Interplay between α-thalassemia and β-hemoglobinopathies: Translating genotype–phenotype relationships into therapies
Abstract
α-Thalassemia represents one of the most important genetic modulators of β-hemoglobinopathies. During this last decade, the ongoing interest in characterizing genotype–phenotype relationships has yielded incredible insights into α-globin gene regulation and its impact on β-hemoglobinopathies. In this review, we provide a holistic update on α-globin gene expression stemming from DNA to RNA to protein, as well as epigenetic mechanisms that can impact gene expression and potentially influence phenotypic outcomes. Here, we highlight defined α-globin targeted strategies and rationalize the use of distinct molecular targets based on the restoration of balanced α/β-like globin chain synthesis. Considering the therapies that either increase β-globin synthesis or reactivate γ-globin gene expression, the modulation of α-globin chains as a disease modifier for β-hemoglobinopathies still remains largely uncharted in clinical studies.
INTRODUCTION
The β-hemoglobinopathies are the most prevalent inherited monogenic disorder, caused by mutations affecting adult β-globin chain synthesis.1 The most clinically significant phenotypes of β-hemoglobinopathies are the β-thalassemias, HbE, and sickle cell disease (SCD). The World Health Organization (WHO) has conservatively estimated that 5%–7% of the world population are carriers of various types of hemoglobinopathies and estimated that over 300,000 severely affected patients are born worldwide each year.1 More than 300 β-globin gene mutations have been reported to cause β-hemoglobinopathies.1 Remarkably, the global distribution and gene frequency of β-globin gene mutations overlap with the geographic distribution of malaria.1 While several factors can be attributed to their high gene frequency, the natural selection favoring heterozygous individuals afforded protection against lethal malaria is the main mechanism.2, 3 Although β-hemoglobinopathies are most common in African, Mediterranean, Middle Eastern, and Asian regions, the outcome of historical and recent immigration trends means these disorders are encountered with increasing frequency in many parts of the world, including Northern Europe, North America, and Australia.1
In regions where β-hemoglobinopathies are prevalent, there is also an increased prevalence of α-thalassemia, which similarly protects against malaria.4, 5 Considering the high frequency of both α- and β-globin gene mutations in the malaria-endemic regions, it is not uncommon to encounter individuals that co-inherit both α- and β-globin gene mutations, producing considerable clinical heterogeneity.6 In β-thalassemia or SCD patients, the coinheritance of a single α-globin deletion or inactivation generally has a minimal impact, but two or three mutated α-globin genes can have a profound impact by reducing or even eliminating the clinical course on disease.7-10 Although several genetic modifiers of the disease have been identified, α-thalassemia remains one of the most frequent and significant modifiers of β-hemoglobinopathy phenotypes globally.7-10
REGULATION OF THE HUMAN α-GLOBIN LOCUS
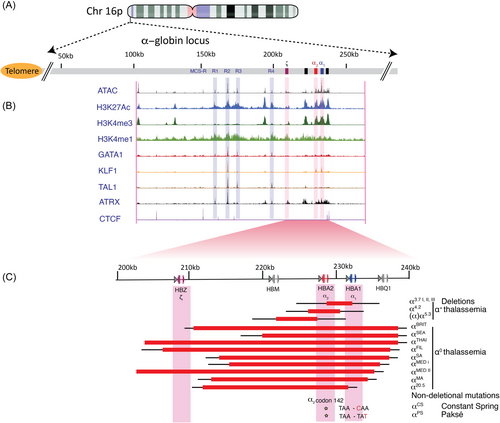
The human α-globin cluster is located near the telomeric end of the short arm of chromosome 16 (16p13.3), containing an embryonic α-like globin gene ζ-globin (HBZ) and two adult α-globin genes (α2 and α1, HBA2 and HBA1, respectively). Upstream of the α-globin cluster, there are four highly conserved enhancer regions identified as multispecies conserved sequences (MCS) R1-R4, critical in the regulation of the α-like globin genes (Figure 1A). Although the four upstream elements are classified as enhancers, they are not functionally equivalent. Results from chromosome conformation capture (3C) assays have shown the MCS elements cooperate to interact with the α-globin gene to form long-range chromatin looping in human erythroid precursor cells.15, 16 Notably, the MCS-R2 element (previously known as HS-40) was identified as the major enhancer capable of driving high levels of α-globin gene expression in erythroid cells, whereas MCS-R1, R3, and R4 represent weaker enhancers.15, 16 The critical component of the MCS-R2 is a 260 bp core sequence that contains several conserved erythroid transcription factor-binding sites.17, 18 Individually, the transcription factors GATA-binding factor 1 (GATA1), Krüppel-like factor 1 (KLF1 or formerly known as EKLF), nuclear factor-erythroid 2 (NF-E2), and stem cell leukemia/T-cell acute leukemia 1 (SCL/Tal1) are recruited to MCS-R2.19, 20
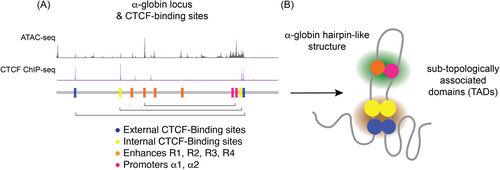
Chromatin structure and chromosomal conformation have a critical role in determining the outcome of gene activity. In conjunction with the other MCS enhancer regions and recruitment of E2-alpha (E2A), LIM domain-binding protein 1 (LDB1), Lim-only 2 (LMO2) and other chromatin architectural proteins such as CCCTC-binding factor (CTCF), facilitate chromatin looping into dynamic sub-topologically associated domains (TAD) confining α-globin enhancer interactions with receptive promoters to control gene activity during erythroid differentiation (Figure 2).19, 21-24 Recent three-dimensional chromatin conformation studies of the mouse α-globin locus have delineated structural re-organization of chromatin architecture whereby specific interactions between enhancers and promoters form a dynamic folded hairpin conformation as chromatin accessibility increases during early erythroid differentiation.19, 21
