In vitro hepatic differentiation of human mesenchymal stem cells
Abstract
This study examined whether mesenchymal stem cells (MSCs), which are stem cells originated from embryonic mesoderm, are able to differentiate into functional hepatocyte-like cells in vitro. MSCs were isolated from human bone marrow and umbilical cord blood, and the surface phenotype and the mesodermal multilineage differentiation potentials of these cells were characterized and tested. To effectively induce hepatic differentiation, we designed a novel 2-step protocol with the use of hepatocyte growth factor and oncostatin M. After 4 weeks of induction, cuboidal morphology, which is characteristic of hepatocytes, was observed, and cells also expressed marker genes specific of liver cells in a time-dependent manner. Differentiated cells further demonstrated in vitro functions characteristic of liver cells, including albumin production, glycogen storage, urea secretion, uptake of low-density lipoprotein, and phenobarbital-inducible cytochrome P450 activity. In conclusion, human MSCs from different sources are able to differentiate into functional hepatocyte-like cells and, hence, may serve as a cell source for tissue engineering and cell therapy of hepatic tissues. Furthermore, the broad differentiation potential of MSCs indicates that a revision of the definition may be required. (HEPATOLOGY 2004;40:1275–1284.)
In vitro models of parenchymal liver cells are of great importance in toxicology and in bioartificial liver research,1, 2 because primary cultures of hepatocytes have been hindered by their short life span and the rapid loss of hepatic functions under in vitro conditions.3
Stem cells responsible for self-repair and regeneration are found in various organs of the human body.4 However, human hepatic stem cells have remained illusive and the mechanism responsible for the regenerative capacity of liver has been of much controversy.5 It has long been thought that the differentiation potential of adult stem cells is limited to their germ layer of origin, but recent studies have demonstrated that adult stem cells are more plastic than once believed.6, 7 Although bone marrow (BM) is considered an extrahepatic origin of hepatic progenitor cells, this concept is primarily attributed by in vivo animal studies.7-15 For example, Lagasse et al.15 showed that transplanted purified hematopoietic stem cells could give rise to hepatocytes and even completely restore liver function in the fumarylacetoacetate hydrolase-deficient mice, while in humans, female recipients of male BM were found to have hepatocytes containing the Y chromosome, using fluorescent in situ hybridization analysis,12, 13 suggesting that hepatocytes could be derived from BM cells. However, the paradigm that BM cells can turn into hepatocytes in vivo is not without controversy.16 Several lines of evidence indicated that transplanted BM cells adopt the phenotype of hepatocytes and restore liver function by cell fusion rather than differentiation,17-19 and in vivo studies of BM transplantation provide little insight as to which cell populations in the BM contribute to hepatic regeneration.
Bone marrow is a reservoir of various stem cells, including hematopoietic stem cells, mesenchymal stem cells (MSCs), and, allegedly, multipotent adult progenitor cells (MAPCs).20 To date, MAPCs are the only adult stem cells reported to be capable of differentiating into functional hepatocyte-like cells, under in vitro conditions.21 However, the prerequisite of prolonged in vitro culture prior to the emergence of MAPCs raises the question whether such cells naturally exist in postnatal tissues,22 due to the extremely low frequency. In contrast, MSCs, widely studied over the past decade, are readily accessible from BM and have been shown to be capable of neuroectodermal differentiation in addition to those of the mesoderm.23 However, the endodermal differentiation potential of bone marrow MSCs has yet to be demonstrated and, to our knowledge, is not reported in the English literature.
The aim of this study was to investigate the hepatic differentiation potential of human MSCs. We hypothesized that MSCs obtained from different sources are capable of differentiating into functional hepatocyte-like cells in vitro.
Abbreviations:
BM, bone marrow; MSC, mesenchymal stem cell; HGF, hepatocyte growth factor; UCB, umbilical cord blood; MAPCs, multipotent adult progenitor cells; bFGF, fibroblast growth factor-basic; PAS, periodic acid-Schiff; LDL, low-density lipoprotein; PROD, pentoxyresorufin-O-dealkylase.
Materials and Methods
Cytokines
Fibroblast growth factor-basic (bFGF), hepatocyte growth factor (HGF), and oncostatin M were purchased from R&D Systems (Minneapolis, MN). Epidermal growth factor was obtained from Becton Dickinson (Flanders, NJ).
Antibodies
Antibodies against human antigens CD13, CD29, CD34, CD44, CD45, CD71, CD73, CD90, and CD105 were purchased from Becton Dickinson. Antibodies against human antigen CD133 were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Antibodies against human antigens SH-2 and SH-3 were purified from SH-2 and SH-3 hybridoma cell lines (American Type Culture Collection, Rockville, MD). Antibodies against human albumin, type II collagen, and secondary goat antimouse antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibody against human 9B2-antigen was a kind gift from Dr. Cheng-Po Hu (Veterans General Hospital, Taipei, Taiwan).
