Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: Role of cytochrome P450 2E1 and 2A5†
Potential conflict of interest: Nothing to report.
Abstract
The mechanisms by which alcohol causes liver injury are still not certain. Either LPS or CYP2E1 are considered independent risk factors involved in alcoholic liver disease, but mutual relationships or interactions between them are unknown. In the present study, the possible synergistic action of CYP2E1 and LPS in liver injury was investigated by evaluating the effects of pyrazole (inducer of CYP2E1), Chlormethiazole (CMZ), an inhibitor of CYP2E1, and CYP2E1-knockout mice. Mice were injected with pyrazole (150 mg/kg, ip) daily for 2 days, followed by LPS injection (4 mg/kg, ip). CMZ (50mg/kg, ip) was administered 15 h before and 30 min after LPS treatment, respectively. LPS-induced liver injury was enhanced by pyrazole, as indicated by pathological changes and increases in ALT and AST, and positive TUNEL staining. LPS-induced oxidative stress was also enhanced by pyrazole as indicated by increases in 4-hydroxy-2-nonenal and 3-nitrotyrosine adduct formation. CMZ protected against the pyrazole enhanced LPS liver injury and oxidative stress. CYP2E1 but also CYP2A5 were increased by the pyrazole/LPS treatment. CMZ decreased the elevated CYP2E1 activity by 90%, but CYP2A5 activity was also lowered (30%-50%). CYP2E1-knockout mice exhibited only minor liver injury after treatment with pyrazole/LPS, but wild-type mice exhibited severe liver injury. While no CYP2E1 was present in the CYP2E1 knockout mice, CYP2A5 activity was also lower. In conclusion, induction of CYP2E1 plays an important role in the enhancement of LPS liver injury by pyrazole, but some contribution by CYP2A5 cannot be excluded. (HEPATOLOGY 2006;44:263–274.)
The mechanisms by which alcohol causes liver injury are still not certain. Oxidative stress and lipopolysaccharide (LPS)-induced inflammatory signaling are two potential mechanisms that have been elucidated. Increased production of reactive oxygen species (ROS) has been observed in alcoholic liver disease (ALD), and antioxidants appear to mediate some protective effects in experimental ALD.1 Oxidative stress has been suggested to play a central role in mechanisms of alcohol-induced damage.2 Many pathways have been suggested to play a key role in how oxidative stress is induced by ethanol, including Kupffer cell activation by LPS and induction of CYP (cytochrome P450) 2E1.3
CYP2E1 is an abundant protein in liver that can cause oxidative stress in the liver especially after induction by ethanol. Inhibitors of CYP2E1, such as malotilate and chlormethiazole (CMZ), are effective in preventing the development of experimental ALD.4 LPS is a component of the outer wall of gram-negative bacteria that normally inhabit the gut. LPS penetrates the gut epithelium only in trace amounts; however, LPS absorption can be elevated under ALD.5 LPS directly causes liver injury by mechanisms involving inflammatory cells such as Kupffer cells, and chemical mediators such as superoxide(O2·−), nitric oxide (NO), and cytokines.6-9
Based on the above, mutual relationships or interactions between CYP2E1 and LPS in ALD is of interest to evaluate. We previously demonstrated that LPS-induced rat liver injury is enhanced by pretreatment with the CYP2E1 inducer pyrazole and postulated that elevated CYP2E1 might play an important role in the pyrazole/LPS-induced liver injury.10 However, conclusive evidence for a role of CYP2E1 in this enhanced liver injury is lacking, e.g., pyrazole can dramatically inhibit hepatic catalase activity11, 12 and potential induction of other cytochrome P450s such as CYP2A513, 14 needs to be considered. In this report, studies with the CYP2E1 inhibitor CMZ and CYP2E1-knockout mice were carried out to directly address the role of CYP2E1 in pyrazole-enhanced LPS liver injury.
CMZ is thought to be a specific inhibitor of CYP2E1,15 and it has been shown to ameliorate CYP2E1-mediated liver damage in rats treated chronically with ethanol,16 in mice treated with acetaminophen17 or mice treated with Jo2/pyrazole.12 CYP2E1-knockout mice have been used in studies on toxicity of acetaminophen,18 carbon tetrachloride,19 chloroform,20 and alcohol.21 We therefore applied CMZ and CYP2E1-knockout mice to examine the role of CYP2E1 in pyrazole/LPS-induced liver injury.
Abbreviations
LPS, lipopolysaccharide; ROS, reactive oxygen species; ALD, alcoholic liver disease; CYP, cytochrome P450; CMZ, chlormethiazole; O2· −, superoxide; NO, nitric oxide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; 3-NT: 3-nitrotyrosine; HNE, 4-hydroxy-2-nonenal; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling; ONOO−,peroxynitrite; H2O2,hydrogen peroxide.
Materials and Methods
Animals and Treatments.