In addition, epigenetic alterations modify the activation of α-globin gene expression. First, the α-globin gene cluster is in a gene-dense region and in an open chromatin conformation. However, in nonerythroid cells, active epigenetic mechanisms silence α-globin gene expression. The polycomb repressive complex 2 (PRC2), which has histone methyltransferase activity, binds to α-globin genes and primarily methylates histone H3 on lysine 27 to increase the histone 3 lysine 27 trimethylation (H3K27me3) chromatin mark, an epigenetic modification associated with transcriptional repression.25 In erythroid cells, PRC2 is displaced and the H3K27me3 chromatin marks are removed by histone lysine demethylases, such as KDM6B/JMJD3, that specifically demethylates di- or trimethylated K27 of histone H3.26 Importantly, the interplay between KDM6B and MCS-R2, located 40 kb upstream from the ζ-globin gene, facilitates the removal of repressive H3K27me3 epigenetic marks to upregulate α-globin gene expression in erythroid cells.25, 26 This interaction is also supported by a naturally occurring MCS-R2 deletion, contributing to a dramatic reduction in α-globin gene expression, to <5% of regular expressions, thus demonstrating the crucial role of MCS-R2 α-globin gene expression during erythropoiesis.27, 28 Of the highly expressed functional α-like globin genes, ζ-globin constitutes the embryonic globin gene, while α1 and α2-globins are the dominant α-globin genes throughout all other stages of development. Considering α1 and α2-globin genes encode identical proteins, α2-globin is expressed at levels 2–3-fold higher than α1-globin at both the transcriptional and translational levels.29, 30 This is apparently due to the location of the α2-globin gene positioned closer to the upstream MCS R1-4 enhancer region and the unequal distribution of epigenetic marks across both α-globin genes.29, 31
α-THALASSEMIA
The most common genetic defects in α-thalassemia are deletions of the α-globin genes. When α-globin synthesis is reduced to ~25% or less, patients experience a moderate to severe hemolytic anemia caused by the accumulation of excess β-globin chains and formation of β-globin-tetramers (HbH) within erythrocytes, resulting in HbH disease.14 Furthermore, the instability of HbH leads to the production of inclusion bodies in red blood cells and a variable degree of hemolytic anemia. However, when α-globin synthesis is abolished during early intrauterine development, excess γ-globin chains produce γ-globin tetramers (known to as Hb Bart), which has very high oxygen affinity, resulting in effectively no oxygen delivery.32 Consequently, the profound intrauterine hypoxia and chronic effects on the developing fetus lead to severe developmental abnormalities and perinatal demise, known as Hb Bart's hydrops fetalis.14, 32 Besides the functional properties of HbH and Hb Bart, the clinical severity is based on which of the two α-globin loci are affected. Over 120 known molecular defects, both gene mutations and deletions, cause α-thalassemia.33, 34 The most common forms of α-thalassemia (-α3.7 or -α4.2), are deletions resulting from homologous recombination between misaligned chromosomes involving one or both globin genes (HBA1 and HBA2).14 The overall distribution of α-thalassemia follows a similar pattern to that of β-thalassemia, extending from Sub-Saharan Africa, throughout the Mediterranean region, Middle East, and East and Southeast Asian countries.14 For instance, the frequency of α-thalassemia varies from 10% to 20% in some regions of Africa to 40% in some Middle Eastern countries. In several Southeast Asian countries, the prevalence of α-thalassemia can range from 20% in Thailand to 51% in Vietnam.35, 36 Remarkably, in the north coastal region of Papua New Guinea, the α-thalassemia deletion (-α4.2) is found in >80% of the indigenous population.37
Deletions that remove the majority of the α-globin gene cluster, including both α-globin genes such as in Mediterranean (--MED), and Southeast Asian (--SEA) deletions, result in α°-thalassemia.14 Intriguingly, in some cases, persistent expression of the embryonic ζ-globin gene has been reported at levels >1% in carriers with --MED and --SEA deletions.38-40 Normally, ζ-globin is expressed in the yolk sac until approximately 6 weeks of gestation. It is then silenced and replaced by α-globin expression. While substantial efforts have been made to understand the switch from γ- to β-globin, the expression of ζ- to α-globin has remained poorly understood. Previous studies have also shown that ζ-globin expression in mouse and/or human erythroid cells may be upregulated when the expression of key transcription factors such as MYB, KLF1, SOX6, BCL11A, and LRF are perturbed.11, 41 Examining ζ-globin expression among different α°-thalassemia carriers will elucidate the mechanisms underlying ζ-globin silencing and facilitate new therapeutic strategies for HbH patients or Hb Bart's survivors by de-repressing ζ-globin expression to compensate for the loss of α-globin.42
In the cases of nondeletion α-thalassemia, a series of over 70 mutations have been identified, including point mutations affecting RNA splicing, polyadenylation, messenger RNA (mRNA) translation, frameshift mutations, and chain termination mutations.14, 43 Many nondeletion mutants occur in the HBA2 gene and, therefore, have a more severe effect on α-globin gene expression. For example, common nondeletional HBA2 mutations, such as Hb Constant Spring (HbCS) and Hb Paksé, result from α2-globin stop codon mutations (TAA → CAA) and (TAA → TAT), respectively, and elongate the α-globin chain by 31 amino acids before the next in-frame termination codon. These α-Hb variants are extremely unstable, causing insoluble inclusions and red cell instability. HbCS appears to be the most prevalent α-globin chain variant since it has been identified in higher frequency in several populations from Southeast Asia, including northeast Thailand (1%–2%) to southern China (5%–8%).43