MSC Isolation and Culture
Isolation.
Human bone marrow for this study was aspirated from the iliac crest of healthy donors (n = 15). Umbilical cord blood (UCB) was collected upon delivery (n = 11). All samples were collected with informed consent. Mononuclear cells were obtained by negative immunodepletion of CD3, CD14, CD19, CD38, CD66b, and glycophorin-A positive cells using a commercially available kit (RosetteSep, StemCell Technologies, Vancouver, BC, Canada), as per the manufacturer's instructions, followed by Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) density-gradient centrifugation (1.077 g/cm3), and plated in tissue culture flasks (Becton Dickinson, Franklin Lakes, NJ) in expansion medium. Expansion medium consists of Iscove's modified Dulbecco's medium (IMDM, Gibco BRL, Grand Island, NY) and 10% fetal bovine serum (Hyclone, Logan, UT) supplemented with 10 ng/mL epidermal growth factor, 10 ng/mL bFGF, 100 units of penicillin, 1000 units of streptomycin, and 2 mmol/L L-glutamine (Gibco BRL). Cells were allowed to adhere overnight, and nonadherent cells were washed out with medium changes. Medium changes were performed twice weekly thereafter.
Limiting Dilution.
To obtain single cell–derived, clonally-expanded MSCs, the isolated plate-adhering second-passage cells were serially diluted and plated on to 96-well plates (Becton Dickinson), in expansion medium, at the final density of 30 cells per 96-well plate (average density, 0.3 cells/well). Colonies that grew, exhibiting relatively homogeneous fibroblastic morphology, were selected for culture expansion and tested for their differentiation potential. Limiting dilution was performed for both UCB-derived and BM-derived cells.
Maintenance and Culture Expansion.
Once adherent cells reached 1.0 to 1.2 × 104/cm2, they were detached with 0.25% trypsin-EDTA (Gibco BRL), washed twice with phosphate-buffered saline (Gibco BRL), with centrifugation, 1000 rpm, 5 minutes, and replated at 0.3 to 0.4 × 104/cm2 under the same culture conditions.
In Vitro Differentiation
Osteogenic Differentiation.
To induce osteogenic differentiation, 5th- to 13th-passage cells were treated with osteogenic medium for three weeks with medium changes twice weekly. Osteogenic medium consists of IMDM supplemented with 0.1 μmmol/L dexamethasone (Sigma-Aldrich, St. Louis, MO), 10 mmol/L β-glycerol phosphate (Sigma-Aldrich), and 0.2 mmol/L ascorbic acid (Sigma-Aldrich). Osteogenesis was assessed by alkaline phosphatase and von Kossa stainings.
Chondrogenic Differentiation.
To induce chondrogenic differentiation, 5th- to 13th-passage cells were transferred into a 15-mL polypropylene tube and centrifuged at 1000 rpm, 5 minutes, to form a pelleted micromass at the bottom of the tube, then treated with chondrogenic medium for 3 weeks. Chondrogenic medium consists of high-glucose Dulbecco's Modified Eagle Medium (Bio-fluid, Rockville, MD) supplemented with 0.1 μmmol/L dexamethasone, 50 μg/mL ascorbic acid, 100 μg/mL sodium pyruvate (Sigma-Aldrich), 40 μg/mL proline (Sigma-Aldrich), 10 ng/mL transforming growth factor-β1, and 50 mg/mL ITS+ (insulin, transferrin, selenium) premix (Becton Dickinson, 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenious acid, 1.25 mg/mL BSA, and 5.35 mg/mL linoleic acid). Medium changes were performed twice weekly, and chondrogenesis was assessed by immunohistochemical staining for type II collagen.
Adipogenic Differentiation.
To induce adipogenic differentiation, 5th- to 13th-passage cells were treated with adipogenic medium for 3 weeks. Medium changes were performed twice weekly. Adipogenic medium consists of IMDM supplemented with 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 1 μmol/L hydrocortisone (Sigma-Aldrich), 0.1 mmol/L indomethacin (Sigma-Aldrich), and 10% rabbit serum (Sigma-Aldrich). Adipogenesis was assessed by Oil Red O staining.
Hepatogenic Differentiation.
