Metabolism in hematology: Technological advances open new perspectives on disease biology and treatment
Abstract
The term metabolism refers to the multi-faceted biochemical reactions within a cell or an organism that occur to maintain energy homeostasis, cell growth, and oxidative balance. Cells possess a high metabolic plasticity, allowing them to adapt to the dynamic requirements of their functional state and environment. Deregulated cellular metabolism is a hallmark of many diseases, including benign and malignant hematological conditions. In the last decade, multiple technological innovations in the metabolism field have made in-depth metabolic analysis broadly applicable. Such studies are shedding new light on normal and malignant hematopoiesis and open avenues to a better understanding of the biology of hematological diseases. In this review, we will first give a brief overview of central metabolic processes. Furthermore, we discuss the most commonly used methods to study metabolism. We begin by elaborating on the use of next-generation sequencing to detect metabolism-related genomic mutations and study transcriptional signatures. Furthermore, we discuss methods for measuring protein expression, such as mass spectrometry (MS), flow cytometry, and cytometry time-of-flight. Next, we describe the use of nuclear magnetic resonance spectroscopy, MS, and flow cytometry for metabolite quantification. Finally, we highlight functional assays to probe metabolic pathways in real-time. We illustrate how these technologies and their combination have advanced our understanding of the role of metabolism. Our goal is to provide hematologists with a comprehensive guide to modern techniques in metabolism research, their benefits and disadvantages, and how they guide our understanding of disease and potentially future personalized therapy decisions.
INTRODUCTION
The term metabolism (from the Greek metaballein, to change) describes the biochemical reactions that occur to maintain cellular and organismal function by converting nutrients into energy and structural components. Both anabolic processes, building complex bioblocks for growth and proliferation, and catabolic pathways, which generate energy by breaking down macromolecules, are essential parts of metabolism. While the importance of metabolism has been recognized for more than a century, biomedical research in the field has achieved impactful advances in the last two decades due to the substantial improvement in technologies allowing us to probe metabolism at the cellular level with precision and resolution exceeding what was possible before. In the field of hematology, these advances have helped us to gain a deeper understanding of the metabolism of healthy and malignant hematopoietic and immune cells, thus opening new perspectives on disease biology and treatment.
In this review, we describe the various technologies used to study cellular metabolism and comment on their advantages and limitations. We discuss applications of metabolism research in hematology and immunology, how they have uncovered previously unknown biological aspects of disease, and their current and potential future use. For the sake of conciseness, our review focuses on discussing technological advances rather than the role of each individual metabolic pathway in a specific context. When describing metabolic processes, we implement simplifications in the interest of readability and allow ourselves to refer to excellent reviews in the field for further reading.
METABOLISM REGULATES ENERGY HOMEOSTASIS, CELL GROWTH, DIFFERENTIATION PROGRAMS, REDOX STATE, AND SIGNALING EVENTS
Cellular metabolism is composed of a network of biochemical reactions that convert one or multiple substrate molecules into one or multiple product molecules. If molecules undergo a series of reactions, this series can be referred to as a metabolic pathway and the metabolites in this pathway as intermediates. Biochemical reactions are catalyzed by enzymes, which are often found in a specific sub-cellular location. For example, the enzymes of glycolysis are located in the cytosol, whereas the enzymes composing the tricarboxylic acid (TCA) cycle are located within mitochondria. This compartmentalization of the metabolic network into organelles allows the concentration of intermediate metabolites closer to the enzymes, leading to higher reaction rates and containment of harmful intermediates (e.g., H2O2 in peroxisomes). An additional effect of compartmentalization is that transport proteins (sometimes called shuttles) are required to connect the metabolic sub-networks of the organelles, and perturbations of transporters can have drastic metabolic effects.1 Metabolism has pleiotropic functions at the cellular and organismal levels. Below, we will briefly describe the central aspects of energy homeostasis, cell growth, cell differentiation programs, redox state, and signaling events (Figure 1).