Several α-globin mutations have also been identified to restrict binding to the Alpha-Hemoglobin Stabilizing Protein (AHSP). AHSP is an abundant erythroid-specific molecular chaperon that plays an important role in maintaining cellular proteostasis by supporting α-globin stability, folding, and hemoglobin tetramer assembly.44 After α-globin synthesis, AHSP binds to a basic and hydrophobic (BH) interface, forming an αHb-AHSP complex and protecting against the deleterious effects arising from α-globin aggregation and precipitation.45 AHSP is eventually displaced by β-globin to form α1β1 heterodimers, which then bind to another heterodimer to form a mature HbA tetramer. Interestingly, the two elongated α-Hb variants, HbCS and Paksé, have also been reported to impair binding to AHSP.46 Besides the elongated α-Hb variants, point mutations mostly located within the G and H helices (103–124aa), such as Groene Hart P119S, Foggia F117S, and Questembert S131P, are at the AHSP binding interface, and play a major role in limiting the α-globin-AHSP interactions and give rise to a mild α-thalassemia phenotype.45, 47 Conversely, when AHSP gene mutations are co-inherited in patients with β-thalassemia, α-globin chain instability, and precipitation are exaggerated, worsening the disease.48, 49
Moreover, very rare and unusual cases of α-thalassemia are caused by mutations in the α-thalassemia mental retardation X-linked (ATRX) gene.50 Affected individuals are characterized by mild to severe intellectual disability and developmental delay in males.14, 51, 52 The ATRX gene, located on the long arm (q) of X chromosome (Xq13.3), encodes for the ATRX protein, belongs to the SWI/SNF2 family of ATP-dependent chromatin remodeling complexes, which execute a broad range of biological functions such as transcriptional regulation, DNA repair, and chromosome segregation.53 More recently, ATRX mutations have been shown to play a key role in controlling α-globin expression by reducing chromatin accessibility and associated chromatin modifications within the α-globin regulatory elements.13 ChIP-seq data for ATRX occupancy sites revealed an enriched region within the MCS-R2 site that overlaps with GATA1, KLF1, and TAL1 erythroid transcription factors-binding sites and, H3K4me1, H3K4me3, and H3K27ac histone modifications (Figure 1). Consequently, various ATRX mutations have been identified to disrupt α-globin gene expression to different degrees, which is reflected in the unusual proportions of red cells containing HbH inclusions and microcytic hypochromic anemia.14
β-THALASSEMIA
In β-thalassemia, the reduction or the absence of β-globin synthesis generates the α/β-globin chain imbalance. More than 300 disease-causing mutations have been identified, with the vast majority caused by point mutations, affecting almost every known stage of gene expression.54 For example, CD41/42(-TCTT), CD17, and IVS2-654 represent common β-mutations located in Southeast Asia, while other β-mutation such as CD39, IVS1-1, and IVS1-110 represent the most common splicing mutations in the Mediterranean region.55 Early symptoms of β-thalassemia appear soon after birth when the fetal γ-globin gene is progressively silenced and replaced by defective β-globin gene synthesis. The pathophysiology of the disease can be very heterogeneous, ranging from nearly asymptomatic to life-threatening severe anemia. The latter has major effects on erythroid precursors resulting in the formation of insoluble α-globin, α-hemichromes, and free iron, which contribute to the generation of reactive oxygen species (ROS) and apoptosis. Normally, when free α-globin is in excess, cellular protein quality control (PQC) pathways help maintain erythroid proteostasis by resolving unpaired α-globin, decreasing protein misfolding and aggregation. In particular, the Heat Shock Protein 70 (HSP70) chaperone system has emerged as playing a vital role in modulating PQC activities at different stages of erythropoiesis.56 However, in β-thalassemia, excess α-globin inundates the PQC pathways contributing to proteotoxic stress during erythropoiesis. Under such conditions, α-globin sequesters the molecular chaperone HSP70 in the cytoplasm, preventing it from performing its normal physiological role of protecting GATA1 from caspase-3-mediated proteolytic cleavage.56, 57 Consequently, the early degradation of GATA1 drives the expansion of early-stage erythroid precursors and is associated with apoptosis of late-stage precursors and anemia.56, 57 The expansion of erythroid precursors in the bone marrow results in skeletal deformities, osteoporosis, while extramedullary erythropoiesis leads to splenomegaly and hepatomegaly.55, 58 Additionally, elevated systemic levels of growth differentiation factor 15 (GDF15) and erythroferrone (ERFE) are released from the large pool of erythroid progenitor during stress erythropoiesis to suppress hepcidin expression.59, 60 In this situation, dietary iron absorption, and availability is increased, which contributes to systemic iron overload and increased ROS production, ultimately causing end-organ damage.6, 61 Treatment options are limited, consisting mainly of chronic RBC transfusions that unavoidably exacerbate iron deposition and toxicity, damaging the liver, heart, pancreas, thyroid, and other endocrine glands and invariably increasing the morbidity and mortality amongst patients. Chelating agents allow for the active elimination of excess iron; however, chelation therapy is not sufficient to prevent iron overload-related complications in all patients.6, 61 Allogeneic hematopoietic stem cell transplantation (HSCT), and more recently approved gene therapy options, including gene addition or gene editing, have increased the curative treatment options available for patients.62-64