For hepatogenic differentiation, 5th- to 13th-passage cells, at 1.0 to 1.3 × 104/cm2, were serum deprived for 2 days, in IMDM supplemented with 20 ng/mL epidermal growth factor and 10 ng/mL bFGF, prior to induction by a 2-step protocol. Differentiation was induced by treating MSCs with Step-1 differentiation medium, consisting of IMDM supplemented with 20 ng/mL HGF and 10 ng/mL bFGF, nicotinamide 0.61 g/L, for 7 days, followed by treatment with step-2 maturation medium, consisting of IMDM supplemented with 20 ng/mL oncostatin M, 1 μmol/L dexamethasone, and 50 mg/mL ITS+ premix, thereafter. Medium changes were performed twice weekly and hepatogenesis was assessed by reverse-transcription–polymerase chain reaction for liver-associated genes listed in Table 1, by immunofluorescence analysis for albumin production, and by in vitro assays for functions that are characteristic of liver cells.
| Primer | Sequence | Product |
|---|---|---|
| αFP | S: 5′-TGCAGCCAAAGTGAAGAGGGAAGA-3′A: 5′-CATAGCGAGCAGCCCAAAGAAGAA-3′ | 216 bp |
| Albumin | S: 5′-TGCTTGAATGTGCTGATGACAGGG-3′A: 5′-AAGGCAAGTCAGCAGGCATCTCATC-3′ | 161 bp |
| CK-18 | S: 5′-TGGTACTCTCCTCAATCTGCTG-3′A: 5′-CTCTGGATTGACTGTGGAAGT-3′ | 148 bp |
| TAT | S: 5′-TGAGCAGTCTGTCCACTGCCT-3′A: 5′-ATGTGAATGAGGAGGATCTGAG-3′ | 358 bp |
| TO | S: 5′-ATACAGAGACTTCAGGGAGC-3′A: 5′-TGGTTGGGTTCATCTTCGGTATC-3′ | 299 bp |
| G-6P | S: 5′-GCTGGAGTCCTGTCAGGCATTGC-3′A: 5′-TAGAGCTGAGGCGGAATGGGAG-3′ | 350 bp |
| CYP2B6 | S: 5′-GACGCTACGTTTCAGTCTTTC-3′A: 5′-GCTGAATACCACGCCATAG-3′ | 204 bp |
| HNF4 | S: 5′-CCAAGTACATCCCAGCTTTC-3′A: 5′-TTGGCATCTGGGTCAAAG-3′ | 295 bp |
| β-actin | S: 5′-TGAACTGGCTGACTGCTGTG-3′A: 5′-CATCCTTGGCCTCAGCATAG-3′ | 174 bp |
- Abbreviations: αFP, alpha-fetoprotein; S, sense; A, antisense; bp, base pair; CK-18, cytokeratin 18; TAT, tyrosine-aminotransferase; TO, tryptophan 2,3-dioxygenase; G-6P, glucose-6-phosphatase; CYP2B6, cytochrome P450 2B6; HNF4, hepatocyte nuclear factor-4; β-actin, beta-actin.
Cytochemical, Histological, and Immunocytochemical Analysis
Cytochemical Staining.
For evaluation of mineralized matrix, cells were fixed with 4% formaldehyde and assayed by von Kossa staining using 1% silver nitrate (Sigma-Aldrich) under ultraviolet light for 45 minutes, followed by 3% sodium thiosulfate (Sigma-Aldrich) for 5 minutes, and then counterstained with Van Gieson (Sigma-Aldrich) for 5 minutes. For alkaline phosphatase detection, cells were stained by Rutenberg's method, as described.24 For Oil Red O staining, cells were fixed with 4% formaldehyde, stained with Oil Red O (Sigma-Aldrich) for 10 minutes, and counterstained with Mayers hematoxylin (Sigma-Aldrich) for 1 minute.
Histological Analysis.
Chondrogenic differentiation was evaluated after pellets were fixed in 4% formaldehyde, dehydrated in serial ethanol dilutions, and embedded in paraffin blocks. Blocks were cut and sections stained with type II collagen antibody (Santa Cruz Biotechnology).
Immunofluorescence.
For staining of intracellular proteins, cells were fixed overnight with 4% formaldehyde at 4°C, and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 10 minutes. Slides and dishes were incubated with mouse primary antibodies against human albumin (1:50) for 1 hour, followed by fluorescein- or phycoerythrin-coupled goat antimouse immunoglobulin G secondary antibody for 1 hour. Between incubations, samples were washed with phsophate-buffered saline.
Flow Cytometry.
For cell surface phenotyping, fifth- to seventh-passage cells were detached and stained with fluorescein- or phycoerythrin-coupled antibodies and analyzed with FACSCalibur (Becton, Dickinson).
Total RNA Isolation and Reverse-Transcription Polymerase Chain Reaction
RNA was extracted from 3 to 30 × 105 MSCs, differentiating cells, or differentiated cells using RNEasy (Qiagen, Stanford, Valencia, CA) per the manufacturer's instructions. The messenger RNA was reverse transcribed to complementary DNA using Advantage RT-for-PCR (Clontech, Palo Alto, CA) per the manufacturer's instructions. complementary DNA was amplified using ABI GeneAmp PCR System 2400 (PerkinElmer Applied Biosystems, Boston, MA) at 94°C for 40 seconds, 56°C for 50 seconds, and 72°C for 60 seconds for 35 cycles, after initial denaturation at 94°C for 5 minutes. Primers used are listed in Table 1.
