Lack of chemokine receptor CCR5 promotes murine fulminant liver failure†
Potential conflict of interest: Nothing to report.
Abstract
Fulminant liver failure (FLF) consists of a cascade of events beginning with a presumed uncontrolled systemic activation of the immune system. The etiology of FLF remains undefined. In this study, we demonstrate that CCR5 deficiency promotes the development of acute FLF in mice following Con A administration by preventing activated hepatic CD1d-restricted NKT cells (but not conventional T cells) from dying from activation-induced apoptosis. The resistance of CCR5-deficient NKT cells from activation-induced apoptosis following Con A administration is not due to a defective Fas-driven death pathway. Moreover, FLF in CCR5-deficient mice also correlated with hepatic CCR5-deficient NKT cells, producing more IL-4, but not IFN-γ, relative to wild-type NKT cells. Furthermore, FLF in these mice was abolished by IL-4 mAb or NK1.1 mAb treatment. We propose that CCR5 deficiency may predispose individuals to the development of FLF by preventing hepatic NKT cell apoptosis and by regulating NKT cell function, establishing a novel role for CCR5 in the development of this catastrophic liver disease that is independent of leukocyte recruitment.
Ajuebor MN, Aspinall AI, Zhou F, Le T, Yang Y, Urbanski SJ, Sidobre S, Kronenberg M, Hogaboam CM, Swain MG. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells. J Immunol 2005;174:8027–8037. (Copyright 2005 The American Association of Immunologists, Inc. Reprinted with permission.)
Comment
CCR5 is a CC chemokine receptor that is expressed on various cell types including activated Th1 cells, cytotoxic T cells, natural killer (NK) cells, natural killer T (NKT) cells, macrophages, and dendritic cells.1, 2 The overall function of chemokines and their receptors is recruitment of various leukocyte subtypes to sites of inflammation. However both, CCR5 as well as the CXC chemokine receptor CXCR4 are the main co-receptors used by HIV-1 for entering their CD4+ target cells.3 Notably, 1% of the Caucasian population harbors a homozygous polymorphism for CCR5, i.e., CCR5Δ32, which is a loss of function mutation. These individuals are resistant to HIV infection and even persons harboring only one CCR5Δ32 allele, thus being heterozygous for this mutation, have delayed progression of HIV disease.3 Therefore, these chemokine receptors have been intensively studied as novel targets for development of antiviral drugs. Besides some modified chemokines with antiviral activity, several low-molecular weight CCR5 and CXCR4 antagonists with potent antiviral activity have been described.3 With respect to chronic viral hepatitis, hepatitis C virus (HCV) carriers homozygous or heterozygous for CCR5Δ32 developed milder hepatic inflammation4, 5 which was associated with an increased incidence of spontaneous viral clearance in one study.5 However, the other study reported an increase in severe hepatic fibrosis associated with the mutation.4 Moreover, in patients with primary sclerosing cholangitis, the frequency of CCR5Δ32 was clearly increased among patients with severe liver disease6 and the CCR5Δ32 mutation seems to be a risk factor for development of ischemic-type biliary lesions after orthotopic liver transplantation.7
Recently, two reports provided evidence for a significant role of CCR5 in attenuation of immune-mediated hepatitis in the model of concanavalin A (ConA) induced liver injury in mice.8, 9 ConA-induced immune hepatitis depends on CD4+ conventional T cells, NKT cells, Kupffer cells as well as on a Th1 cytokine response with tumor necrosis factor (TNF) α and interferon (IFN) γ representing major mediators of liver damage.10 Notably, interleukin (IL) 4, a cytokine being produced at high concentrations together with IFN-γ upon NKT cell activation, also seems to mediate ConA hepatitis.10 Moreover, the Fas-driven death receptor pathway seems to be involved in hepatocyte death in this model.10