STRUCTURAL HEMOGLOBIN VARIANTS
The most frequent and clinically significant structural hemoglobin variants are HbS, HbC, and HbE. These variants differ from normal hemoglobin (HbA) by a single amino acid residue caused by the point mutation in the β-globin gene. HbS results from a glutamic acid to valine substitution at codon 6 of the β-globin gene (common in sub-Saharan Africa and the Middle East), while HbC is characterized by a glutamic acid to lysine substitution also at codon 6 (dominant in West Africa). The homozygous state for the HbS results in SCD, while the compound heterozygous state for the HbS and HbC genes results in HbSC disease, although milder than HbS, still has significant health risks.65 In SCD, the pathogenesis of the disease is reflected in the tendency for HbS to polymerize and form intercellular fibers causing red blood cells to adopt a poorly deformable sickled configuration under hypoxic conditions. Moreover, HbS is unstable and is almost entirely responsible for ROS generation inside RBCs.66 Consequently, sickled RBCs are fragile contributing to intravascular hemolysis, promoting activation of endothelial cells and inflammation. These interactions lead to vascular obstruction, tissue ischemia, and painful vaso-occlusive events. Ultimately, the disease progresses to significant end-organ damage involving the bone marrow, spleen, and kidneys, as well as pulmonary and neurologic complications.65, 67
The HbE variant results from a glutamic acid to lysine substitution at codon 26 of the β-globin gene, producing a structurally abnormal hemoglobin and activating a cryptic splice site, causing aberrantly spliced β-globin mRNA. HbE, in its heterozygous and homozygous states, does not pose major clinical issues but, because the HbE β-globin is produced at a reduced level, can interact with β0-mutations to produce a condition called HbE/β0-thalassemia.68 This condition is by far the most common severe form of β-thalassemia globally, and is particularly prevalent in India, Bangladesh, and throughout Southeast Asia, such as in Thailand, Laos, and Cambodia.68 Additionally, structural hemoglobin variants may interact with other β-thalassemia mutations to produce remarkable clinical variability, ranging from asymptomatic to life-threatening anemia requiring transfusions from an early age. Currently, the reasons for this extraordinary clinical heterogeneity are not fully understood. However, information on allele frequency and genetic diversity amongst various populations has been used to identify several genetic factors that influence the clinical phenotype. For example, several genome-wide association studies (GWAS) in different ethnic groups have identified three major quantitative trait loci (QTLs) with increased expression of γ-globin gene: (1) Gγ XmnI polymorphism, (2) HBS1L-MYB intergenic region, and (3) BCL11A gene.36, 69-71 Polymorphisms in these three loci were found to be responsible for the variation in HbF and F-cell levels, which together account for up to 50% of the genetic variation affecting HbF levels.36, 70 However, among the different genetic modifications attributed to clinical variability in HbE, β-thalassemia or SCD, the high prevalence of α-thalassemia mutations, contributing to variable α-globin gene expression is additionally considered a major genetic modifier of disease.8, 35, 36
α-GLOBIN AS A MAJOR DISEASE MODIFIER OF β-HEMOGLOBINOPATHIES
In 1961, Fessas, Stamatoyannopoulos, and Karaklis first reported α-globin as a potential modifier gene in the context of β-thalassemia and proposed that lowering levels of α-globin may improve clinical outcomes.30, 72 Subsequently, Kan and Nathan also suggested that combinations of α- and β-thalassemia genes may be favorable for a normal hemoglobin phenotype.73 This led the field to further discover cohorts of β-thalassemia patients with different combinations of α/β-globin gene compositions with decreased disease severity, thereby demonstrating α-thalassemia as a significant predictor for a milder thalassemia phenotype.9, 74-77
Similar association studies have been reported for HbE/β0-thalassemia, which represents one of the most common β-thalassemia subtypes identified throughout parts of Southeast Asia.68 The first description of HbE/β0-thalassemia was reported in 1956; however, it was much later that detailed reports emerged from Thailand describing the interaction of various forms of α-thalassemia with HbE/β0-thalassemia patients.78, 79 Intriguingly, coinheritance of α-thalassemia appeared to have a pronounced and beneficial impact on HbE/β0-thalassemia patient cohorts. These individuals were either asymptomatic or displayed a very mild phenotype that did not require medical attention.68 A single α-globin gene deletion (-α/αα) has minimal impact, but two α-globin gene deletions (-α/-α or --/αα) were found to have a major beneficial impact on the disease severity by normalizing α:β-globin chain balance and minimizing the harmful effects of α-globin chain precipitation and cell damage to erythrocytes and their precursors.8, 30, 58, 80 In contrast to the beneficiary findings of α-and β-thalassemia co-inheritance, the co-inheritance of excess of α-globin genes (α-globin gene triplication; αα/ααα, and ααα/ααα) can worsen disease severity by increasing the globin chain imbalance.8, 81-83