Periodic Acid-Schiff (PAS) Stain for Glycogen
Culture dishes containing cells were fixed in 4% formaldehyde, permeabilized with 0.1% Triton X-100 for 10 minutes and were not incubated or were incubated with Diastase for 1 hour, 37°C. Samples were then oxidized in 1% periodic acid for 5 minutes, rinsed 3 times in de-ionized (d)H2O, treated with Schiff's reagent for 15 minutes, and rinsed in dH2O for 5 to 10 minutes. Samples were counterstained with Mayer's hematoxylin for 1 minute and rinsed in dH2O and assessed under light microscope.
Uptake of Low-Density Lipoprotein (LDL)
The Dil-Ac-LDL staining kit was purchased from Biomedical Technologies (Stoughton, MA) and assay was performed per the manufacturer's instructions.
Pentoxyresorufin O-Dealkylase (PROD) Assay
After 6 weeks of differentiation, cells were maintained under the same conditions in the presence or absence of 1 mmol/ phenobarbital for 72 hours followed by treatment with 10 μmol/L pentoxyresorufin, and assessed under fluorescence microscope.
Urea Assay
Urea concentrations were determined by colorimetric assay (640-1, Sigma-Aldrich) per the manufacturer's recommendations and, analyzed with BioPhotometer 6131 (Eppendorf, Hamburg, Germany). Step-2 hepatocyte maturation medium, as described in Materials and Methods, was used as a negative control.
Results
Isolation and Characterization of Bone Marrow–Derived Mesenchymal Stem Cells
We previously reported the isolation of single cell–derived clonally expanded MSCs from UCB by negative immunoselection and limiting dilution, and the multidifferentiation ability of these cells was also demonstrated.25 Using the same approach, single cell–derived colonies of MSC-like cells were also isolated from BM and rapidly growing colonies exhibiting homogeneous morphology were selected for culture expansion. The fibroblast-like morphology of BM-derived cells, at 0.3 × 104/cm2 (Fig. 1Ai) and at 1.2 × 104/cm2 after expansion (Fig. 1Aii), as well as their surface phenotype (Fig. 1B), as determined by flow cytometry, were consistent with those reported in the literature for MSCs.26 These BM-derived cells were negative for CD13, CD34, CD45, and CD133 but were positive for CD29, CD44, CD73, CD90, and CD105, as well as for human MSCs markers SH-2 and SH-3. The doubling time of these BM-derived cells was found to be between 40 to 52 hours (data not shown).
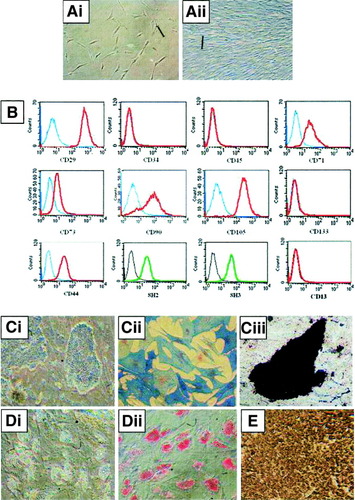
(A) Characterization of bone marrow–derived MSCs. Morphology of MSCs at lower confluence (i) and at higher confluence (ii). (B) Analysis by flow cytometry shows that bone marrow (BM)-MSCs are negative for the expression of CD13 CD34, CD45, and CD133, but positive for the expression of CD29, CD44, CD71, CD73, CD90, CD105, SH-2, and SH-3. (C) MSCs are induced to differentiate into osteoblasts (i) and stain positive for alkaline phosphatase (ii) and for mineralized matrices by von Kossa assay (iii). (D) Under adipogenic conditions, BM-MSCs accumulate neutral lipid vacuoles (i), which are positively stained by Oil Red O assay (ii). (E) Under chondrogenic conditions BM-MSCs differentiate into chrondrocyte-like cells and stained positively for type II collagen by immunohistochemical analysis. Scale bars, 100 μm. (Original magnification, ×100 [A, Ci, Ciii, D]; ×200 [Cii].)
Under the induction of osteogenic medium for 3 weeks, BM-derived MSCs could differentiate into osteoblasts (Fig. 1Ci) showing positive alkaline phosphatase (Fig. 1Cii) and Von Kossa stainings (Fig. 1Ciii). Under adipogenic induction conditions for 3 weeks, the formation of intracellular microdroplets was noted (Fig. 1Di), and it stained positive for Oil Red O (Fig. 1Dii). Under chondrogenic conditions for 4 weeks, chondrocyte-like cells were noted, and positive immunohistochemical staining for type II collagen was shown (Fig. 1E).
