The effect of bile salts on carbonic anhydrase
David E. Milov
Department of Pediatrics, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Search for more papers by this authorWou-Seok Jou
Department of Biochemistry and Molecular Biology, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Search for more papers by this authorRachel B. Shireman
Department of Food Science and Human Nutrition, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, Florida 32611
Search for more papers by this authorCorresponding Author
Paul W. Chun
Department of Biochemistry and Molecular Biology, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Department of Biochemistry and Molecular Biology, College of Medicine, Box 100245, J.H.M.H.C., University of Florida, Gainesville, FL 32610–0245===Search for more papers by this authorDavid E. Milov
Department of Pediatrics, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Search for more papers by this authorWou-Seok Jou
Department of Biochemistry and Molecular Biology, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Search for more papers by this authorRachel B. Shireman
Department of Food Science and Human Nutrition, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, Florida 32611
Search for more papers by this authorCorresponding Author
Paul W. Chun
Department of Biochemistry and Molecular Biology, Division of Gastroenterology, College of Medicine, University of Florida, Gainesville, Florida, 32610
Department of Biochemistry and Molecular Biology, College of Medicine, Box 100245, J.H.M.H.C., University of Florida, Gainesville, FL 32610–0245===Search for more papers by this authorAbstract
Bile salts are potent inhibitors of bovine carbonic anhydrase and human carbonic anhydrase I and human carbonic anhydrase II. To further characterize the binding of bile salts to carbonic anhydrase, rate constants for the CO2 hydration reaction in the presence of deoxycholate, cholate, glycocholate and taurocholate were determined using stop-flow experments. Values for the Michaelis-Menton dissociation constant for bovine carbonic anhydrase, human carbonic anhydrase I and human carbonic anhydrase II were found to be 5.2, 9.2 and 13.2 mmol/L, respectively. The inhibition constant values for the various bile salts tested ranged from 0.1 to 1 mmol/L for bovine carbonic anhydrase, 1.6 to 2.4 mmol/L for human carbonic anhydrase I and 0.09 to 0.7 mmol/L for human carbonic anhydrase II. Our results suggest a mechanism of noncompetitive carbonic anhydrase inhibition for bile salts.
Bile-salt binding to carbonic anhydrases as measured by scanning molecular sieve chromatography resulted in an increase in partition radius, molecular volume and surface area. The partition radius increased from 24 Å to 28 Å in the presence of 2.5 mmol/L sodium deoxycholate at critical micelle concentration. As determined by sedimentation equilibrium measurements, approximately 1 gm of carbonic anhydrase will bind 0.03 gm of deoxycholate, suggesting three to six binding sites for bile salt on the carbonic anhydrase molecule. The conformational changes and inhibition of carbonic anhydrases resulting from bile-salt binding may be important to the regulation of enzymatic activity in tissues along the enterohepatic circulation; by limiting bicarbonate availability this interaction may also contribute to the metabolic derangements seen in patients with cholestatic liver disease. (HEPATOLOGY 1992;15:288-296).
References
- 1 Linskog S, Coleman JE. The catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci USA 1973; 70: 2505–2508.
- 2 Deutch HF. Carbonic anhydrases. Int J Biochem 1987; 19: 101–103.
- 3 Maren TH, Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants and bacteria. Ann Rev Pharmacol Toxicol 1983; 23: 439–459.
- 4 Pocker Y, Tanaka N. Inhibition of carbonic anhydrase by anions in the carbon dioxide-bicarbonate system. Science 1978; 199: 907–909.
- 5 Hardison WGM, Wood CA. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol 1978; 235: E158–E164.
- 6 Barnhart JL, Combs B. Characterization of SC2644-induced chloresis in the dog: evidence for canalicular bicarbonate secretion. J Pharmacol Exp Ther 1978; 206: 190–197.
- 7 Dunmount M, Erlinger S, Uchman S. Hypercholeresis induced by ursodeoxycholic acid and 7-ketolithocholic acid in the rat: possible role for bicarbonate ion. Gastroenterology 1980; 79: 82–89.
- 8 Strasberg SM, Ilson RG, Paloheimo JE. Bile salt associated electrolyte secretion and the effect of sodium taurocholate on bile flow. J Lab Clin Med 1983; 101: 317–326.
- 9 Dodgson SJ, Forster RE, Storey BT. The role of carbonic anhydrase in hepatocyte metabolism. Ann NY Acad Sci 1984; 429: 516–524.