Relatedly, α-thalassemia is also regarded as one major genetic modifier of SCD. Notably, up to 55% of SCD individuals from West Africa have been reported to co-inherit α-thalassemia, which has a protective role against many disease-related complications.84 The α-thalassemia deletion (-α3.7) is the most common α-thalassemia in sub-Sahara African populations with varied prevalence reported from different populations with SCD, including 37% in Cameroon, 43% in Nigeria, and 56% in Tanzania.85, 86 The beneficial effects of α-thalassemia are from the reduced intracellular hemoglobin concentration in RBCs, as measured by a lower mean cell hemoglobin concentration (MCHC), a parameter for sickle RBC density and better RBC deformability.7, 87-93 Notably, co-inheritance of α-thalassemia in SCD improved blood rheology, which was most apparent in SCD patients carrying one or two -α3.7-thalassemia mutations (i.e., -α/αα; -α/-α), exhibiting reduced intracellular HbS concentration, HbS polymerization, and hemolysis.7, 87-93 The presence of α-thalassemia reduced the adhesion of sickled cells to the endothelium in vivo,94, 95 and consequently, individuals had fewer vaso-occlusive complications and reduced pulmonary hypertension, leg ulcers, and stroke.84, 85, 96-99 Furthermore, the median age of SCD diagnosis increased with one or two α-globin gene deletions and improved the overall survival rate of SCD patients, which could possibly explain the higher proportion of α-thalassemia gene deletions (-α/αα; -α/-α) among SCD patients than controls, particularly in Sub-Saharan Africa.98, 100, 101 Similarly, a higher prevalence of α-thalassemia was found in patients ≥10 years of age than in the younger group, suggesting a possible advantageous effect of α-thalassemia on the survival of patients with SCD in India.102
More recently, in one of the largest single-center studies conducted on 614 SCD patients from Brazil, ranging from 8 to 67 years old, described the influence of α-thalassemia -α3.7 deletion (αα/-α and -α/-α) on clinical outcome.98 In agreement with previous reports, co-inheritance of the -α3.7 deletion in SCD patients, lowered the degree of hemolysis and was therefore protective in the development of stroke, priapism, and cholelithiasis.98 While the impact of co-inheritance of α-thalassemia has been widely studied in SCD, its interaction with individuals with sickle cell trait (SCT) is now being recognized as a key modifier of recently accepted clinical complications.103 For example, in a large cohort study of 2916 African Americans with SCT, α-thalassemia stratified by -α3.7 deletion copy number status, significantly lowered the risk of chronic kidney disease (CKD) among individuals with SCT, whereas SCT carriers without α-thalassemia displayed a 2.6-fold increased risk of CKD.103-105 Additionally, as an example of evolving genetic complexity, a common α-globin regulatory variant located within the MCS-R2 enhancer element (rs11865131) was identified to mitigate the protective effect of the -α3.7 deletion on stroke among 1139 HbSS patients.103 While the molecular mechanism by which this SNP increased HBA1/HBA2 expression was not resolved, it indicates that co-inheritance of SNPs within key regulatory elements may have important implications for risk stratification and clinical management of SCT and SCD patients.103
In contrast to the beneficial effects of α-globin gene deletions, excess of α-globin gene copy number was independently associated with greater prevalence of CKD and end-stage kidney disease among Americans of African descent, thus highlighting the importance of understanding the role of α-globin expression in renovascular pathophysiology, independent of the co-existing SCT in this population.106 Taken together, these studies highlight the substantial impact α-globin gene expression can have on disease. It also emphasizes the importance of understanding the mechanism of α-globin gene regulation and how its expression may be exploited to mitigate many of the clinically relevant phenotypes in β-hemoglobin disorders.84, 85, 96-99
NOVEL THERAPIES TARGETING α-GLOBIN EXPRESSION
Modulation of the ubiquitin-proteasome system (UPS)
During erythropoiesis, HbA synthesis is intricately coordinated to minimize the accumulation of free α-globin. The molecular chaperone, AHSP, binds to a basic and hydrophobic (BH) domain of free α-globin and prevents misfolding and protease digestion prior to HbA assembly, whereas unpaired or excess α-globin is selectively eliminated by the UPS (Figure 3).110-112 The UPS is central to the regulation of all cellular processes, such as transcriptional regulation, signal transduction, and stress response, and defects in this system can result in several human disorders.113 During normal erythroid differentiation, several quality control factors are upregulated, required for the degradation and elimination of various intracellular organelles. One such factor is the ubiquitin-conjugating enzyme E2O (UBE2O), which displays both E2 ubiquitin-conjugating enzyme and E3 ubiquitin ligase activities and determines the flow of ubiquitinated proteins through the 26 S proteasome system (Figure 3B)114 The induction of UBE2O during erythropoiesis remodels the erythroid proteome during the transitional reticulocyte stage.114 In such a way, UBE2O helps to shape the complex erythroid proteome into an erythrocyte containing predominantly hemoglobin. Additionally, UBE2O acts as an independent quality control factor, recognizing misfolded and unassembled proteins, mediating a broad ubiquitination program including mono-ubiquitination and poly-ubiquitination of several substrates.112 Notably, UBE2O plays a pivotal role in targeting excess α-globin to the proteasome for degradation.114, 115 UBE2O, binds to the exposed BH domain in the monomeric free α-globin to mediate its ubiquitination and achieve globin chain equilibrium during hemoglobin assembly.