In Vitro Hepatic Differentiation of Bone Marrow–Derived Mesenchymal Stem Cells
In the absence of serum cell proliferation arrested, and in the presence of HGF and bFGF, the fibroblastic morphology (Fig. 2Ai) of MSCs was lost and cells developed a broadened, flattened morphology (Fig. 2Aii) 1 week postinduction. In the presence of oncostatin M, dexamethasone and ITS+, a retraction of elongated ends was observed (Fig. 2Aiii) 2 weeks postinduction, and the cuboidal morphology of hepatocytes developed with increasing time of differentiation (Fig. 2Aiv-vi). After prolonged culture, the hepatocytic morphology further matured with the appearance of abundant granules in the cytoplasm (Fig. 2Avii) and was retained for over 12 weeks (Fig. 2Aviii).
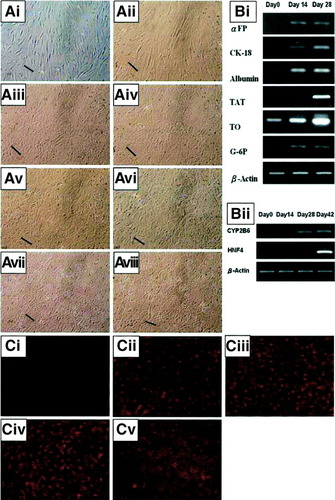
(A) In vitro hepatic differentiation of bone marrow–derived MSCs. Morphological changes of undifferentiated MSCs to differentiated hepatocytes under hepatogenic conditions. Undifferentiated MSCs (i); 1 week postinduction (ii); 2 weeks post-induction (iii); 3 weeks postinduction (iv); 4 weeks postinduction (v); 5 weeks postinduction (vi). The morphology of matured hepatocyte-like cells is achieved at 6 weeks postinduction (vii) and is retained for 12 weeks or more (viii). (B) Differentiated cells express hepatocyte-specific marker genes, by reverse-transcription–polymerase chain reaction at the indicated time points. (C) Immunofluorescence analysis showed that undifferentiated MSCs stain negative for albumin, (i) while positive staining was detected for hepatocyte-like cells at 2 weeks postinduction (ii), 4 weeks postinduction (iii), and 6 weeks postinduction (iv). Positive immunofluorescence staining for albumin is also detected in hepatoma cell lineHep3B (v). Scale bars, 100 μm. (Original magnification, ×100.) αFP, alpha-fetoprotein; CK-18, cytokeratin 18; TAT, tyrosine-aminotransferase; TO, tryptophan 2,3-dioxygenase; G-6P, glucose-6-phosphatase; β-Actin, beta-actin; HNF4, hepatocyte nuclear factor-4.
Reverse-transcription–polymerase chain reaction analysis showed the expression of alpha-fetoprotein and glucose 6-phosphatase by day 14, while tyrosine-aminotransferase, a late marker gene of hepatocytes,27 was detected by day 28. Expression of cytokeratin-18, albumin, and tryptophan 2,3-dioxygenase was detected at all time points and increased with time of differentiation, whereas undifferentiated cells did not express alpha-fetoprotein, tyrosine-aminotransferase, or glucose 6-phosphatase but did express low levels of albumin and cytokeratin-18, and tryptophan 2,3-dioxygenase (Fig. 2Bi). In addition, expression of cytochrome P450 2B6 was detectable by 4 weeks postinduction, and the expression of hepatocyte nuclear factor-4, a transcription factor largely expressed in adult liver,28 was detected at 6 weeks postinduction (Fig. 2Bii). Undifferentiated cells were negative for albumin by immunofluorescence staining (Fig. 2Ci), while differentiated cells were strongly positive at 2 weeks, 4 weeks, and 6 weeks postinduction (Fig. 2Cii-iv). Hep3B cells were used as positive control (Fig. 2Cv).