In the first report investigating the role of CCR5 in ConA-induced hepatitis, Ajuebor et al.8 described that genetic deficiency of CCR5 aggravated liver damage by preventing activated hepatic NKT cells from dying by activation-induced apoptosis. NKT cells, the majority of which is characterized by a restricted T cell receptor (TCR) repertoire, i.e. an invariant Vα14-Jα18 chain in mice and the homologous Vα24-Jα18 chain in man, both paired to a restricted set of Vβ chains, have been implicated in either up- or downregulation of immune responses.11 Most of them express a phenotype of activated T cells as well as NK cell determinants and the majority of hepatic NKT cells are CD4+ or double negative. NKT cells recognize glycolipid antigens, e.g., the surrogate antigen α-galactosylceramide, presented by the monomorphic MHC class I-like molecule CD1d. Upon activation in vivo, NKT cells secrete high amounts of cytokines, mainly IL-4 and IFN-γ.11 As mentioned above, these cells have been shown to mediate the immune-pathology in the Con A model. In their report, Ajuebor and colleagues8 showed that shortly after activation, NKT cells, identified by CD1d-α-galactosylceramide tetramer, disappeared from liver tissue of Con A-treated mice by dying from apoptosis, possibly in terms of immune-regulation. However, in CCR5-deficient mice, the percentage and absolute number of annexin V-positive hepatic NKT cells was significantly lower and, vice versa, the absolute number of viable NKT cells was significantly higher than those observed in wild-type mice 90 min following Con A injection. The resistance of CCR5-deficient NKT cells to apoptosis was not due to a defective Fas-driven apoptosis pathway,8 however, one could speculate that CCR5 facilitates death signals by itself.12 Obviously, hepatic CCR5-deficient NKT cells produced more IL-4, but not IFN-γ, relative to wild-type NKT cells, which mediated the aggravation of liver damage. The effect of IL-4 could have been due to either increased FasL expression on NKT cells,8 or to potentiation of TNF-α production.13 Taken together, this gene deletion approach substantiates the previous findings mentioned above regarding the critical role for CCR5 in potentiating immune-mediated liver damage.
In human liver, where invariant NKT cells represent only a minority of the T cell population in healthy persons, CD1d-reactive NKT cells with more diverse TCR are present at higher frequency.14 These cells become activated to a Th1-biased cytokine response in patients infected with HCV and might contribute either to protective as well as destructive immune-responses in the liver. Hence, a blockade of their CCR5 might result in aggravation of the Th1-response because of defective apoptosis of these cells, possibly associated with increased virus clearance on the one hand, but also with enhancement of the Th1-related immune-pathology on the other hand. However, in chronic viral hepatitis, hepatic invariant NKT cells increase in number and seem to contribute to cirrhosis progression by a Th2-biased cytokine response,15 indicating again that inhibition of NKT cell apoptosis might have deleterious consequences during liver disease.
In the second study published in HEPATOLOGY by Moreno et al.9 the authors also investigated the outcome of ConA-induced hepatitis in CCR5-/- mice. They obtained similar results with respect to increased susceptibility of the knockout animals towards Con A. Moreover, they found a strong up-regulation not only of IL-4 and TNF-α but also of the CCR5 ligands CCL3, CCL4, and CCL5. Importantly, the authors detected increased numbers of intrahepatic CD4+, CD8+, CD3+NK1.1+ as well as CD11b+ cells and a strong up-regulation of CCR1+ expressing liver mononuclear cells 8 hours following Con A administration compared to wild-type mice. In view of the fact that CCL3 and CCL5 also bind to other chemokine receptors, i.e. CCL3 binds CCR1, and CCL5 binds both, CCR1 and CCR3, it seems obvious that increased plasma or tissue concentrations of the chemokines led to enhanced recruitment of CCR1+ mononuclear cells to livers of CCR5-/- mice. Indeed, CCR1 has been shown to be an important chemokine receptor in the pathogenesis of Con A hepatitis, mediating the recruitment of CD4+ IFN-γ-producing effector T cells to the liver.16 Accordingly, either neutralization of CCL5 in CCR5-/- mice9 or antagonistic methionylated CCL5 or a dual CCR1/CCR5 peptide antagonist16 prevented Con A-induced hepatitis. Therefore, the absence of CCR5 might result in an increased production of its ligands in terms of counter-regulation or, alternatively, in a lack of trapping the chemokines by CCR5. Increased concentrations of the chemokines CCL3 and CCL5 might enhance the recruitment of harmfully activated CD4+ effector T cells to the liver in dependence of CCR1+, thereby exacerbating liver damage in the absence of CCR5. In the presence of CCR5, however, CCR5+ lymphocytes are recruited to the liver at time points after the initial pro-inflammatory cytokine storm,9 indicating that these cells might exhibit immune-regulatory functions.
