for 3 hours and subjected to AMPK immunocomplex kinase assays. In vitro kinase activity was assessed using purified rat liver AMPK incubated with oltipraz or compound C (1 μM). (C) Reversal by DN-AMPKα of oltipraz's inhibition of S6K1 activity in HepG2 cells (oltipraz, 30 μM, 1 hour). (D) Oltipraz's effect on LKB1 phosphorylation. (E) Effects of PKC inhibitors (1 μM PMA, 18 hours; 10 μM GF109203X, 1 hour) on oltipraz's activation of AMPK. (F) Reversal by DN-AMPKα of oltipraz's protection of insulin signals.
We then examined the causal relationship between AMPK activation by oltipraz and S6K1 inhibition. Transfection of dominant negative AMPKα (DN-AMPKα) reversed the ability of oltipraz to inhibit the phosphorylations of S6K1 and S6 (Fig. 5C). In this experiment, HepG2 cells were used to observe S6K1 inhibition by oltipraz because basal S6K1 phosphorylation was higher in these cells than in H4IIE cells. Immunocomplex kinase assays verified antagonism by DN-AMPKα of S6K1 activity repression by oltipraz. In HepG2 cells, oltipraz treatment unaffected LKB1 phosphorylation (Fig. 5D). We additionally tested the possible role of PKC pathway in oltipraz's activation of AMPK. When H4IIE cells were treated with PMA (18 hours) or GF109203X (PKCδ inhibitor, 1 hour), ACC phosphorylations by oltipraz were unchanged (Fig. 5E). Moreover, we analyzed the inhibitory effects of DN-AMPKα on insulin action promoted by oltipraz. As expected, the introduction of DN-AMPKα abolished the ability of oltipraz to protect the insulin-stimulated phosphorylations of IRβ/IRS (tyrosine) and Akt/GSK3β against TNF-α (Fig. 5F). Our findings show that oltipraz activates AMPK, which then inhibits S6K1 and protects insulin sensitivity.
Functional Improvements in Glucose Production and Glucose Uptake by Oltipraz.
To link the protected insulin signals by oltipraz to functional improvements, we examined glucose homeostasis in hepatocyte, myotube, and adipocyte cell lines. Incubation of H4IIE cells with oltipraz resulted in a significant decrease in glucose production (Fig. 6A, left), the extent of which was comparable to that caused by insulin. However, because TNF-α treatment reduced glucose production in hepatocytes,16 the combined effect of oltipraz and TNF-α could not be assessed. In HepG2 cells, oltipraz completely inhibited glucose production, which was much greater than that caused by insulin treatment (Fig. 6A, right).
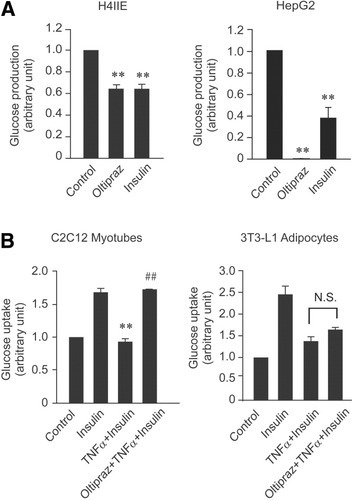
Oltipraz improves glucose homeostasis. (A) Inhibition of the basal glucose output by oltipraz. Cells were cultured in serum-free DMEM with or without 30 μM oltipraz or 10 nM insulin for 6 hours and continuously incubated with oltipraz or insulin in glucose-free DMEM for 3 hours. Glucose levels were measured in collected culture media. (B) The effects of oltipraz on insulin-induced glucose uptake. Cells were incubated in serum-free media and exposed to 10 ng/ml TNF-α with or without oltipraz for 6 hours. The cells were then treated with insulin for 10 minutes, and cellular glucose uptake was subsequently determined. Data represent the mean ± SE for at least 4 separate experiments (significant compared with insulin [**P < 0.01] or TNF-α plus insulin [##P < 0.01]).
To determine whether oltipraz has an insulin-sensitizing effect, we used C2C12 myotubes. Incubation of myotubes with TNF-α completely inhibited the glucose uptake stimulated by insulin, whereas oltipraz pretreatment abrogated this inhibition (Fig. 6B). However, in differentiated 3T3-L1 adipocytes, glucose uptake was not significantly changed by oltipraz treatment.
In Vivo Inhibition of Hepatic Insulin Resistance by Oltipraz and Its Hypoglycemic Effect in Murine Models.