- 10 Scharschmidt BF, Van Dyke RW. Mechanisms of hepatic electrolyte transport. Gastroenterology 1983; 85: 1199–1214.
- 11 Hofmann AF. The liver biology and pathobiology. 2nd ed. In: IM Arias, WB Jakoby, H Popper, D Schachter, DA. Shafritz New York: Raven Press, Ltd., 1988: 553–569.
- 12 Osborn WRA, Tashian RE. An improved method for the purification of carbonic anhydrase isozymes by affinity chromatography. Anal Biochem 1975; 64: 297–303.
- 13 CRC Handbook. International critical tables. Vol II. 1980: P260.
- 14
Pocker Y,
Bjorkquist DW.
Comparative studies of bovine carbonic anhydrase in H2O and D2O stop-flow studies of kinetics of interconversion of CO2 and HCO
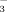 .
Biochemistry
1977;
16:
5698–5707.
.
Biochemistry
1977;
16:
5698–5707.
- 15 Khalifah RC. The carbon dioxide hydration activity of carbonic anhydrase. J Biol Chem 1971; 246: 2561–2573.
- 16 Kalifah RG, Strader DJ, Bryant SH, Gibson SH. Carbon-13 nuclear magnetic resonance probe of active-site ionizations in human carbonic anhydrase B. Biochemistry 1977; 16: 2241–2247.
- 17 Wilkinson GN. Statistical estimations in enzyme kinetics. Biochem J 1961; 80: 324–332.
- 18 Cleland WW. The statistical analysis of enzyme kinetic data. Adv Enzymol 1969; 29: 1–32.
- 19 Tu C, Wynns GC, Silverman DN. Inhibition by cupric ions of O18 exchange catalyzed by human carbonic anhydrase II. J Biol Chem 1981; 256: 9466–9470.
- 20 Silverman DN, Tu CK, Lindskog S, Wynns G. Rate of exchange of water from the active site of human carbonic anhydrase C. J Am Chem Soc 1979; 101: 6734–6740.
- 21 Haussinger D, Kaiser S, Steble T, Gerok W. Liver carbonic anhydrase and urea synthesis. Biochem Pharmacol 1986; 35: 3317–3322.
- 22 Brumbaugh EE, Saffen EE, Chun PW. Scanning molecular sieve chromatography of interacting protein systems. Biophys Chem 1979; 9: 299–311.
- 23 Saffen EE, Chun PW. Scanning molecular sieve chromatography of interacting protein systems IV: the difference profile method as applied by the Gibbs-Duhem expression in the analysis of the dimer-tetramer equilibria of oxyhemoglobin A. Biophys Chem 1979; 9: 329–344.
- 24 Oswein JQ, Chun PW. Human serum low density lipoproteinsodium deoxycholate interaction. J Biol Chem 1983; 258: 3645–3654.
- 25 Oswein JQ, Chun PW. Interaction of human serum apolipoprotein B with sodium deoxycholate. Biophys Chem 1983; 18: 35–51.
- 26 Yphantis DA. Equilibrium ultracentrifugation of dilute solutions. Biochemistry 1964; 3: 297–317.
- 27 Reisler E, Haik Y, Eisenberg H. Bovine serum albumin in aqueous guanidine hydrochloride solutions: preferential and absolute interactions and comparison with other systems. Biochemistry 1977; 16: 197–203.
- 28
Eisenberg H.
Forward scattering of light, X-rays and neutrons.
Q Rev Biophys
1982;
14:
141–172.
10.1017/S0033583500002237 Google Scholar
- 29 Casassa EF, Eisenberg H. Thermodynamic analysis of multicomponents solutions. Adv Protein Chem 1964; 19: 287–395.
- 30 Van Holde KE. In: L Hager, F Wold, eds. Physical biochemistry. Englewood Cliffs, NJ: Prentice-Hall, Inc., 1971: 146–147.
- 31 Milov DE, Shireman RB, Chun PW. Bile salt—induced swelling of carbonic anhydrase [Abstract]. FASEB J 1990; 4: A731.
- 32
De Sanctis SC.
Location of guest molecules in inclusion compounds of deoxycholic acid by means of Van der Waals energy calculations.
Acta Crystallogr
1983;
B39:
366–372.
10.1107/S0108768183002554 Google Scholar
- 33 Jou WS, Chun PW. Molecular mechanics of the formation of cholic acid micelles [in press]. J Mol Graphics, 1991.