In Vitro Functional Characterization of MSC-Derived Hepatocyte-Like Cells and Expression of Bile Canaliculi-Specific Antigen
While undifferentiated MSCs did not exhibit the ability to uptake LDL (Fig. 3Ai), differentiated cells showed low levels of LDL uptake at 2 weeks and 4 weeks postinduction (Fig. 3Aii-iii). After 6 weeks of differentiation, hepatocyte-like cells demonstrated the ability to uptake significant levels of LDL (Fig. 3Aiv). Hep3B cells were used as positive control (Fig. 3Av). Pentoxyresorufin is a nonfluorescent compound O-dealkylatable by cytochrome P450 (mainly cytochrome P450 2B and 2F isofamilies29) into resorufin, emitting a red fluorescence. At 2 weeks postinduction, the supplementation of pentoxyresorufin to differentiated cells did not yield significant levels fluorescence in the absence (Fig. 3Bi) or presence of phenobarbital (Fig. 3Bii). At 4 weeks postinduction, the supplementation of pentoxyresorufin to differentiated cells resulted in low levels of fluorescence in the absence of phenobarbital (Fig. 3Biii), and a small increase in intensity was noted in the presence of phenobarbital (Fig. 3Biv), suggesting the presence of endogenous P450 enzymes in differentiated cells. At 6 weeks postinduction, differentiated cells demonstrated the ability to metabolize pentoxyresorufin in the absence of phenobarbital (Fig. 3Bv), and a significant increase in fluorescence activity was observed in the presence of phenobarbital (Fig. 3Bvi). Hep3B cells were used as positive control in the absence and presence of phenobarbital (Fig. 3Bvii-viii). The presence of stored glycogen, as determined by PAS staining, was not observed in differentiated cells at 2 weeks postinduction (Fig. 3Ci) but was visualized at 4 weeks postinduction (Fig. 3Cii). By 6 weeks postinduction, approximately 50% of differentiated cells stored glycogen (Fig. 3Ciii). Undifferentiated cells did not show the ability to store glycogen (Fig. 3Civ). When pretreated with diastase to digest glycogen, differentiated cells stain negative for glycogen (Fig. 3Cv). Hep3B cells were used as positive control (Fig. 3Cvi). The afore-tested functions were sustained for 12 weeks or more by the differentiated cells (data not shown). Secretion of urea by differentiated cells was measured, at weekly intervals, using the Sigma Urea assay kit according to manufacturer instructions. Background values resulting from the presence of ammonia were subtracted from the yielding values, and the production of urea was expressed as pg/cell/h. Urea production was detectable after 6 weeks postinduction (n = 3) and increased in a time-dependent manner with prolonged treatment in the presence of maturation medium, while undifferentiated MSCs, at day 0, did not produce urea (Fig. 3D).
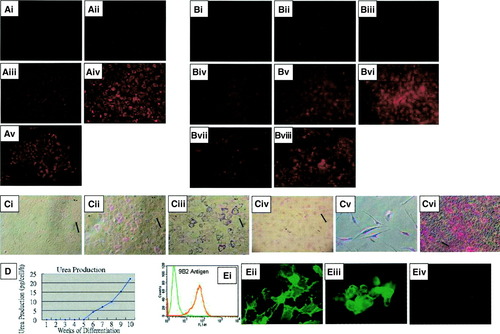
(A) In vitro functional characterization of hepatocytes differentiated from bone marrow–derived MSCs. Uptake of low-density lipoprotein is not detected in undifferentiated MSCs (i) but is detected in differentiated cells at 2 weeks postinduction (ii), at 4 weeks postinduction (iii), and at 6 weeks postinduction (iv). Uptake of low-density lipoprotein by hepatoma cell line Hep3B (v). (B) Cytochrome P450 enzyme activity, as evaluated by pentoxyresorufin-O-dealkylase assay, is not detectable at 2 weeks postinduction without phenobarbital (i) or with phenobarbital (ii); barely detectable without phenobarbital at weeks postinduction (iii) but can be induced in the presence phenobarbital (iv); and detectable at 6 weeks postinduction without phenobarbital (v) and can be further induced in the presence of phenobarbital (vi). Hepatoma cell line Hep3B also show P450 activity without phenobarbital (vii) and can be induced in the presence of phenobarbital (viii). (C) PAS staining for glycogen showed that differentiated cells do not store glycogen at 2 weeks postinduction (i), but begin to show glycogen storage at 4 weeks postinduction (ii), and approximately 50% of differentiated cells store glycogen at 6 weeks postinduction (iii). Glycogen stored in differentiated cells can be digested by pretreating with diastase and result in negative PAS staining (iv). Undifferentiated MSCs stain negative for glycogen storage (v) while hepatoma cell line Hep3B stains positive for glycogen storage (vi). (D) Differentiated cells produce urea in a time-dependent manner. (E) Differentiated cells express bile canaliculi-specific antigen 9B2 as determined by flow cytometry (i), and immunofluorescent staining shows that the antigen 9B2 is predominantly localized on the junction between adjacent cells (ii). Immunofluorescence analysis on Hep3B cell line reveals the expression of 9B2 antigen in a similar pattern (iii), while undifferentiated MSCs stain negative for 9B2 antigen by immunofluorescence analysis (iv). Scale bar, 100μm. (Original magnification, ×100 [A, B, Ci-iv, Cvi; ×200 [Cv, Eii-iv].)
The monoclonal antibody 9B2 is a liver-specific antibody found to react with an antigen expressed on the bile canaliculi formed between adjacent hepatocytes.30 Analysis by flow cytometry (Fig. 3Ei) revealed that differentiated hepatocyte-like cells were positive for the expression of antigen 9B2, and immunofluorescence assays (Fig. 3Eii) further showed that the antibody was predominantly localized on the surface membrane bordering adjacent differentiated cells. Immunofluorescence analysis on hepatoma cell line Hep3B show a similar staining pattern (Fig. 3Eiii), while undifferentiated MSCs stained negative (Fig. 3Eiv).