Endotoxemia, which accompanies large increases in cytokines, provides an experimental model for investigating the relationship between elevated TNF-α levels and insulin resistance. Here, we first assessed the hypoglycemic effect of oltipraz in an insulin-resistance state induced by endotoxemia. Mice administered lipopolysaccharide (LPS) exhibited hyperglycemia 40-80 minutes after administration (Fig. 7A), consistent with a previous observation.17 Mice treated with oltipraz in combination with LPS displayed hypoglycemic responses versus LPS-treated controls. Moreover, oltipraz did not change normal blood glucose levels in animals not administered LPS. Marked increase in plasma TNF-α levels were confirmed after LPS challenge (Fig. 7B), and although oltipraz treatment tended to reduce LPS-induced TNF-α production, the amount of TNF-α in the blood was substantial, indicating that the hypoglycemic effect of oltipraz is not achieved by inhibiting TNF-α production.
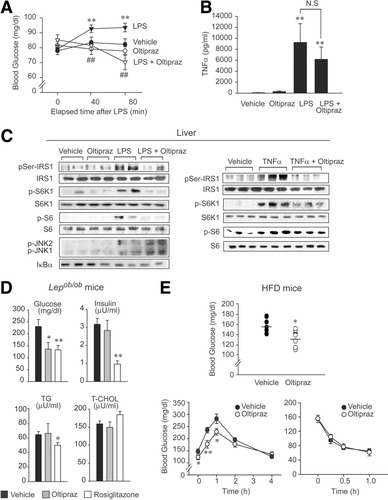
In vivo inhibition of insulin resistance by oltipraz. (A) Hypoglycemic effect of oltipraz in endotoxemic mice. Mice were gavaged with a single dose of 30 mg/kg oltipraz and 90 minutes later were intraperitoneally injected with 20 mg/kg LPS. Fasting blood glucose levels were measured (n = 6 animals) and were significant compared with LPS (##P < 0.01). (B) Serum TNF-α contents. TNF-α levels were measured 80 minutes after LPS challenge in mice treated as described in (A). (C) Immunoblot analyses. Liver homogenates were prepared from LPS-treated mice, as described in panel A. Similarly, mice were treated with oltipraz, intraperitoneally injected with 10 μg/kg TNF-α, and 1 hour later liver homogenates were prepared for immunoblottings. (D) Hypoglycemic effect of oltipraz in Lepob/ob mice. The parameters in blood were determined 1 hour after final dosing in Lepob/ob mice orally administered vehicle, oltipraz (30 mg/kg/day, every 2 days, 3 times) or rosiglitazone (10 mg/kg/day, 5 consecutive days) for 1 week (nonfasting) (n = 6 animals). (E) Hypoglycemic effect of oltipraz in mice fed a high-fat diet. Fasting blood glucose contents (upper) were monitored in mice that had been fed a high-fat diet for 9 weeks and administered oltipraz (30 mg/kg/d, every 2 days, 3 times) during the last week (n = 8 animals). Glucose tolerance (lower left) and insulin tolerance (lower right) tests were conducted in mice fed a high-fat diet for 10 weeks and administered oltipraz during the last 2 weeks (n = 8 animals). Data represent the mean ± SE (significant compared with vehicle [*P < 0.05, **P < 0.01]).
As expected, oltipraz was found to completely abolish the ability of LPS to induce Ser307 phosphorylation of IRS1 in liver, and this paralleled its hypoglycemic action. Oltipraz treatment abrogated the phosphorylations of both S6K1 and S6 by LPS (Fig. 7C), but the abilities of LPS to phosphorylate JNK1/2 and to degrade IκBα were unaffected. We also examined the effects of oltipraz on IRS1 and S6K1 phosphorylations in the livers of mice injected with TNF-α. TNF-α administration was found to markedly enhance IRS1 serine phosphorylation and to concomitantly activate S6K1, and both were notably diminished by pretreating mice with oltipraz. To test the hypoglycemic action of oltipraz under another insulin-resistant condition, we next assessed its effect in Lepob/ob mice, which show insulin resistance, hyperglycemia, and sustained insulin secretion. The results presented in Fig. 7D show that oltipraz exhibited a significant hypoglycemic effect, as did rosiglitazone. Moreover, oltipraz did not reduce plasma insulin levels in Lepob/ob mice, whereas rosiglitazone treatments did, suggesting that the mechanistics underlying the hypoglycemic effect of oltipraz differ from those of rosiglitazone. This possibility was strengthened by the finding that oltipraz has no transactivation potential of peroxisome proliferator-activated receptor γ compared with rosiglitazone (Supplementary Table 1). In addition, it was noted that the elevated plasma triglyceride and total cholesterol levels in Lepob/ob mice were unchanged by oltipraz. Oltipraz was also administered to determine its effects on insulin resistance in mice that had been fed a high-fat diet for 9 weeks, and the basal or glucose-stimulated blood glucose levels were found to be significantly lower in oltipraz-treated animals (Fig. 7E). Insulin tolerance test, however, showed no significant change. Oltipraz's effect on plasma insulin level could not be assessed in this model, because high-fat diet feeding did not increase it. The in vivo hypoglycemic effect of oltipraz shown here is in agreement with the cell culture data (Fig. 6) because liver and skeletal muscle are major contributors to glucose homeostasis in blood.