Indeed, a recent report by Wysocki et al. provided evidence for a critical role of CCR5 in the function of CD4+CD25+ regulatory T cells (Tregs).17 Tregs have been recognized to mediate peripheral tolerance, and the absence of this cell population is associated with autoimmune disease.18 Moreover, Tregs also inhibit allograft rejection.18 In the report mentioned above,17 CCR5 was upregulated on Tregs following T cell activation in a murine model of graft-versus-host disease (GVDH). CCR5 upregulation seemed to occur in lymphoid tissue and was critical for migration of Tregs to specific organs — including liver — where GVDH took place.17 Moreover, we have observed recently that active hepatitis in the ConA model is followed by a state of immune-suppression in case of remission, which is mediated by Tregs and IL-10 (A. Erhardt, M. Biburger, G. Tiegs, unpublished). Therefore, one can hypothesize that, according to the observations made in GVDH, Con A might stimulate CCR5 up-regulation on Tregs in the mesenteric lymph nodes thereby enhancing their capacity to migrate into the inflamed liver and to downmodulate the proinflammatory cytokine response of CD4+ effector T cells and NKT cells, most likely via production of IL-10 (Fig. 1). In analogy to the observation that CD4+CD25+ Tregs bind IL-2 via CD25, thereby trapping IL-2 thus preventing proliferation of effector T cells, an alternative explanation with respect to CCR5 expressing Tregs could be that these cells “neutralize” CCL3 and CCL5, which would otherwise activate CCR1 (Fig. 1).
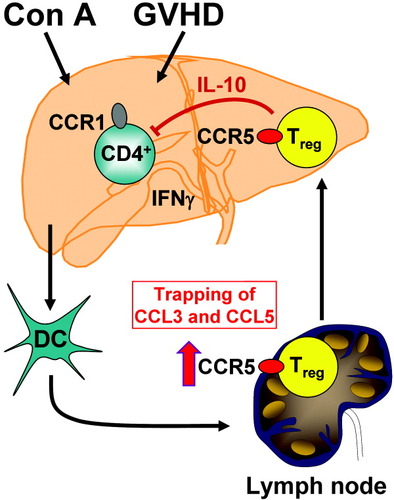
Hypothesis for the immune-regulatory function of CCR5 in hepatotoxicity. As in the case of graft-versus-host disease (GVDH) (17), concanavalin A (Con A) might activate regulatory T cells (Tregs) in the mesenteric lymph nodes to up-regulate CCR5. Thereby these cells become activated to migrate into the inflamed liver and to down-modulate the pro-inflammatory cytokine response of harmfully activated CD4+ effector T cells probably via release of interleukin (IL)-10. Alternatively, CCR5 expressing Tregs might trap CCL3 as well as CCL5 thereby preventing activation of CCR1. Antagonisation of CCR5 might worsen inflammation in the liver and probably also in other organs.
Thus, in view of the enhanced hepatotoxicity observed in the absence of CCR5 in the Con A model as well as in patients with the CCR5Δ32 loss of function mutation together with the newly described role of CCR5 in the function of Tregs and regulation of NKT cells activity, treatment of HIV patients with CCR5 antagonists might evoke hepatotoxicity, in particular in case of underlying liver disease, e.g., in case of co-infection with HCV. Indeed, two cases of severe hepatotoxicity have been reported recently in phase II clinical trials with a CCR5 entry inhibitor in combination with other anti-HIV drugs.19 This clearly demonstrates that occurrence of hepatotoxicity during treatment of HIV patients with CCR5 antagonists has to be carefully considered.