Male C57BL/6 mice (20g) were purchased from Charles River Breeding Laboratories (Boston, MA). CYP2E1-knockout mice were kindly provided by Dr. Frank J. Gonzalez (Laboratory of Metabolism, NCI, Bethesda, MD), and the offspring's of these mating pairs were used in this study. All mice were housed in temperature-controlled animal facilities with 12-hour light/dark cycles and permitted consumption of tap water and Purina standard chow ad libitum. The mice received humane care and experiments were carried out according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals and with approval of the Mount Sinai Animal Care and Use Committee.
Mice were injected intraperitoneally with pyrazole (Sigma, St. Louis, MO), 150 mg/kg body weight, once per day for 2 days, to induce liver CYP2E1, or 0.9% saline as control. After an overnight fast, LPS (Sigma), serotype 055:B5, biological activity (Limulus Amebocyte Lysate) no less than 500,000 EU/mg, 4 mg/kg body weight, or 0.9% saline as control were injected intraperitoneally between 9:00-10:00 AM. CMZ (Sigma) was injected at dosage of 50 mg/kg body weight 15 hours before and 30 minutes after LPS treatment, respectively. The total dose of CMZ was 100 mg/kg body weight. The mice were sacrificed at 3, 8, or 24 hours after LPS or saline injection. Blood was collected, and serum was separated. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed using diagnostic kits (Thermo Electron, Louisville, CO). Livers were excised into fragments; one aliquot of tissue was placed in 10% formalin solution for pathology while the other aliquots were stored at −20°C for subsequent assays.
Liver Pathology and Immunohistochemistry.
Liver sections were stained with H&E for pathological evaluation. Immunohistochemical staining for 3-nitrotyrosine (3-NT) and 4-hydroxy-2-nonenal (HNE) adducts were performed by using anti-nitrotyrosine adducts IgG (Upstate, Lake Placid, NY) and anti-4-hydroxy-2-nonenal Michael adducts IgG (Calbiochem, La Jolla, CA) and a rabbit ABC staining system (Santa Cruz Biotechnology, Santa Cruz, CA).
Caspase-3 Activity.
Caspase-3 activity was determined by measuring enzymatic cleavage of the substrate Ac-DEVD-AMC (Alexis Biochemicals, San Diego, CA) as previously described.10
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling (TUNEL) Assay.
DNA fragmentation (apoptosis) was assessed by TUNEL assay using an ApopTag in situ apoptosis detection kit (Chemicon, Temecula, CA). The numbers of positive stainings with apoptotic morphology were counted in 20 random fields per sample, and averaged numbers were calculated.
Index of Nitric Oxide Production.
Production of endogenous NO was determined by measuring nitrite+nitrate using a nitrate/nitrite colorimetric assay kit (Cayman Chemical Company, Ann Arbor, MI).
Cytochrome P450 2E1 and 2A5 Activities.
Hepatic microsomes were prepared as described.22 CYP2A5 activity was measured by assessing coumarin 7-hydroxylase activity.13, 14 CYP2E1 activity was measured by the rate of oxidation of p-nitrophenol to p-nitrocatechol.23
Western Blotting.
Western blotting for CYP2E1 was performed as described.10 Anti-CYP2E1 antibody was a gift from Dr. Jerome Lasker, Hackensack Biomedical Research Institute, Hackensack, NJ.
Measurement of Catalase Activity.
Catalase activity in homogenate was determined as described.24
Statistics.
Results are expressed as mean ±SD. Statistical evaluation was carried out by analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test. Results were considered statistically different if P was less than .05.
Results
Pyrazole Enhances LPS-Induced Liver Injury in C57BL/6 Mice.
We previously demonstrated the presence of LPS/pyrazole synergistic toxicity in rats.10 Initial experiments were carried out to establish the LPS/pyrazole synergistic toxicity model in mice in order to take advantage of the CYP2E1-knockout mouse model. In addition, for unknown reasons, CMZ added to LPS-treated rats resulted in their death (not seen with mice—described below). So the role of CYP2E1 in this synergistic toxicity could not be directly evaluated in the rat model. Saline, pyrazole, or LPS treatment alone did not cause significant increases in ALT and AST activities at different time points (Fig. 1A-B). In the LPS/pyrazole group, serum ALT and AST were not significantly increased at 3 hours and 8 hours, but dramatically increased at 24 hours (Fig. 1A-B). Pathological examination showed that in the LPS/pyrazole group, single necrotic cells were casually observed at 3 hours, some small necrotic foci were seen at 8 hours, but widespread small necrotic foci and large necrotic areas were seen at 24 hours (Fig. 1C, panels d, e and f). In the LPS group, necrosis was not observed at the three time points (Fig. 1C, panels a, b and c). The saline and pyrazole groups did not show any necrosis (data not shown). Caspase-3 activity did not change in the saline and pyrazole groups at any of the time points; however, in the LPS group caspase-3 activity increased at 3 hours, peaked at 8 hours, but decreased at 24 hours after the LPS administration. In the LPS/pyrazole group, an increase in caspase-3 activity was delayed to 24 hours after LPS administration (Fig. 2A). Saline and pyrazole groups had no TUNEL positive staining with apoptotic morphology; in the LPS group, TUNEL positive staining with apoptotic morphology casually appeared at 3 hours, peaked at 8 hours, and decreased at 24 hours (compared to 8 hours) (Fig. 2B). In the LPS/pyrazole group, positive staining with apoptotic morphology casually appeared at 3 hours, increased at 8 hours (comparable to the LPS group), and further increased at 24 hours. Quantification of the TUNEL staining is shown in Fig. 2C. The results on aminotransferase levels, liver morphology, and TUNEL staining indicate that pyrazole dramatically enhanced LPS-induced liver injury at 24 hours after LPS administration in C57BL/6 mice.