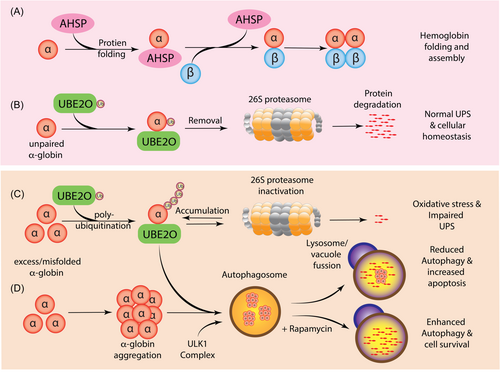
In β-thalassemia, where the level of free α-globin chain exceeds the detoxification capacity of the proteasome-mediated degradation system, toxic α-globin aggregates, along with the accumulation of hemichromes, constitute a major source of intracellular ROS production and oxidative stress (Figure 3).116-119 Notably, the tightly regulated UPS is also a target of oxidative stress. Under prolonged oxidative stress, the entire ubiquitin-proteasome system can malfunction, by both increasing ubiquitination activity and inhibiting the 26 S proteasome system, resulting in the accumulation of polyubiquitinated proteins potentially affecting virtually all cellular processes (Figure 3C).107-109 Oxidative stress and oxidative modifications play a significant role in β-thalassemia and SCD pathogenesis, contributing to extensive erythroid ubiquitination.116-119 Intriguingly, the loss of UBE2O expression was found to lower levels of ubiquitinated α-globin and mitigate the effects of excess α-globin and extramedullary erythropoiesis in a mouse model of β-thalassemia.114 These definitive results demonstrate the biological and clinical relevance of the posttranslational modification (PTM) activity of UBE2O and suggest the UBE2O-ubiquitin axis constitutes a potential therapeutic target. It is, therefore, crucial to precisely delineate how reduced UBE2O expression and even how components of the ubiquitin proteolytic pathway may be targeted to influence the clinical phenotype of β-thalassemia and SCD. More recently, hydroxyurea (HU) treatment of SCD patients was found to reverse the oxidative stress-induced PTMs in sickle RBCs to levels observed in controls, thus providing novel insights into the multifaceted therapeutic effects of HU.119
Upregulation of autophagy
In the erythroid lineage, autophagy plays an important role in erythroid maturation, including the elimination of mitochondria and ribosomes during the final stages of erythroid differentiation. In addition, autophagy constitutes a cytoprotective response activated by cells to cope with cellular stress.120, 121 However, if the stress is excessive and the induced injury irreversible, autophagy can turn into a cell death mechanism and substitute for apoptosis (Figure 3C).122 Interestingly, enhanced autophagy at the early stage of erythroid differentiation in β-thalassaemic mice and β-thalassemia/HbE patients was associated with decreased apoptosis.123 Conversely, inhibition of autophagy by chloroquine significantly increased erythroblast apoptosis, thus highlighting that autophagy plays a protective role in β-thalassemia erythroblasts during stress erythropoiesis.123 Recent studies have shown that rapamycin, a mammalian target of rapamycin complex 1 (mTORC1) inhibitor, increased clearance of excess α-globin chains via the induction of Unc-51-like autophagy-activating kinase 1 (ULK1) (Figure 3D). By stimulating this pathway, rapamycin improved the terminal maturation of erythroblasts and anemia in a mouse model of β-thalassemia.110 However, the loss of the Ulk1 gene in β-thalassaemic mice reduced autophagic clearance of excess α-globin in erythroblasts and exacerbated disease phenotypes.110 Recent studies have shown that the bicistronic microRNA miR-144/45, which is the highest expressed miR locus in terminal erythroid cells, is a genetic modifier of β-thalassemia.124 The disruption of the miR-144/451, released the mTORC1-mediated repression of ULK1-mediated autophagy of free α-globin to alleviate ineffective erythropoiesis and hemolysis in β-thalassemia mice.124 The identification of ULK1 as a central regulator of excess α-globin has drawn attention to the mTORC1 signaling pathway and potential clinical application of mTORC1 inhibitors to enhance autophagy and decrease ineffective erythropoiesis in β-thalassemia patients with substantially residual β- or γ-globin chain synthesis.110
Additional studies have tested rapamycin in combination with RAP-536 in β-thalassaemic mice.125 RAP-536, the murine analog of luspatercept, selectively binds transforming growth factor-β (TGF-β) superfamily ligands to reduce SMAD2/3 signaling and promote erythroid maturation. Interestingly, combination treatment significantly increased Hb levels by >40% in β-thalassaemic mice, while the single agents achieved modest increases in Hb levels (19.2% with RAP-536 alone, 13.0% with rapamycin alone).125 Altogether, these encouraging preclinical results provide a rationale for combining rapamycin and potentially other mTORC1 inhibitors with luspatercept to treat β-thalassemia. Moreover, rapamycin has been reported to