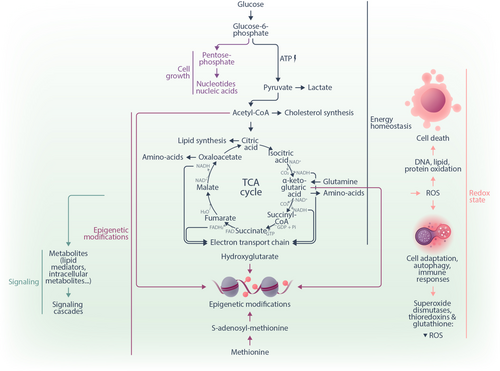
The best-studied function of metabolism is the generation of energy in the form of adenosine triphosphate (ATP) by covalently coupling free phosphate to adenosine diphosphate (ADP). The energy stored in ATP can subsequently power a large number of enzymatic reactions and cellular transport processes, resulting in the formation of free phosphate and ADP as by-products. Cells regenerate ATP mainly through glycolysis and the TCA cycle coupled to the electron transport chain (ETC). During glycolysis, one glucose molecule is broken down into two pyruvate molecules in the cytosol through a series of biochemical reactions. In the process, a net regeneration of two molecules of ATP and reduction of two molecules of nicotinamide adenine dinucleotide (NAD+) to NADH occurs. Pyruvate can then be transported to the mitochondria, where it is metabolized to acetyl-CoA, which enters the TCA cycle in a reaction catalyzed by citrate synthase.2 The TCA cycle produces CO2 and reduced redox equivalents such as NADH and flavin adenine dinucleotide (FADH2). These redox metabolites function as electron donors for the ETC, which generates a proton gradient across the inner mitochondrial membrane, ultimately resulting in the regeneration of up to 34 molecules of ATP (per one molecule of glucose) by the ATP synthase. Of note, the ETC uses oxygen (O2) as a terminal electron acceptor. Consequently, it can be limited by insufficient O2 availability. In this case, cells produce lactate from pyruvate, thereby oxidizing NADH to NAD+ to maintain cytosolic redox homeostasis. Energy production by mitochondrial oxidative phosphorylation (OXPHOS) is more efficient than glycolysis alone, nevertheless, some cells might convert pyruvate to lactate even in the presence of sufficient O2. One rationale to understanding this phenomenon is to consider that OXPHOS requires a large biosynthetic investment by cells as it involves many additional enzymes and a complex organelle (the mitochondrion). Fermentation of glucose to lactate under conditions of sufficient O2 supply is also called “aerobic glycolysis” or “the Warburg effect” and was discovered more than a century ago by Otto Warburg.3 It was initially believed to be characteristic only of cancer cells and thus represents the first example of a neoplastic metabolic remodeling.4, 5 However, the Warburg effect has since emerged as a metabolic pathway also employed by distinct rapidly proliferating cell types, including healthy blood cells, such as effector T lymphocytes.6, 7 A detailed review of the metabolic pathways for energy homeostasis is provided in Rigoulet et al.8
While glycolysis and the TCA cycle yield the intermediates ultimately required for energy generation, both of these central pathways also generate precursors for biomass synthesis and thus for cell growth and division. For instance, glucose-6-phosphate, the product of the first step in glycolysis, can be rerouted to the pentose phosphate pathway (PPP), generating ribose-5-phosphate, a precursor for nucleotides and nucleic acids.9 Furthermore, the TCA cycle can be seen as a biosynthetic hub as much as a bioenergetic pathway.2, 10 Citrate can be exported from the mitochondria to the cytosol and enable the production of cytosolic acetyl-CoA, a substrate for fatty acid and cholesterol synthesis. Oxaloacetate and α-ketoglutarate (αKG) provide carbon skeletons for the synthesis of amino acids, such as aspartate, asparagine, glutamate, proline, and others. Aspartate can further be utilized as a substrate to form purine bases. These examples illustrate how the same metabolic process can feed both energy generation and biomass production. For additional literature regarding the metabolic control of cell growth and proliferation, we refer to other sources.11, 12
Another increasingly recognized function of metabolism is its contribution to determining cell fate and differentiation. Several metabolites can directly influence epigenetic programs by regulating DNA, RNA, and histone modifications.13 For example, S-adenosyl-methionine (derived from the proteinogenic amino acid methionine) is the substrate for DNA methyltransferases and histone methyltransferases.13 Demethylation of DNA and histones by Ten-eleven translocation (TET) enzymes requires αKG as co-substrate and yields succinate as co-product. Limiting the availability of αKG can inhibit this reaction.13 Another metabolite prominently involved in epigenetic regulation is 2-hydroxyglutarate (2-HG), a competitive inhibitor of DNA and histone demethylation.14 In particular, 2-HG has been in the focus of hematologists as an oncometabolite in the context of acute myeloid leukemia (AML).14, 15 2-HG is produced from the TCA intermediate αKG by mutated isocitrate dehydrogenases 1 and 2 (IDH1/2), while wildtype IDH1/2 catalyze the conversion of isocitrate to αKG.16 Accumulation of 2-HG induces a global DNA hypermethylation14 due to its activity as a competitive inhibitor of αKG-dependent dioxygenases.17 Multiple other connections between metabolism, epigenetics, and cell fate exist and have been reviewed elsewhere.13, 18