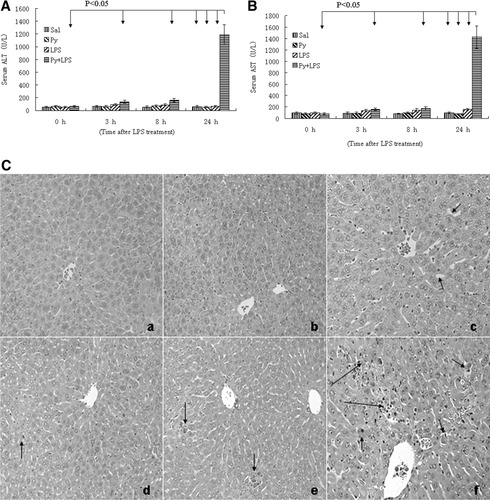
LPS-induced hepatic necrosis is enhanced by pyrazole in C57BL/6 mice. Pyrazole (150mg/kg) or saline was injected ip daily for 2 days followed by LPS (4mg/kg) or saline injection intraperitoneally. Blood and liver samples were collected at the indicated times after treatment with LPS, and aminotransferase levels were assayed as described in Materials and Methods. Serum ALT (A) and AST (B): Data are presented as mean+SD (n = 6). H&E stained liver sections (C): 3 hour (a) and 8 hour (b) after LPS injection to saline-treated mice, normal liver histology is observed; 24 hour (c) after LPS injection, sinusoid dilatation (arrow) is observed; 3 hour (d) after LPS plus pyrazole treatment, casual single necrotic cell (arrow) is found; 8 hours (e) after LPS plus pyrazole treatment, some small necrotic foci (arrows) are observed; 24 hours (f) after LPS plus pyrazole treatment, widspread small necrotic foci (short arrows) and large necrotic area (long arrow) are seen. Sal, saline; Py, pyrazole; LPS, lipopolysaccharide.
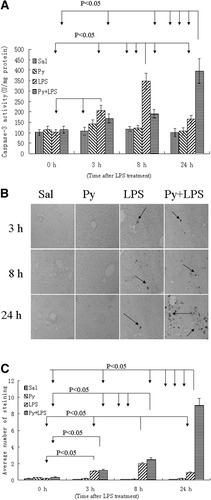
Caspase 3 activity and TUNEL staining in C57BL/6 mice. Pyrazole (150mg/kg) or saline was injected intraperitoneally daily for 2 days followed by LPS (4mg/kg) or saline injection intraperitoneally. Liver samples were collected at the indicated times after treatment with LPS, and caspase-3 activities and TUNEL were assayed as described in Materials and Methods. (A) Caspase-3 activities. Data are presented as mean±SD (n = 6). (B) TUNEL staining in liver sections. Arrows show positive staining. (C) Quantification of the number of TUNEL positive staining. Data are presented as mean±SD (n = 6). Sal, saline; Py, pyrazole; LPS, lipopolysaccharide.
Pyrazole Enhances LPS-Induced Oxidative andNitrosative Stress in C57BL/6 Mice.
In the LPS group, the content of NO (nitrite+nitrate) increased at 8 hours after LPS treatment, and remained at a high level at 24 hours. In the LPS/pyrazole group, although AST and ALT were elevated at 24 hours, the contents of NO were lower at 8 and 24 hours, as compared with the LPS group (Fig. 3A). Peroxynitrite (ONOO−), formed by the rapid reaction between NO and O2· −, has been shown to nitrate free and protein-associated tyrosine residues and produce nitrotyrosine protein adducts.25 As shown in Fig. 3B, in the LPS/pyrazole group, staining for formation of 3-NT adducts can be detected at 8 hours after LPS administration, with strongest signals at 24 hours. However, in the LPS group, 3-NT adducts were undetectable at 8 hours, and only weak signals were observed at 24 hours; in the saline and pyrazole groups 3-NT adducts were undetectable at all time points (Fig. 3B).
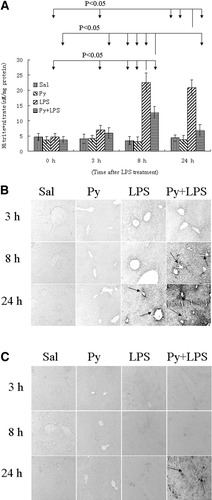
Production of nitric oxide and formation of 3-NT and HNE adducts in C57BL/6 mice. (A) Contents of nitrite and nitrate in liver homogenates. Nitrite and nitrate were assayed to reflect production of nitric oxide as described in Materials and Methods. Data are presented as mean±SD (n = 6). Immunohistochemistry staining for 3-NT (B) and HNE adducts (C) in liver sections. Arrows show positive staining. 3-NT: 3-nitrotyrosine; HNE: 4-hydroxy-2-nonenal. Sal, saline; Py, pyrazole; LPS, lipopolysaccharide.