increase γ-globin mRNA expression,126 HbF production in human cell cultures,127 SCD mice and patients.128-130 The ongoing interest in rapamycin as a potential drug candidate for β-hemoglobinopathies is related to the fact that it is a Food and Drug Administration (FDA)-approved immunosuppressant (also known as sirolimus) with validated data related to the pharmacokinetic and pharmacodynamic properties.131 Hence, drug repurposing forms a key role in identifying new candidate medications for β-hemoglobinopathies. Subsequently, sirolimus was granted orphan drug designation by the European Medicinal Agency (EMA, Europe) and by the FDA (USA) for both β-thalassemia and SCD in two ongoing clinical trials (ClinicalTrials.gov: NCT03877809 and NCT04247750).132, 133
Targeting epigenetic regulation of α-globin gene expression
The understanding of epigenetic mechanisms and their effect on globin gene expression has paved the way for the development of new treatments for β-hemoglobinopathies. Considering that the chromatin environment of the α- and β-globin locus have very clear differences, it may be possible to identify drugs with differential effects on α- and β-globin expression. Interestingly, epigenetic downregulation of α-globin gene expression was demonstrated using a derivative of 8-hydroxyquinoline (IOX1) in primary human erythroid progenitor cells.134 IOX1, is a broad-spectrum 2-oxoglutarate (2OG)-dependent oxygenase inhibitor. 2OG-oxygenases catalyze a diverse range of reactions involving histone and nonhistone targets. The largest of these subgroups are the iron-dependent histone lysine demethylases (KDMs), that contain the Jumonji-C (JmjC) domain, which catalyze lysine demethylation of histones.135 Histone lysine demethylation is a fundamental process in epigenetic gene expression, controlling different methylation states and supporting transcriptional activation or suppression.136 IOX1 was found to exert its inhibitory effect by chelating Fe (II) ions in the catalytic site of KDMs.137, 138 Previous reports on IOX1 demonstrated that it acts as a broad-range inhibitor of histone demethylase enzymes.137, 138 Accordingly, IOX1 was demonstrated to increase H3K27me3 and H3K9me3 occupancy at the α-globin promoter reducing its expression via the formation or maintenance of key repressive epigenetic marks.134 However, attempts to evaluate the efficacy of IOX1 in HbE/β0-thalassemia erythroid progenitor cells failed to reduce α-globin gene expression.139 KDM enzymes known to act at the α-globin promoter include KDM6A and KDM6B; however, attempts to replicate the effect of IOX1 by knocking down individual enzymes were not successful, suggesting α-globin gene expression, particularly in β-thalassemia may be regulated by other KDMs or via alternative pathways.26, 134
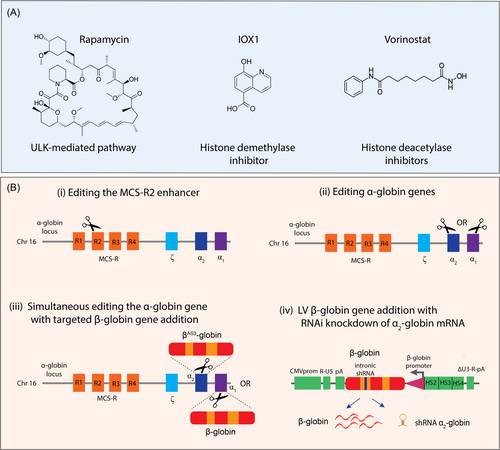
Further investigations on epigenetic agents tempering α-globin gene expression identified the pan-histone deacetylase inhibitor (HDACi) Vorinostat (also known as suberoylanilide hydroxamic acid (SAHA) and approved for the treatment of cutaneous T cell lymphoma), was able to reduce α-globin gene expression while inducing γ-globin expression.134, 140 Moreover, Vorinostat established cellular stability without affecting erythroid cell viability and differentiation in cord blood-derived HSCs.141 Overall, these studies revealed a crucial synergistic role of Vorinostat and, therefore, has been considered as a potential therapeutic agent for β-thalassemia and SCD patients. Notably, a phase 2 clinical trial assessing the safety and efficacy of Vorinostat in adult SCD patients resistant to HU was initiated (ClinicalTrials.gov: NCT01000155). Encouragingly, these findings offer potential new avenues for the modulation of α-globin expression, which may deliver an adaptable approach for fine-tuning globin gene expression. Considering the need for lifelong treatment, research examining a range of therapeutic doses and toxicity profiles for specific agents will become particularly relevant, as has been demonstrated for HU.142-145
Reducing α-globin gene expression by genome editing strategies