Pharmacological Effects of Dithiolethione Analogs.
Based on the novel pharmacological effects of oltipraz, we synthesized a series of new dithiolethione analogs to identify candidates capable of inhibiting S6K1. Among the dithiolethione derivatives synthesized, we identified several compounds that activate AMPK and would thus be expected to inhibit S6K1. Figure 8A shows that dithiolethione compounds containing a pyrazine ring and a major metabolite (M1) of oltipraz activated AMPK and inhibited S6K1, as evidenced by changes in ACC and S6 phosphorylations. Moreover, other dithiolethiones in Fig. 8A also promoted ACC phosphorylation but reduced S6 phosphorylation. Thus, our data support the concept that an active moiety capable of AMPK activation exists in dithiolethione.
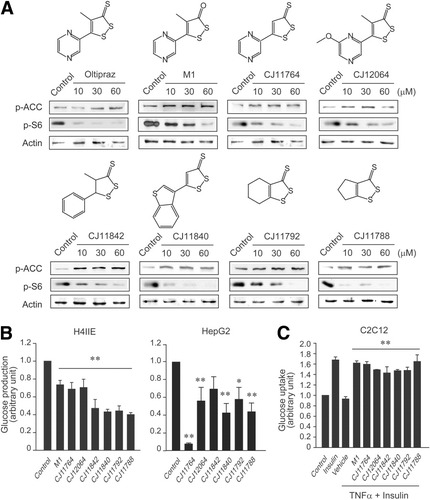
Comparative activity of dithiolethione derivatives to inhibit insulin resistance. (A) Chemical structures of dithiolethiones and their effects on ACC and S6 phosphorylations. H4IIE cells were treated with each agent for 6 hours. (B) The basal glucose output (significant compared with control [**P < 0.01]). (C) Insulin-induced glucose uptake. Glucose output and uptake were measured in the cells treated with 30 μM each candidate, as described in the legends to Fig. 6. Data represent the mean ± SE for at least 4 separate experiments (significant compared with TNF-α plus insulin [**P < 0.01]).
We next examined the effects of candidates on glucose production in H4IIE or HepG2 cells. All of the derivatives that increase ACC phosphorylation significantly reduced glucose production like oltipraz in H4IIE cells (Fig. 8B). In HepG2 cells, these compounds comparably inhibited glucose production with a notable inhibition being observed after CJ11764 treatment. Finally, we assessed whether these compounds block the ability of TNF-α to inhibit insulin-dependent glucose uptake into C2C12 myotubes. All of the derivatives examined almost completely restored glucose uptake (Fig. 8C), which supports our conclusion that this novel class of dithiolethione compounds controls glucose homeostasis and improves insulin action via AMPK-mediated S6K1 inhibition.
Discussion
The present study demonstrates that oltipraz efficaciously inhibits TNF-α–induced IRS1 serine phosphorylation in H4IIE cells, C2C12 myotubes, and 3T3-L1 adipocytes. As reported by others and ourselves, the daily dose of oltipraz required to effectively treat cirrhosis in humans is approximately 100 mg, and its estimated upper plasma Cmax in clinical situations is approximately 1.7 μM.18 Because levels of oltipraz in liver tissue are 20-fold greater than in plasma,18 the concentration required for the effective inhibition of insulin resistance appears to be pharmacologically achievable in target tissue.
The mTOR–S6K1 pathway underlies the emergence of insulin resistance. A study that used an S6K1 knockout model suggested the crucial role of S6K1 and the physiological importance of negative feedback from mTOR–S6K1 to PI3K for insulin resistance in vivo.5 The inhibitory effects of a high-fat diet on the IR–PI3K pathway may also be mediated by S6K1. Nevertheless, no therapy targeting S6K1 is currently available. TNF-α treatment causes activation of S6K1 by phosphorylating Thr389, a site specifically phosphorylated by mTOR. A key role for mTOR–S6K1 signaling in IRS1 phosphorylation is suggested by the initial observation that rapamycin inhibited IRS1 phosphorylation at Ser307.19 Our finding that rapamycin inhibits S6K1 activity and prevents IRS1 serine phosphorylation also confirms the S6K1 pathway to be a therapeutic target in cases of insulin resistance. In the present study, we investigated the effects of oltipraz on insulin resistance in an effort to identify molecular targets. We observed that oltipraz prevents S6K1 activation and protects tissues from IRS1-mediated insulin resistance, an observation strengthened by the results of our DN-S6K1 and CA-S6K1 experiments.