Characterization of Hepatocyte-Like Cells Differentiated From UCBDerived MSCs
The UCB-derived MSCs (Fig. 4A), under the same 2-step induction protocol, differentiated into hepatocyte-like cells (Fig. 4B) with expression of liver-specific genes, as previously reported,25 and expressed cytochrome P450 2B6 and hepatocyte nuclear factor-4 in the same pattern as the BM counterpart (Fig. 4C). Differentiated cells also showed production of albumin (Fig. 4D), and demonstrate the ability to uptake low-density lipoproteins (Fig. 4E). In the presence of pentoxyresorufin, differentiated cells exhibit a bright fluorescence (Fig. 4F) while undifferentiated cells did not (data not shown). Differentiated cells stain positive for glycogen by PAS assay (Fig. 4G) while undifferentiated cells and diastase-treated hepatocytes did not (data not shown). Hepatocyte-like cells differentiated from UCB-derived MSCs also demonstrate similar capacity for urea production (n × 3, Fig. 4H).
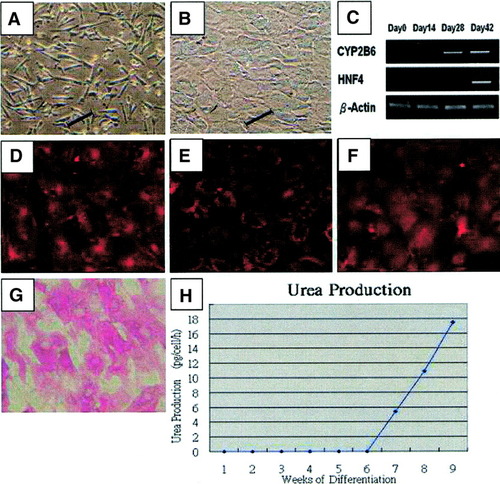
Hepatic differentiation of umbilical cord blood–derived MSCs. Undifferentiated mesenchymal stem cells ((A), under the same hepatogenic conditions, differentiate into hepatocyte-like cells (B) expressing liver-specific genes (C), produce albumin (D), and uptake low-density lipoprotein (E). Differentiated cells possess cytochrome P450 activity as determined by pentoxyresorufin-O-dealkylase assay (F), store glycogen which stain positive by PAS assay (G) and secrete urea in a time-dependent manner (H). Scale bar, 100μm. (Original magnification, ×100.)
Discussion
Previously, MSCs have been isolated from BM by plating total mononuclear cells followed by culture-expansion of the plate-adhering population. However, BM contains a mixture of different stem or progenitor cells, and plate-adhering cells obtained would comprise of heterogeneous cell types. Differentiation of such population into various cell lineages raises the question whether different progenitor cells within are responsible for the results observed. In this study, we adopt an approach previously reported for isolating multipotent MSCs from UCB by negative immunoselection and limiting dilution to obtain single cell-derived populations. Although the approach shares similarities with that for the isolation of MAPCs, culture conditions established for our cells are different to those for MAPCs. Further, the surface phenotype of our cells is consistent with classical MSCs,26 while MAPCs express CD13 but not CD44, SH-2, and SH-3, which have been reported to be some of the key differences between MSCs and MAPCs.20
Although previous studies have shown that different colonies may show varying differentiation potentials31 all colonies selected for culture-expansion under our criteria demonstrate equal potential for all cell lineages assayed while retaining normal karyotype (data not shown).
During embryonic development, the production of growth factors such as HGF and FGFs have been associated with endodermal specification.32, 33 In addition, the first event occurring after partial hepatectomy is a large increase in the blood level of HGF,34 suggesting that HGF plays a greater role in the early stages of hepatogenesis. Oncostatin M is a member of the interleukin 6 family cytokines and was originally identified by its ability to inhibit growth of A375 melanoma cells,35 and later studies demonstrated the progression of hepatocytic development toward maturation, in primary cultures, stimulated by oncostatin M.36, 37 Hence, we designed a 2-step serum-free protocol to effect the hepatic differentiation from MSCs. Prior to initiation of differentiation, cells are preconditioned by serum-withdraw but retaining the growth factors used in the culture medium to hinder cell proliferation while maintaining multidifferentiation potentials.
Under hepatogenic conditions, the fibroblastic morphology of MSCs gradually progressed toward the polygonal morphology of hepatocytes in a time-dependent manner and became apparent by 4 weeks postinduction. However, the mature cuboidal morphology with granulated structures is not fully developed until 6 weeks postinduction. In vitro functional assays on differentiated cells at different time points were consistent with the morphological changes. With the exception of albumin, which is strongly detectable by immunofluorescence analysis at all time points, other assays for functionality were found to be negative at 2 weeks postinduction but gradually became evident by 4 weeks postinduction. By 6 weeks postinduction, differentiated cells acquired complete functionality of all assays performed and are sustained to 12 weeks postinduction or later.