Next, metabolism is at the core of maintaining the redox balance of cells. Physiological metabolic processes result in the generation of reactive oxygen species (ROS), highly reactive chemicals formed from diatomic oxygen, hydrogen peroxide, and water.19 ROS are byproducts of the normal metabolism of oxygen, frequently generated in the mitochondria or peroxisomes.19 Sources of ROS are the ETC, mitochondrial P450 systems, and the NADPH oxidase (NOX) pathway.19 ROS regulate physiological processes, such as hypoxia adaptation, autophagy, and immune responses.20 Excess amounts of ROS can lead to the permanent oxidation of DNA, lipids, and proteins with irreversible damage and, ultimately, cell death.19, 21 Multiple lines of antioxidant defense exist to prevent such damage, most notably superoxide dismutase (SOD) enzymes, thioredoxin, and glutathione.22 For additional information on the interplay between metabolism and redox homeostasis in health and disease, we refer to other sources.19, 23
Finally, metabolites can serve as signaling molecules directly activating intracellular cascades to modulate phenotypes.24 For instance, adenosine monophosphate (AMP) activates AMP kinase (AMPK) to signal a low energy state in the cell and inhibit the mammalian target of rapamycin (mTOR) complex 1 (mTORC1), a protein complex that controls cell growth and protein synthesis.25 Furthermore, TCA intermediates and byproducts serve as signaling molecules in physiological and pathological settings.2 Lipids also fulfill multiple essential signaling functions in cells. One example is phosphatidylinositides, and importantly phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidylinositol-3,4,5-triphosphate (PIP3). These plasma-membrane localized lipids are substrates for a number of essential signaling proteins or precursors of other signaling molecules, such as the second messengers diacylglycerol (DAG) and inositol triphosphate (IP3).26 For a comprehensive review of the signaling functions of metabolites, we refer to other studies.2, 24
Taken together, metabolic processes are not only essential for energy and biomass generation but also affect cellular and organismal phenotypes by maintaining redox homeostasis, orchestrating epigenetic programs, and mediating signaling events (Figure 1). Understanding how these processes regulate hematopoietic and immune cells in health and disease adds an additional layer to our biological understanding and has the potential to inform innovative diagnostic and therapeutic approaches.
METABOLIC REGULATION IN HEALTHY AND DISEASED HEMATOPOIETIC CELLS
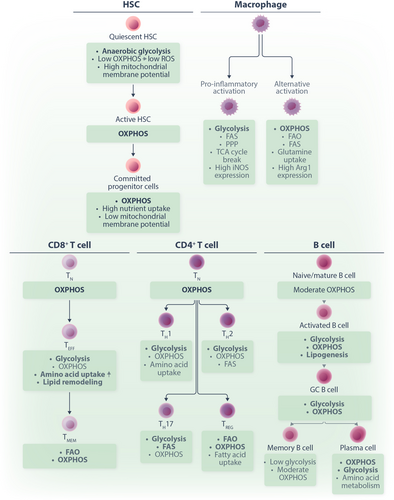
Hematopoietic cells display metabolic programs adapted to the function and differentiation state of the individual lineages. Hematopoietic stem cells (HSCs) are marked by quiescence and low metabolic activity with a predominant dependence on glycolysis and fatty acid oxidation (FAO) to generate ATP.27-30 Nevertheless, mitochondrial activity and biogenesis remain central to the proper maintenance of the HSC pool and their differentiation.27 HSC also exhibit a strong dependence on certain amino acids, for instance, the branch-chained amino acid valine.31 Dietary depletion of valine in mice emptied the BM niche and allowed engraftment of donor HSCs.32 Upon healthy differentiation, progenitor cells undergo metabolic reprogramming to support their cell fate programs. For instance, a recent study showed that genetic defects in glutaminolysis and FAO in mice (by conditional deletion of glutaminase and carnitine palmitoyl transferase 2) did not affect most hematopoietic lineages.1 In contrast, silencing of the mitochondrial pyruvate carrier subunit 2 (MPC2) resulted in significantly slower engraftment of myeloid subsets.1 This and other studies33-35 demonstrate that an orchestrated metabolic remodeling is central to the maintenance of a quiescent HSC pool and the physiological differentiation of the distinct hematopoietic subsets. Finally, mature cells display metabolic features related to their specific function. For instance, red blood cells lack mitochondria and rely on glycolysis and the PPP for the generation energy and redox equivalents.36 The glycolytic intermediate 1,3-bisphophoglycerate serves as a precursor of 2,3-bisphosphoglycerate (2,3-BPG), an allosteric regulator of hemoglobin. The binding of 2,3-BPG to hemoglobin stabilizes the deoxygenated form of hemoglobin, thus enhancing the release of oxygen in hypoxic tissues.36 Interestingly, metabolic enzyme deficiencies are relatively frequent causes of hereditary erythrocyte disorders.37 The most notable examples