Is the FXR the fix for cholesterol gallstone disease?†
Potential conflict of interest: nothing to report.
Abstract
Cholesterol gallstone disease is characterized by several events, including cholesterol precipitation in bile, increased bile salt hydrophobicity and gallbladder inflammation. Here, we describe the same phenotype in mice lacking the bile acid receptor, FXR. Furthermore, in susceptible wild-type mice that recapitulate human cholesterol gallstone disease, treatment with a synthetic FXR agonist prevented sequelae of the disease. These effects were mediated by FXR-dependent increases in biliary bile salt and phospholipid concentrations, which restored cholesterol solubility and thereby prevented gallstone formation. Taken together, these results indicate that FXR is a promising therapeutic target for treating or preventing cholesterol gallstone disease.
Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 2004;10:1352–1358. (Reprinted with permission from Nature Publishing Group, at www.nature.com.)
Comments
Cholesterol gallstone disease (CGD) has become increasingly common in recent decades, affecting as much as 10% of the population of the United States, with annual treatment costs in excess of $6 billion.1, 2 Currently, the most common treatment for CGD is cholecystectomy, with over 700,000 such operations performed each year.1, 3 Although this procedure is effective, it is invasive and may cause surgical complications in some patients.3 Furthermore, because of comorbidity, many patients with symptomatic CGD (e.g., the elderly) are not candidates for surgical intervention. Oral administration of ursodeoxycholic acid is an approved therapy for CGD but is not completely successful because of limited efficacy on gallstone resolution, need for prolonged therapy, and frequent relapse of gallstone formation following termination of therapy.3 Indeed, development of effective, noninvasive therapies will have a great impact on many lives and may help to reduce the costs of health care associated with CGD.
Development of cholesterol gallstones is ultimately the result of disrupted homeostasis between cholesterol, bile salt, and phospholipid levels in the bile. In human CGD, this is generally initiated by hypersecretion of cholesterol to the bile2, 4 leading to decreased gallbladder motility and increased mucin production.4 In turn, the bile becomes increasingly hydrophobic, enhancing the biliary secretion of cholesterol.4 Eventually the ability of bile salt and phospholipid mixed micelles to maintain cholesterol solubility is exceeded, and rapid precipitation of cholesterol crystals ensues.2, 4 Over time, these crystals aggregate to form the pathogenic gallstones.
Cholesterol homeostasis is facilitated in the liver through a complex series of pathways involving the uptake of plasma cholesterol, the synthesis of cholesterol from acetate, the direct transport of free cholesterol to bile, and the elimination of cholesterol via synthesis of bile acids.5 At the heart of these pathways lies cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the classic bile acid biosynthesis pathway, catalyzing the conversion of cholesterol into bile acids.5, 6 In the mouse, control of CYP7A1 activity is mediated by ligand-driven activation of two members of the nuclear receptor superfamily, liver X receptor (LXR) and the farnesoid X receptor (FXR)5, 6 (Fig. 1). LXR is a cholesterol sensor, driving the elimination of intracellular cholesterol by inducing the transcription of CYP7A1 and stimulating expression of the Abcg5 and Abcg8 cholesterol transporters.5, 6 Conversely, FXR is a bile acid sensor, stimulating bile acid and phospholipid export through induction of the Abcb11 (BSEP) and Abcb4 (Mdr 2) export pumps, and repressing CYP7A1 transcription as a feedback mechanism to shut down bile acid synthesis before intracellular bile acid levels become toxic5, 6 (see Fig. 1).
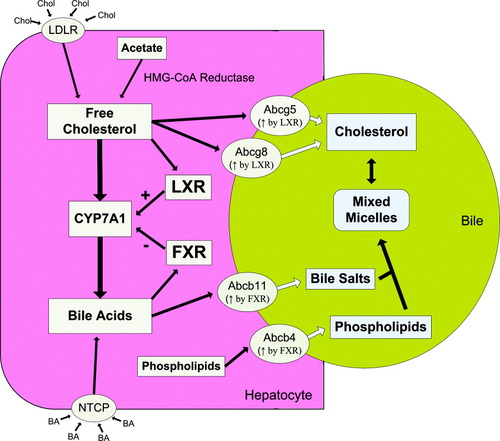
Cholesterol and bile acid homeostasis in the mouse. Intracellular free cholesterol in the hepatocyte is the result of HMG-CoA reductase–mediated conversion of acetate and uptake of plasma cholesterol via the low-density lipoprotein receptor. Elimination of free cholesterol from the hepatocyte occurs through direct transport to the bile via Abcg5 and Abcg8 or by conversion to bile acid through the classic bile acid synthesis pathway, of which CYP7A1 is the rate-limiting step. Both of these processes are self-stimulated through the ligand-dependent activation of LXR. Build-up of intracellular bile acid levels through uptake of circulating bile acids via NTCP and synthesis from cholesterol activates the bile acid sensor FXR, stimulating bile salt and phospholipid elimination via induction of Abcb11 and Abcb4 and repressing bile acid synthesis through downregulation of CYP7A1. Bile salts and phospholipids combine in the bile to form mixed micelles, which are capable of solubilizing cholesterol. Under normal conditions, these pathways interact to achieve a homeostasis of cholesterol and bile acid levels in the cell while providing sufficient cholesterol solubility in the bile. Abbreviations: Chol, cholesterol; LDLR, low-density lipoprotein receptor; LXR, liver X receptor; FXR, farnesoid X receptor; BA, bile acid.