Overproduction of ROS may cause lipid peroxidation, which contributes to liver injury. In the saline, pyrazole, and LPS group, HNE protein adducts could not be detected at all time points after LPS administration (Fig. 3C). In the LPS/pyrazole group, HNE adducts could be detected at 24 hours only (Fig. 3C). In the LPS/pyrazole group, strongest signals for 3-NT and HNE adducts can be detected at 24 hours, at which time point ALT and AST were elevated dramatically. Thus, there appears to be an association between nitrosative and oxidative stress with the liver injury.
Protective effect of CMZ on LPS/PyrazoleIinduced Liver Injury in C57BL/6 Mice.
We evaluated the protective effect of CMZ on the enhancement of LPS liver injury by pyrazole at 24 hours because ALT and AST were elevated at 24 hours in the LPS/pyrazole group. After LPS administration to pyrazole-pretreated mice, serum ALT levels increased about 14-fold, while serum AST increased about 16-fold, as compared with the saline group (Fig 4A). CMZ treatment decreased serum ALT and AST levels about 55% and 65%, respectively (Fig. 4A). Pathological examination showed large necrotic areas in the LPS/pyrazole group, but only small necrotic foci were observed after treatment with CMZ (Fig. 4B). However, the CMZ treatment did not significantly change the number of cells positive for TUNEL staining with apoptotic morphology (Fig. 4C) or caspase-3 activity (Fig. 4D). These results suggest that CMZ attenuated the enhancement of LPS-induced liver injury by pyrazole mainly via decreasing necrosis, not via decreasing apoptosis.
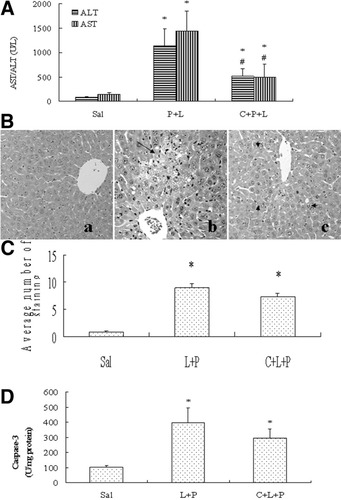
Protective effects of CMZ on liver injury induced by LPS plus pyrazole in C57BL/6 mice. Pyrazole (150mg/kg) or saline was injected ip daily for 2 days followed by LPS (4mg/kg) or saline injection ip. CMZ was injected (50mg/kg intraperitoneally) 15 hour before and 30 minutes after LPS injection. Mice were killed 24 hours after LPS injection, blood was collected and livers were removed. (A) Serum levels of ALT and AST. Results are expressed as mean±SD (n = 6). (B) HE staining. (a) Control group; (b) Py+LPS group, showing a large necrotic area (long arrow); (c) CMZ+Py+LPS group, showing only small necrotic foci (arrow heads). (C) Quantification of the number of TUNEL positive staining. Data are shown as mean ±SD (n = 6). (D) Caspase-3 activity. Data are shown as mean±SD (n = 6). * P < .05, compared with saline group; # P < .05, compared with P+L group. Sal, saline; P, pyrazole; L, lipopolysaccharide; C, chlormethiazole.
Effect of CMZ on Pyrazole/LPS-Induced Oxidative and Nitrosative Stress in C57BL/6 Mice.
Because CMZ is an inhibitor of CYP2E1, an active producer of ROS, it is anticipated that CMZ should possess an antioxidant action against CYP2E1-generated oxidant stress. Strong signals for formation of HNE adducts were detected in the LPS/pyrazole group, but only a weak signal for formation of HNE adducts was detected after treatment with CMZ (Fig. 5A). CMZ did not decrease the elevated content of NO in the LPS/pyrazole group (data not shown), however, CMZ decreased formation of 3-NT adducts dramatically (Fig. 5B).
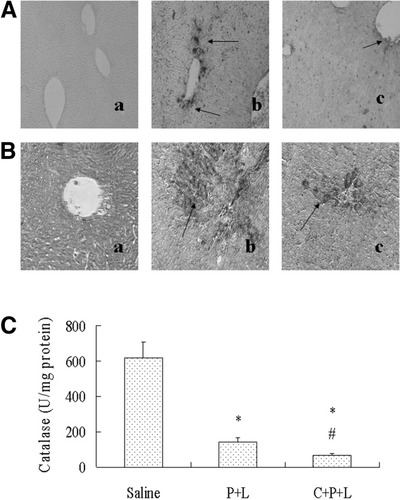
Inhibitory effects of CMZ on hepatic oxidative stress induced by LPS plus pyrazole treatment in C57BL/6 mice. (A) Immunohistochemical staining for HNE. (a) Saline group, no positive staining is observed; (b) LPS/Pyrazole group, showing strong positive staining (arrows); (c) LPS/Pyrazole/CMZ group, showing weaker positive staining (arrows). (B) Immunohistochemical staining for 3-NT. (a) Saline group, no positive staining is observed; (b) LPS/Pyrazole group, showing strong positive staining (arrows); (c) LPS/Pyrazole/CMZ group, showing weaker positive staining (arrows). (C) Catalase activity in liver homogenates. Data are shown as mean±SD (n = 6). * P < .05, compared with saline group; # P < .05, compared with LPS/Pyrazole group. P, pyrazole; L, lipopolysaccharide; C, chlormethiazole; HNE, 4-hydroxy-2-nonenal; 3-NT, 3-nitrotyrosine.