- 34 Engberg P, Millquist E, Pohl G, Lindskog S. Purification and some properties of carbonic anhydrase from bovine skeletal muscles. Arch Biochem Biophys 1985; 241: 628–638.
- 35 Brumbaugh EE, Hilt ML, Shireman RB, Espinosa AJ, Chun PW. Scanning molecular sieve chromatography of interacting protein systems: least-squares analysis of small zone experiments. Anal Biochem 1986; 154: 287–304.
- 36 Chun PW, Baumbaugh EE, Shireman RB. Interaction of human low density lipoprotein and apolipoprotein B with ternary lipid emulsion. Biophys Chem 1986; 25: 223–241.
- 37 Maren TH, Sanyal G. The activity of sulfonamides and anions against the carbonic anhydrases of animals, plants, and bacteria. Ann Rev Pharmacol Toxicol 1983; 23: 439–450.
- 38
Maren TH.
Carbonic anhydrase: chemistry physiology and inhibition.
Physiol Rev
1967;
47:
597–781.
10.1152/physrev.1967.47.4.595 Google Scholar
- 39 Roda A, Cappalleri AR, Roda E, Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res 1982; 23: 490–495.
- 40 Edelhoch JL, Bocian DF, Sudmeier JL. Evidence for direct metal-nitrogen binding in aromatic sulfoamide complexes of cadmium (II)-substituted carbonic anhydrases by cadmium. Biochemistry 1981; 20: 4951–4954.
- 41 Liang J-Y, Lipscomb WN. Hydration of CO2 by carbonic anhydrase: intramolecular proton transfer between Zn+2-bound H2O and histidine 64 in human carbonic anhydrase II. Biochemistry 1988; 27: 8676–8682.
- 42 Tu C, Silverman DN. Role of histidine 64 in the catalytic mechanisms of human carbonic anhydrase II studies with a site-specific mutant. Biochemistry 1989; 28: 7913–7918.
- 43 Eriksson AE, Jones TA, Liljas A. Refined structure of human carbonic anhydrase II at 2.0 Å resolution. In: C Leventhal, ed. Proteins: structure, function, and genetics. New York: Alan R. Liss, Inc., 1988; 4: 274–282.
- 44
Kannan KK.
Crystal structure of carbonic anhydrase. In:
C Bauer,
G Gross,
H Bartels, eds.
Biophysics and physiology of carbon dioxide.
New York:
Springer-Verlag,
1980:
184–196.
10.1007/978-3-642-67572-0_20 Google Scholar
- 45
Hausinger DE,
Gerok W.
Hepatic urea synthesis and pH regulation role of Co2, HCO
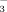 , pH and the activity of carbonic anhydrase.
Eur J Biochem
1985;
152:
381–386.
, pH and the activity of carbonic anhydrase.
Eur J Biochem
1985;
152:
381–386.
- 46 Cohen NS, Kyan FS, Kyan SS, Cheung CW. The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J 1985; 229: 205–211.
- 47 Garcia-Marin JJ, Dumont M, Corbic M, DeCovet G, Erlinger S. Effect of acid-base balance of acetazolamide on ursodeoxycholate-induced biliary bicarbonate secretion. Am J Physiol 1985; 248: G20–G27.
- 48
Grotmol T,
Buanes T,
Raeder MG,
DCCD (N′,N′-decyclohexylcarboiimide) inhibits biliary secretion of HCO
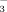 .
Scand J Gastroenterol
1987;
22:
207–21.
.
Scand J Gastroenterol
1987;
22:
207–21.
- 49 Salomoni M, Zuccato E, Granelli P, Montorsi W, Doldi SB, Germineani R, Mussini E. Effect of bile salts on carbonic anhydrase from rat and human gastric mucosa scan. J Gastroenterol 1989; 24: 28–32.
- 50 Mussini E, Salomoni M, Zuccato E. Effect of free conjugate bile salts on human and rat gastric carbonic anhydrase. International workshop on carbonic anhydrase [Abstract]. Palazzo Anaini-Spoleto, Italy, 1990.
- 51 Sanyal G. Comparative carbon dioxide hydration kinetics and inhibition of carbonic anhydrase isozymes in vertebrates. Ann NY Acad Sci 1984; 429: 165–177.
- 52 Kararli T, Silverman DN. Inhibition of the hydration of CO2 catalyzed by carbonic anhydrase III from cat muscle. J Biol Chem 1985; 260: 3484–3489.