It has been long established that feeding a lithogenic diet high in cholesterol and cholic acid leads to gallstone formation in mice.6, 7 Genetic mapping of mouse strains especially susceptible to development of gallstones has implicated a number of genes involved with this process, including FXR.6, 8 Indeed, susceptible mice evidence decreased FXR expression and may harbor various polymorphisms in the FXR gene.6, 8 It is with this knowledge that Moschetta et al. hypothesized that FXR may play a critical role in the prevention of CGD by helping to maintain the proper solubilization of cholesterol in bile. To this end, stimulation of FXR using synthetic ligands could be useful in the prevention and treatment of CGD.
In the first part of the study, Moschetta et al. demonstrate the role of FXR in the development of CGD. Age-matched wild-type and FXR−/− mice were fed a lithogenic diet for 1 week, after which the gallbladder bile and expression of known FXR and LXR target genes were analyzed.6 Inspection of the gallbladder and bile showed increases in inflammation, bile salt hydrophobicity, bile turbidity, and presence of cholesterol monohydrate crystals in the FXR null mice, all phenotypical of CGD.6 Furthermore, bile salt and phospholipid levels were found to be significantly lower in the FXR null mice due to a lack of FXR-mediated expression of Abcb11 and Abcb4.6 Conversely, cholesterol levels were not significantly altered, because regulation of the cholesterol transporters Abcg5 and Abcg8 through LXR occurred independently of FXR.6 Consequently, the cholesterol saturation index was increased in the FXR null mice, driving the early development of cholesterol monohydrate crystals.
In the second part of the study, Moschetta et al. expand their findings by demonstrating that stimulation of FXR with a synthetic agonist can prevent the onset of CGD. Here, CGD-susceptible C57L and FXR−/− mice were fed a lithogenic diet supplemented with the synthetic FXR ligand GW4064 or vehicle control for 1 week.6 As expected, examination of the gallbladder and bile indicated onset of CGD in the vehicle-treated C57L and FXR null mice as well as the GW4064-treated FXR null mice.6 Interestingly, two of the five vehicle-treated C57L mice evidenced more advanced disease sequelae compared with the FXR null mice,6 suggesting that mechanisms in addition to those mediated by FXR may contribute to the increased susceptibility of these mice to CGD. However, GW4064 treatment prevented CGD onset in the C57L mice through FXR-mediated upregulation of Abcb11 and Abcb4, increasing transport of bile salts and phospholipids to the bile, reducing of the cholesterol saturation index, and providing protection from cholesterol monohydrate crystal formation.6
The work of Moschetta et al. provides compelling evidence that synthetic FXR agonists may be useful in treating CGD. However, maintenance of cholesterol and bile acid homeostasis in mice is somewhat different from that of humans. The bile acid pool of mice is more hydrophilic than that of man and thus is less effective in activating FXR.5 Control of CYP7A1-mediated bile acid synthesis from cholesterol in mice is dominated by feed-forward activation through LXR, whereas in humans LXR is not functional in this capacity.9, 10 Instead, control of bile acid synthesis in humans is dominated by feedback repression of CYP7A1 through FXR and other means.10 Thus in humans bile acid synthesis from cholesterol is primarily a means to maintain bile acid homeostasis, whereas in the mouse it is a means for removal of cholesterol.
Despite these differences, FXR action in mice and humans is fundamentally the same. Transcription of CYP7A1 is repressed, while transcription of ABCB11 and ABCB4 transporters are induced. Indeed, there is precedence for using FXR agonists to treat human CGD; the bile acid chenodeoxycholic acid, a potent endogenous FXR agonist, was used to treat gallstones in the past.3, 6, 11 However, the toxicity of chenodeoxycholic acid led to replacement with the less toxic bile acid ursodeoxycholic acid,3, 6 which remediates CGD by reducing bile acid pool hydrophobicity and inducing the ABCB4 phospholipid transporter, resulting in an increased capacity for cholesterol solubility11 nonetheless, with limited efficacy to resolve CGD. The ability of FXR to stimulate ABCB11 transcription facilitating the efflux of bile acids to the bile, potentially resulting in greater cholesterol solubility than that achieved by ursodeoxycholic acid, could prove beneficial in the treatment of CGD. Furthermore, synthetic FXR agonists hold the promise of being more active and less toxic than their endogenous counterparts.
In addition to its potential role in CGD, GW4064 treatment has been shown to be hepato-protective in rat models of intra- and extrahepatic cholestasis through repression of CYP7A1-mediated bile acid synthesis and induction of ABCB11 and ABCB4, decreasing the concentration of toxic bile acids in the liver.12 Furthermore, deficiencies in FXR activity and mutations in ABCB11 (BSEP) and ABCB4 (MDR3) have been identified in subtypes of progressive familial intrahepatic cholestasis,12, 13 evidence that FXR exerts a protective effect in humans. Could FXR agonists ameliorate the intrahepatic accumulation of bile acids present in chronic cholestatic liver disease such as primary biliary cirrhosis and primary sclerosing cholangitis? Although the cause of primary biliary cirrhosis and primary sclerosing cholangitis remains speculative, there are no known mutations in FXR or its downstream targets associated with these disorders that would suggest treatment with FXR agonists to be futile. In fact, ABCB11 levels were shown to be conserved in livers of late-stage primary biliary cirrhosis patients in conjunction with increased expression of ABCB4.14 To this end, treatment with a synthetic FXR agonist alone or in combination with ursodeoxycholic acid could prove useful in improving the cholestasis of primary biliary cirrhosis and primary sclerosing cholangitis patients.
Will manipulation of FXR in humans prove to be the fix for cholesterol gallstone disease? The work of Moschetta et al. provides additional evidence that indeed it may. However, more translational studies are needed to address these findings and investigate the therapeutic potential of synthetic FXR agonists in humans.