Pyrazole can inhibit the activity of catalase.11, 12 In the LPS/pyrazole group, catalase activity was decreased about 80% (Fig. 5C). CMZ did not increase but further decreased catalase activity to about 90% of the saline controls. These results do not support the possibility that CMZ protected against the enhanced liver injury via recovery of catalase activity, indicating that the pyrazole enhancement of LPS-induced liver injury is not via inhibition of catalase activity.
Effects of CMZ on Activities of CYP2E1 and CYP2A5.
Pyrazole is known to elevate CYP2E1 by a posttranscription mechanism stabilizing CYP2E1 against degradation.26 The elevated CYP2E1 levels rapidly decline with time as the inducer is metabolized or removed.27 A significant increase in CYP2E1 activity was observed after 2 days' treatment with pyrazole and an overnight fast as compared to the saline controls (0 hour data in Fig. 6A). This increase was maintained 3 hours and 8 hours later, but it was decreased 24 hours later. There was no difference in CYP2E1 activity between the pyrazole and LPS/pyrazole groups at all times (Fig. 6A). LPS alone caused a significant decrease in CYP2E1 activity at 24 hours but the pyrazole treatment elevated this activity 2-fold (Fig. 6A). CMZ treatment caused a significant decrease in CYP2E1 activity in the LPS/pyrazole group (Fig. 6B). Thus, CMZ treatment nearly completely prevented the elevation in CYP2E1 levels in the LPS/pyrazole group.
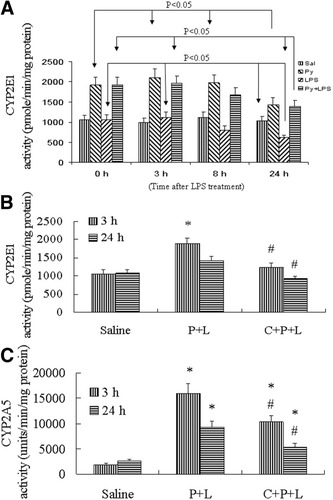
CYP2E1 and CYP2A5 activity in liver microsomes in C57BL/6 mice. (A) Time course of CYP2E1 activity after treatment with saline or pyrazole or LPS or LPS plus pyrazole. The 0 hour refers to treatment with saline or pyrazole for 2 days followed by an overnight fast. Mice were then treated with LPS or saline for 3, 8 or 24 hours. (B) Inhibitory effect of CMZ on CYP2E1 activity in mice treated with LPS plus pyrazole. (C) Inhibitory effect of CMZ on CYP2A5 activity. Data are shown as mean±SD (n = 6). * P < .05, compared with saline group; # P < .05, compared with P+L group. Sal, saline; P, pyrazole; C, chlormethiazole; L, lipopolysaccharide.
Besides CYP2E1, Coumarin 7-hydroxylase, a specific activity for CYP2A5, can also be induced by pyrazole in mice,13 and induction of CYP2A5 by pyrazole may be related to oxidative stress, because antioxidants prevent and pro-oxidants stimulate CYP2A5 mRNA induction by pyrazole.14 CMZ was suggested to be a specific inhibitor of CYP2E1 expression suitable for in vivo study.15 Since CMZ blocked the induction of CYP2E1 by pyrazole, it was important to determine the effects of CMZ on CYP2A5. We expected that CMZ would not inhibit CYP2A5 activity, however, to our surprise, CMZ did inhibit CYP2A5 activity. As shown in Fig. 6C, at 3 hours after LPS administration, CYP2A5 activity increased about 8-fold in the LPS/pyrazole group, compared to saline control. CYP2A5 activity decreased about 30% after CMZ treatment (P < .05), but it was still about 5-fold higher than the saline group. At 24 hours after LPS administration, CYP2A5 activity was still increased 4-fold, compared to saline controls in the absence of CMZ, and about 2-fold higher in the presence of CMZ as CMZ lowered CYP2A5 activity about 50% (P < .05).
CYP2E1-Knockout Mice Exhibit Minor Liver Injury Induced by Pyrazole/LPS.