In well-differentiated hepatoma cell lines, the 9B2 antigen is first detected in cytoplasm and packaged in microvilli-lined vesicles, then vectorially transported to the cell surface and eventually fused with microvilli-lined vesicles from neighboring cells to form bile canaliculi.30 Immunofluorescence analysis on MSCs-derived hepatocyte-like cells and hepatoma cell line Hep3B revealed that the 9B2 antibody stained lightly on the membrane surface with stronger localization on the membrane bordering adjacent cells, suggesting the formation of bile canaliculi in differentiated hepatocyte-like cells under in vitro conditions.
It was also noted that cell density played a significant role in effecting hepatic differentiation. Optimal cell density was found to be 1.0 to 1.3 × 104 cells/cm2, and cells differentiated in homogeneous fashion. Visual inspection under phase-contrast microscopy as well as immunofluorescence analysis for albumin indicated that all cells differentiated into hepatocyte-like cells at optimal density. At suboptimal cell densities, hepatic differentiation was observed in cell clusters, while diffused cells failed to adopt the cuboidal morphology characteristic of hepatocytes. The hepatic differentiation potential of our MSCs was tested between the 5th passage and the 13th passage, and no differences were observed in the hepatic potential.
Although previous studies have suggested that BM and UCB may contain cells of hepatic potential, no evidence was provided to show that the limited results were attributed by stem cells.38, 39 Thus these studies suggest only the presence of hepatic progenitors in BM and UCB, as opposed to well-characterized stem cells' possessing hepatic differentiation potential. To our knowledge, this is the first report demonstrating that classical MSCs clonally derived from BM can differentiate into hepatocyte-like cells, under in vitro conditions, with comprehensive phenotype and functions characteristic of liver cells.
Previously, we reported that multipotent MSCs isolated from human UCB possess the potential to differentiate into hepatocyte-like cells under in vitro conditions.25 However, UCB is perceived to be a relatively “younger” source of stem cells than adult BM; hence, the previously reported stem cell population may encompass a greater differentiation potential than the classical MSC derived from BM. Further, limited characterizations on the differentiated hepatocyte-like cells were performed in the previous study. In the present study, we show that MSCs isolated from human BM as well as from UCB differentiate into hepatocyte-like cells exhibiting morphology, express primitive and mature marker genes in a time-dependent manner, and further acquire, in a time-dependent manner, comprehensive in vitro functions characteristic of liver cells including albumin production, glycogen storage, urea secretion, LDL uptake, and exhibit phenobarbital-inducible cytochrome P450 activity.
The differentiation potential of adult stem cells has long been believed to be limited to the tissue or germ layer of their origin. However, recent studies have demonstrated that adult stem cells may encompass a greater potential then once thought.40-42 Schwartz et al. reported for the first time that an adult marrow–derived stem cell, MAPC, could differentiate into functional hepatocyte-like cells21 under in vitro conditions in addition to mesodermal and ectodermal cell lineages.20, 43-45 While MAPCs exhibit certain similarities to embryonic stem cells,20 which may attribute to the broad potential, our findings indicate that MSCs can also differentiate into cells of endodermal origin in addition to those of the mesoderm, albeit that our MSCs are without embryonic stem cell features.
The in vitro system reported in this study for the derivation of hepatocyte-like cells has numerous advantages. First, cell source: We report a method for systemic derivation of functional hepatocyte-like cells from human MSCs, which are readily accessible from BM and UCB. Second, simplicity: The differentiation of MSCs into hepatocyte-like cells requires only a 2-step procedure without the need of additional surface coatings or treatments to culture vessels.21, 46 Third, longevity: Differentiated hepatocyte-like cells can be sustained for 12 weeks or more, with in vitro functions, making it an ideal candidate for pharmacological and toxicological studies, as well as bioartificial liver studies. Fourth, cell quantity: Undifferentiated MSCs can be extensively culture expanded in vitro and, thus, large quantities of differentiated cells can be generated for potential cell therapy or tissue-engineering applications. Fifth, rapidity: A magnitude of functional hepatocyte-like cells can be generated from a bone marrow aspirate within a reasonably short period of time.
In summary, our findings indicate that MSCs derived from both human bone marrow and umbilical cord blood can differentiate into functional hepatocyte-like cells in vitro, in addition to mesodermal and ectodermal lineages. The results of this study continue to challenge the perspective of the restricted differentiation potential of adult-derived stem cells. Most important of all, MSCs may serve as a cell source for tissue engineering or cell therapy of liver.