are defects in the PPP enzyme glucose-6-phosphate dehydrogenase (G6PDH)38 and in pyruvate kinase,39 the final enzyme in glycolysis, both leading to anemia. Leukocytes also have subtype- and differentiation stage-specific metabolic programs to meet the distinct demands of quiescence and activation. For instance, naive CD8+ T lymphocytes predominantly engage OXPHOS to cover their energetic needs.40 Upon activation, they undergo a metabolic reprogramming upregulating aerobic glycolysis,6, 41 while memory development is characterized by FAO and OXPHOS activity.42, 43 The physiological metabolic programs of lymphocytes have been extensively reviewed elsewhere.44, 45 Figure 2 summarized the energy metabolism of HSCs and several subtypes of immune cells.
Metabolic reprogramming has also been recognized as one of the hallmarks of cancer, highlighting how cancer cells can alter their metabolic pathways to support aberrant growth and increased energy demand.52 Tumor metabolic programs can be diverse and involve changes in glucose and amino acid metabolism as well as lipid synthesis and mitochondrial functions, among others.10 One of the critical drivers of metabolic reprogramming is the acquisition of genetic mutations.53 Oncogenic signaling, such as through mutated MYC, RAS, or TP53, induces changes in gene transcription, which are directly linked to altered metabolic profiles.54-56 Furthermore, therapy-induced cytotoxic pressure and immune pressure can select tumor cells with advantageous metabolic programs.57-59 Hematological malignancies display a range of metabolic aberrations that distinguish them from healthy hematopoietic cells, including rewiring of glucose, amino acid, nucleotide, fatty acid, lipid, vitamin, and mitochondrial metabolism.60 Such metabolic programs could present therapeutic vulnerabilities. One major metabolic vulnerability in AML is mitochondrial OXPHOS, which we discuss in detail in “Functional, proteomic, and genomic metabolism studies identify mitochondrial metabolism as a vulnerability in AML” section. Sphingolipid metabolism represents another prominent therapeutic target in AML. Sphingolipids are a class of bioactive lipids that regulate cell growth, proliferation, cell death, stemness, and immune cell activation.61 Structurally, they are characterized by long-chain sphingoid bases (e.g., sphingosine), linked to a fatty acid and a head group (e.g., phosphocholine, phosphoethanolamine, or sugar residues). Ceramides, composed of sphingosine linked to a fatty acid by an amide bond, are the central hub of sphingolipid metabolism. Acid ceramidase (AC), which catalyzes ceramide breakdown to sphingosine, was reported to be highly expressed in primary AML cells.62 The AC inhibitor LCL204 induced apoptosis in primary patient cells and AML cell lines through the accumulation of ceramides and prolonged the survival of mice engrafted with leukemic C1498 cells.62 AC overexpression in the AML cell line HL-60 conferred resistance to the chemotherapeutic drugs cytarabine, daunorubicin, and mitoxantrone via increased NF-kB activation.63 More recently, another AC inhibitor, LCL805, was reported to induce toxicity in AML cell lines and primary samples via antagonizing Akt signaling and inducing iron-dependent cell death.64 Similarly, sphingosine-1-kinase (SPHK1), which generates sphingosine-1-phosphate from sphingosine, was found to be overexpressed in primary AML patient blasts.65 Targeting SPHK1 induced cell death in AML cell lines, primary AML patient blasts, and isolated AML patient leukemic progenitor/stem cells via downregulation of the pro-survival protein MCL1.65, 66 Importantly, enhanced expression of the sphingosine-1-phosphate-receptor-3 in murine HSCs was sufficient to induce a transplantable leukemia with a myeloid phenotype.67 Targeting sphingolipid metabolism might be particularly effective in specific subtypes of AML. For instance, FLT3-ITD signaling was shown to prevent the formation of intracellular pro-death ceramides by reducing the expression of the synthesis enzyme CERS1.68 A combination of Ceramide Transfer Protein (CERT) inhibitor with FLT3 inhibitors showed synergistic effects in models of FLT3-ITD-mutated AML.69 Based on these and other studies, there are multiple ongoing efforts to target sphingolipid metabolism in AML.61 For excellent in-depth reviews of the role of other metabolic pathways in hematopoietic malignancies, we refer to the following recent publications.60, 70, 71
These examples demonstrate that both healthy and diseased hematopoietic cells exert specific metabolic programs matching their differentiation state, function, and environment. Importantly, metabolism is not just the consequence of a particular cell fate; rather, cell function depends on intact metabolic programs. This concept highlights that by modulating metabolism, we can also actively regulate cell function.