In view of the decrease in both CYP2E1 and CYP2A5 activities by CMZ, to further evaluate the role of CYP2E1 in the LPS/pyrazole toxicity, CYP2E1-knockout mice were used. Because 129Sv is the genetic background strain for CYP2E1-knockout mice, 129SV mice were used as wild-type control. Wild-type 129SV mice were treated with saline or pyrazole or LPS or LPS/pyrazole (same protocol and doses as in C57BL/6 mice). Serum ALT and AST were elevated after 24 hours of LPS/pyrazole treatment, the time point at which time ALT and AST were elevated in the C57BL/6 mice (Supplementary Fig. 1A-B; Supplementary material for this article can be found on the HEPATOLOGY website (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html). No changes in ALT or AST were found with the pyrazole alone or LPS alone groups. CMZ treatment decreased the elevated serum ALT and AST levels of the LPS/pyrazole group (Supplementary Fig. 1A-B). No pathological changes were observed in the saline, pyrazole, or LPS group (Supplementary Fig. 1C, panels a, b and c). Large necrotic areas were observed in the LPS/pyrazole group (Supplementary Fig. 1C, panel d), and almost normal morphology was observed in the LPS/pyrazole/CMZ group (Supplementary Fig. 1C, panel e). Thus, the LPS/pyrazole potentiated liver injury model is also functional and CMZ has a protective effect in 129Sv mice, analogous to results with C57BL/6 mice.
CYP2E1-knockout mice were subjected to LPS/pyrazole treatment; serum ALT and AST levels were lower than that in wild-type mice by 60% and 40%, respectively (Fig. 7A). Results were similar with male and female mice and there was no significant difference in ALT and AST values between males and females. Pathological examination showed large necrotic areas and widespread necrotic foci in wild-type mice, while almost normal histology was found in CYP2E1-knockout mice (Fig. 7B). Again, no sex-dependent difference was observed (data not shown). Positive TUNEL staining with apoptotic morphology was significantly lower in CYP2E1-knockout mice, compared to wild-type mice (Fig. 7C). In CYP2E1-knockout mice, Western blotting showed that CYP2E1 protein was undetectable in males and females, while in wild-type mice, strong signals for CYP2E1 were observed (Fig. 7D). There was not any difference in catalase activity between CYP2E1-knockout and wild-type mice (data not shown). Treatment with LPS/pyrazole increased CYP2A5 activity in male and female 129Sv wild-type mice compared to saline controls (Fig 7E). Again, to our surprise, increases in activity of CYP2A5 by LPS/pyrazole were different between CYP2E1-knockout and wild-type mice. The basal levels of CYP2A5 activity in female CYP2E1-knockout and wild-type mice were the same, but after treatment with LPS/pyrazole, CYP2A5 activity in female wild-type mice was about 2-fold higher than that in female CYP2E1-knockout mice (Fig 7E). The basal level of CYP2A5 activity in male wild-type mice was about 3-fold higher than that in male CYP2E1-knockout mice; after treatment with LPS/pyrazole, CYP2A5 activity increased both in male CYP2E1-knockout and wild-type mice, but CYP2A5 activity in male wild-type mice was 4-fold higher than that in male CYP2E1-knockout mice (Fig. 7E). Thus, levels of CYP2A5 were 2- to 4-fold lower after treatment with LPS/pyrazole in the CYP2E1-knockout mice compared to wild-type mice.
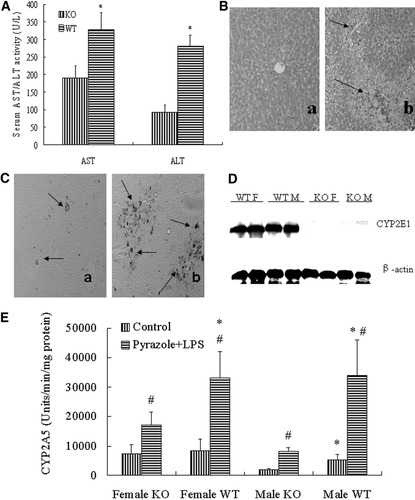
Difference in pyrazole/LPS-induced liver injury between CYP2E1-knockout and wild-type mice. Pyrazole (150mg/kg) or saline was injected intraperitoneally daily for 2 days followed by LPS (4mg/kg) or saline injection intraperitoneally. Blood and liver samples were collected at 24 hours after treatment with LPS or saline, and assays were performed as described in Materials and Methods. (A) Serum ALT and AST: Data are presented as mean±SD (n = 10). * P < .05, compared with the CYP2E1-knockout mice. (B) H&E stained liver sections: liver section from CYP2E1-knockout mice (a) showing almost normal liver histology and liver sections from wild-type mice (b) showing necrotic areas (arrows). (C) TUNEL staining in liver sections from CYP2E1-knockout mice (a) showing less staining compared to wild-type mice (b). Arrows show positive staining. (D) Western blots for CYP2E1 and β-actin in non-treated male and female CYP2E1-knockout mice and wild-type mice. (E) CYP2A5 activity. Data are presented as mean±SD (n = 5). * P < .05, compared with KO; # P < .05, compared with control groups. KO, CYP2E1-knockout mice; WT, wild-type mice; F, female; M, male; LPS, lipopolysaccharide.