The CRISPR-Cas genome editing tool has also been adapted to manipulate the α-globin locus and restore α/β-globin chain imbalance in β-thalassemia. In the first instance, CRISPR/Cas9 was used to delete the MCS-R2 α-globin enhancer region to emulate a natural α-thalassemia mutation in a β-thalassemia background.18 The targeted MCS-R2 deletion ameliorated the α/β-globin chain ratio without perturbing erythroid differentiation or having detectable off-target events.18 Alternatively, genome editing was used to disrupt one of the two α-globin genes to recreate an α-thalassemia trait mutation and correct the pathological phenotype in a cellular model of β-thalassemia.146 In the same study, genome editing was also used to replace the α2-globin gene with the anti-sickling βAS3-globin transgene demonstrating greater potency in attaining balanced α/β-globin chain ratios.146 In a similar study, genome editing was used to replace the α1-globin gene with the β-globin transgene in HSCs. This approach both normalized α/β-globin chain imbalance in β-thalassemia erythroid progenitor cells and restored adult hemoglobin synthesis in RBCs.147 In both settings, a genome editing strategy was used to alter the α-globin expression while increasing therapeutic β-globin transgene expression in order to attain equimolar levels of α- and β-globin chain synthesis and possibly achieve therapeutic efficacy in β-hemoglobinopathy patients irrespective of genotype.
RNA interference (RNAi)-mediated silencing of α-globin gene expression
RNAi has also evolved as a plausible approach in the regulation of α-globin expression. The reduction of α-globin expression via a siRNA-mediated approach was successfully demonstrated at both mRNA and protein levels, resulting in a phenotypic correction in murine β-thalassemia erythroid cells.80, 148 Alternatively, the transduction of HSC with lentiviral (LV) vectors harboring short hairpin RNA (shRNAs) has emerged as an effective delivery strategy for the clinical translation of RNAi-based therapies.149-152 In this strategy, the artificial miRNA scaffold is used to express a shRNA in the cell type of interest.151-155 Such a design principle permits the shRNA to be processed in the same pathway as natural miRNAs and mediate silencing of target mRNA. These findings signify important advances in lineage-specific gene silencing, as this approach can be used as a flexible tool for the analysis of gene function and the development of gene-specific therapeutics.
Recent studies have demonstrated the beneficiary effects of intronic shRNA expression systems with coordinated β-globin expression.151, 152 In this context, the generation of a lentiviral gene therapy vector comprised of the therapeutic anti-sickling βA-T87Q-globin gene was configured with an intronic miR30-shRNA tailored to specifically reduce α2-globin mRNA expression and correct the α/β-globin chain imbalance in β-thalassemia.151 This approach has the important advantage of not only expressing the therapeutic β-globin gene but also reducing excess α-globin expression. These findings define a new approach to improve current LVβ gene therapy vectors and offer new insights into alternative therapeutic approaches for β-hemoglobinopathies. It is anticipated that this vector system may enable improved clinical efficacy in β-thalassemia patients, particularly with the most severe β0-thalassemia genotypes (Figure 4).151
CONCLUSION
Equipped with the increasing understanding regarding the pathophysiology of β-hemoglobinopathies through decades of clinical, genetic, molecular, and experimental evidence, ongoing efforts in exploring disease modifiers have gradually shifted to be multi-dimensional, not solely limited to a single facet of bioactivity but involve interrelated pathways. For instance, pharmacological activation of UPS and autophagy pathways have further exemplified the role of clearing toxic α-globin protein aggregates to alleviate the manifestation of disease.110 Gene therapy/RNAi/gene editing strategies have been used to alter the expression of α-globin while increasing β-globin expression and reversing the α/β-globin chain imbalance in β-thalassemia.146, 147, 151 Pharmacological silencing of α-globin and induction of γ-globin gene expression has demonstrated synergistic activity without affecting erythroid cell viability and differentiation in cord blood-derived HSCs.141 Such studies provide alternative avenues of investigation in re-examining available treatment strategies. The challenge now is to identify tangible pharmacological and genetic approaches that are suitable for widespread clinical application, especially for the multitude of patients in developing countries that have higher incidences of β-hemoglobinopathies and persistent social and economic inequalities.
ACKNOWLEDGMENTS
Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
AUTHOR CONTRIBUTIONS
All authors were involved in the manuscript preparation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This study was supported by the National Health and Medical Research Council (GNT1147867).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.