S6K1 inhibition enhances Akt phosphorylation in response to insulin because Akt lies at the crossroads of growth factor signaling and upstream of mTOR.5 Inhibition of S6K1 by oltipraz allowed cells to protect Akt and GSK3β phosphorylations in response to insulin. Our finding that oltipraz protects the tyrosine phosphorylations of IRβ and IRS1/2 against TNF-α in hepatocytes, and that it promotes glucose uptake by myotubes, adds to the conclusion that oltipraz improves insulin signaling networks. Moreover, enhanced glucose uptake by muscle cells may account for the hypoglycemic effect of oltipraz because skeletal muscle is the major consumer of plasma glucose. Previously, we identified CUGBP1-mediated LIP (CCAAT/enhancer-binding protein β-liver-enriched inhibitory protein) production by oltipraz, which inhibits preadipocyte differentiation.20 However, it is unclear why oltipraz did not improve glucose uptake in differentiated adipocytes, although this might be due to possible adipocyte de-differentiation as occurs after TNF-α treatment.
AMPK, an intracellular sensor of energy status, has been suggested as a drug target for diabetes. Activation of AMPK leads to mTOR inhibition through TSC2.6 Our findings showing that oltipraz activates AMPK and inhibits mTOR phosphorylation suggest that oltipraz's inhibition of S6K1 depends on the AMPK–TSC2–mTOR–S6K1 pathway. Moreover, AICAR, an AMPK activator, mimic the effects of insulin on gluconeogenic gene expression, and thereby inhibits glucose production. Therefore, it is probable that the inhibition of glucose production in hepatocytes by oltipraz might be associated with AMPK activation. Our data showing that S6K1 inhibition via AMPK activation by oltipraz supports the identification of a novel hypoglycemic agent. It appears that oltipraz is not a direct AMPK activator. Our speculations for cellular AMPK activation by oltipraz are: (1) the stimulation of AMPK kinases, such as LKB1 and Ca2+/calmodulin-dependent protein kinase kinase; (2) the concerted transition of the conformation of AMPK, which increases its affinity for substrate binding; (3) the inhibition of AMPK phosphatase, PP2C; and (4) changes in cellular or subcellular AMP/ATP ratios. Further studies are required to fully elucidate the mode of action of oltipraz.
A single dose of oltipraz treatment completely abolished LPS- or TNF-α–induced insulin resistance in liver, confirming the effectiveness of this compound for the prevention of hepatic insulin resistance. Moreover, this study demonstrates that oltipraz has blood glucose–lowering effects in LPS-induced hyperglycemic or Lepob/ob mice or in mice fed a high-fat diet. In the Lepob/ob mice experiment, the equivalent daily dose and the hypoglycemic efficacy of oltipraz in the 1-week acute dose regimen were found to be comparable to those of rosiglitazone. It has been shown that the hypoglycemic effects of peroxisome proliferator-activated receptor γ agonists led to the inhibition of hyperinsulinemia. Our finding that oltipraz did not affect levels of blood insulin suggests that oltipraz has a hypoglycemic mechanism, which distinguishes it from peroxisome proliferator-activated receptor γ agonists. This suggestion is supported by the finding that oltipraz does not activate peroxisome proliferator-activated receptor γ. No effect by oltipraz on hyperinsulinemia may have resulted from its possible insulin secretion; this aspect requires further study. Oltipraz treatment therapeutically decreased elevated blood glucose levels in mice fed a high-fat diet, verifying its bona fide effect against insulin resistance.
In this study, we identify other dithiolethione analogs as candidate activators of AMPK and thus inhibitors of S6K1 activity. All of these compounds reduced glucose production in hepatocytes and increased glucose uptake in muscle cells, much like oltipraz. Presumably, their AMPK activation would contribute to the inhibition of glucose production in hepatocytes, whereas S6K1 inhibition mediated by AMPK activation would sensitize glucose uptake in muscle cells. In addition, we confirmed the effectiveness of dithiolethiones in terms of reversing the insulin resistance caused by hyperosmolarity. Future in vivo pharmacodynamic and pharmacokinetic studies of these candidates are required to confirm their therapeutic effectivenesses. The fact that IRS1-mediated signaling is protected by dithiolethiones via S6K1 inhibition and that S6K1 inhibition depends on AMPK offers a route toward unraveling the AMPK–S6K1 pathway as a novel approach to pharmaceutical intervention in cases of insulin resistance. In terms of therapeutic potential, the findings presented here demonstrate the existence of a new class of compounds that may function in a unique manner and offer the possibility of treatment for hepatic insulin resistance.