Effect of LPS Alone on Wild-Type and CYP2E1-Knockout Mice.
In view of the lower CYP2A5 activity after LPS/pyrazole treatment in CYP2E1-knockout mice compared to wild-type mice, to more specifically evaluate the role of CYP2E1 in the LPS toxicity, an LPS alone-induced liver injury model was used. The absence of pyrazole would diminish induction of CYP2A5. Female CYP2E1-knockout mice and female wild-type mice were selected, because there is no difference in basal levels of CYP2A5 activity between female CYP2E1-knockout mice and female wild-type mice (Fig. 7E). A higher dose of LPS (10 mg/kg instead of 4 mg/kg) was administered to induce liver injury in the absence of pyrazole treatment. Blood was collected 3 hours and 7 hours after LPS administration. Serum ALT and AST levels in CYP2E1-knockout mice were slightly but not significantly lower than that in wild-type mice at 3 hours, but significantly lower at 7 hours by 60% and 50%, respectively (Fig. 8A-B), suggesting that CYP2E1 plays an important role. However, although CYP2A5 activity was not different at 3 hours between CYP2E1-knockout and wild-type mice, it was lower in CYP2E1-knockout mice at 8 hours (Fig. 8C).
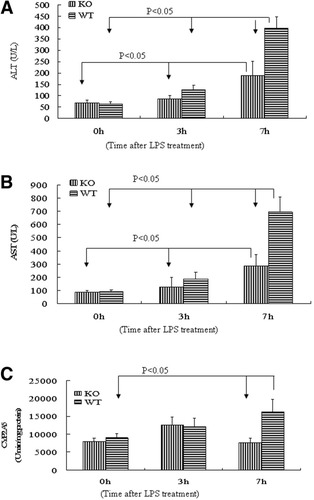
Difference in liver injury induced by LPS alone between CYP2E1-knockout and wild-type mice. LPS (10 mg/kg) or saline was injected intraperitoneally. Blood and liver samples were collected at the indicated times after treatment with LPS, and assays were performed as described in Materials and Methods. (A) Serum ALT and (B) AST: Data are presented as mean ± SD (n = 6). (C) CYP2A5 activity. Data are presented as mean± SD (n = 6). KO, CYP2E1-knockout mice; WT, wild-type mice; LPS, lipopolysaccharide.
Discussion
In the present study, we evaluated the possible synergistic action of CYP2E1 and LPS in potentiating liver injury. A mouse model was developed which involves injecting LPS to pyrazole-pretreated mice, analogous to a similar model in rats.10 In the rat model, at 8 hours after LPS injection, enhanced liver injury was observed. In the mouse model, the enhancement of toxicity was delayed to 24 hours after LPS administration. In the rat model, pyrazole enhanced LPS-induced hepatic necrosis, but not apoptosis. However, in this mouse model, LPS-induced apoptosis was enhanced, in addition to necrosis. In addition to species difference, these variations may be due to the different doses of pyrazole and LPS used in the two models, e.g., in the rat model, the doses of pyrazole and LPS are 200 mg/kg and 10 mg/kg, respectively, but in the mouse model, the doses of pyrazole and LPS were lowered to 150 mg/kg and 4 mg/kg, respectively. These doses were chosen to avoid any significant toxicity by either pyrazole alone or by LPS alone, in order to obtain conditions which promote potentiation or synergistic interactions between pyrazole and LPS. Nevertheless, in both mice and rats, pyrazole can enhance LPS-induced liver injury. Because the CYP2E1 inducer pyrazole enhanced LPS-induced liver injury both in mice and rats, the CYP2E1 inhibitor CMZ protected against pyrazole/LPS-induced liver injury in mice, and CYP2E1-knockout mice also exhibited a minor liver injury induced by pyrazole/LPS or LPS alone, it appears that CYP2E1 plays an important role in the enhancement of LPS-induced liver injury by pyrazole.
LPS can inhibit several cytochrome P450s including CYP2E1 at transcription and translation levels.28 In the rat model, enhanced liver injury was observed at 8 hours after treatment with LPS/pyrazole, at which time CYP2E1 activity did not yet decrease. However, in the mouse model, enhanced liver injury was observed at 24 hours after treatment with LPS/pyrazole, at which time CYP2E1 activity did decrease. Complete absorption of LPS from the peritoneum requires several hours,29 and inflammatory responses were initiated after 3 hours of LPS treatment,30 at which time CYP2E1 activity was high in this mouse model (Fig. 6A) but ALT and AST activities had not elevated yet (Fig. 1A-B). Enhanced liver injury could be readily observed at 24 hours, even though CYP2E1 activity was lower. Similarly, CYP3A2 can be downregulated by LPS,31 but LPS-induced liver injury was aggravated by CYP3A2 induction.32
CYP2E1 is known to bioactivate many hepatotoxins such as carbon tetrachloride and acetaminophen to reactive intermediates.33-37 In addition, CYP2E1 itself is also an effective enzyme for ROS production, exhibits enhanced NADPH oxidase activity, and elevated rates of production of O2·− and hydrogen peroxide (H2O2) even in the absence of substrate.38-40 By producing excessive ROS, CYP2E1 may sensitize toxicity of hepatotoxins such as alcohol,41 Fas agonist Jo2,12 and cisplatin.42 To our knowledge, there are no data to show that LPS can be bioactivated by CYP2E1, therefore, we hypothesize that CYP2E1-mediated oxidative stress may play an important role in the potentiation of LPS-induced liver injury by CYP2E1. LPS activates Kupffer cells to produce O2·− via NADPH oxidase,43 which may synergize with CYP2E1-mediated oxidative stress.
Overproduction of ROS may cause lipid peroxidation and form MDA or HNE adducts. LPS can also induce NO production; e.g., O2·− derived from CYP2E1 can react with NO rapidly to form OONO−, the latter can nitrate tyrosine and cause the formation of 3-NT.25 Interestingly, even though NO levels were lower in the LPS/pyrazole group compared to the LPS alone group, 3-NT protein adducts were higher, perhaps suggesting that the lower content of NO may be due to formation of ONOO− and 3-NT adducts. Because NO levels were not decreased, it is likely that CMZ decreases OONO− formation through lowering production of O2·− following inhibition of CYP2E1 activity.
Through induction of CYP2E1, pyrazole may enhance LPS-induced oxidative and nitrosative stress. Indeed, HNE and 3-NT adducts could be detected at 24 hours in the LPS/pyrazole treated mice, at which time point enhanced liver injury was observed. It appears that HNE and 3-NT adducts are associated with LPS/pyrazole liver injury. But further studies with antioxidants or inhibitors of NO synthase will be required to establish cause-and-effect relationships.
In addition to CYP2E1 induction, pyrazole induces CYP2A5 activity and inhibits catalase activity, both of which may enhance hepatotoxin-induced toxicity and oxidative stress. CMZ, an inhibitor of CYP2E1, protected against pyrazole-enhanced LPS liver injury and oxidative stress, but CMZ did not increase catalase activity under these conditions; In addition, there was not any difference in catalase activity between CYP2E1-knockout and wild-type mice while these mice did show a different severity of liver injury after treatment with LPS/pyrazole. These results do not support a role for catalase in the LPS/pyrazole liver injury.
Results with respect to a possible role for CYP2A5 in the pyrazole/LPS toxicity proved to be more complex and difficult to clearly distinguish from the role of CYP2E1. When LPS/pyrazole-induced liver injury was protected by CMZ, besides CYP2E1 activity, CYP2A5 activity was also significantly inhibited. In CYP2E1-knockout mice where lower liver injury was observed after treatment with LPS/pyrazole or LPS alone, levels of CYP2A5 were also lower, compared with wild-type mice. Interestingly, CYP2A5 levels were also found to be diminished in CYP2G1-knockout mice,44 suggesting that changes in one cytochrome P450 may influence expression of other cytochrome P450s. We are not aware of any report of a CYP2A5-knockout mouse to assess LPS or LPS/pyrazole toxicity. It is therefore possible/likely that CYP2A5 in addition to CYP2E1 plays a role in the LPS/pyrazole-induced liver injury. We believe that CYP2E1 plays the key role in LPS/pyrazole liver injury for the following reasons: (1) Although LPS/pyrazole induced CYP2A5 activity was significantly inhibited by CMZ, activity still remained about 5-fold higher than the saline group (Fig. 6C), in contrast to the complete prevention by CMZ of CYP2E1 induction (Fig. 6B); (2) the magnitude of CYP2A5 inhibition by CMZ (30%) was much less than that of the elevated CYP2E1 activity (90%); (3) although CYP2A5 levels were lower after treatment with LPS/pyrazole in CYP2E1-knockout mice than the wild-type mice, levels were still several folds higher than the saline controls, in contrast to the total absence of CYP2E1; (4) there was a more than 2-fold difference in CYP2A5 activity between female and male CYP2E1-knockout mice after treatment with LPS/pyrazole, but no difference in liver injury was observed between the male and female CYP2E1 knockout mice (Fig. 7); (5) in rats, where pyrazole also enhances LPS-induced liver injury,10 CYP2A5 activity was undetectable both in the control and LPS/pyrazole groups (data not shown), which is consistent with a previous report in the literature showing the absence of CYP2A5 in rat liver.45
In conclusion, the CYP2E1 inducer pyrazole enhanced LPS-induced liver injury. The CYP2E1 inhibitor CMZ protected against the pyrazole-enhanced LPS-induced liver injury and the oxidative stress. CYP2E1-knockout mice exhibited minor liver injury, compared with wild-type mice. The CYP2E1 inhibitor CMZ also inhibited CYP2A5 activity; and in CYP2E1-knockout mice, CYP2A5 activity was also decreased. These results suggest that CYP2E1 plays an important role in the potentiation of LPS liver injury by pyrazole, however, some contribution by CYP2A5 cannot be excluded.
Acknowledgements
We thank Dr. Frank Gonzalez, NCI/NIH for the provision of CYP2E1 knockout mice.