TECHNOLOGICAL INNOVATIONS IN METABOLISM RESEARCH
Over the last decades, profound technological advancements such as high-throughput sequencing, novel imaging techniques, and metabolomics have significantly broadened our understanding of metabolic processes and their regulation in health and disease. These techniques provide detailed insights into the complex biochemical pathways of cellular metabolism, not only helping researchers better comprehend disease pathology but also adding to the identification of new therapeutic targets. In this section, we will describe state-of-the-art technologies to probe metabolism and discuss their advantages and disadvantages. We have chosen a classification of the technologies depending on whether they allow us to infer information on a genomic, transcriptomic, phenotypic, or functional level.
Genomic aberrations and transcription signatures to profile metabolism
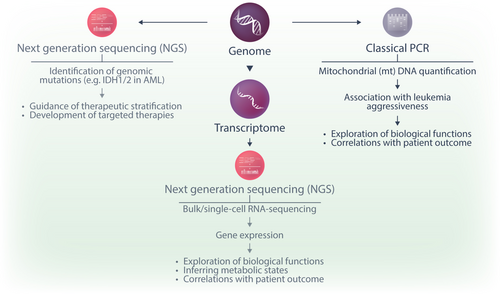
A first layer of understanding the role of metabolism in disease biology is offered by the detection of genetic mutations in metabolic enzymes and other related molecules. Genomic analysis is an integral part of the diagnostic workup in acute leukemias, although the vast majority of affected genes are unrelated to metabolism. Nevertheless, with the emergence of next-generation sequencing (NGS) and primarily through projects such as the cancer genome atlas (TCGA),72 BeatAML,73 and BeatAML2.0,74 researchers were able to identify IDH1 and 2 mutations as recurrent aberrations in AML.75 The neomorphic activity of mutant IDH1 and 2 leads to the production of the oncometabolite 2-HG, which supports leukemia progression by epigenetic remodeling.14, 15, 17 To interfere with this pathway, small molecule inhibitors of mutant IDH1 (ivosidenib) and IDH2 (enasidenib) were successfully developed and FDA-approved in 201876 and 2017.77 While mutations in IDH1 and 2 have a clear impact on disease biology by generating an epigenetically active oncometabolite, they remain, to date, the only therapeutically relevant genetic aberrations in metabolic enzymes in acute leukemias.
Genomic analysis by PCR has been applied to quantify mitochondrial DNA (mtDNA) content in leukemic cells.78, 79 Changes in mtDNA content can reflect alterations in mitochondrial biogenesis, function, and metabolic adaptation in cancer cells. After the isolation of genomic DNA from cell samples, mtDNA content is determined by a quantitative real-time PCR using primers for a mitochondrial gene (e.g., cytochrome B, CYTB).79 The relative amount of mtDNA can be defined as a ratio between the expression of this gene and the expression of a nuclear-encoded gene (such as pyruvate kinase, PKLR, or hemoglobin subunit beta, HBB).79 As mitochondrial metabolism is a central therapeutic vulnerability in AML,80 and AML cells often exhibit metabolic plasticity, increased mtDNA content could indicate a higher reliance on OXPHOS and mitochondrial respiration for energy production. Given this, patients with elevated mtDNA levels may be particularly sensitive to mitochondria-targeted therapies.
NGS is further employed to characterize transcriptional programs. Bulk RNA-sequencing data from large cohorts of patient samples are available through TCGA,72 the BeatAML,73 and BeatAML2.0 trials,74 the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) AML projects (https://www.cancer.gov/ccg/research/genome-sequencing/target) for pediatric leukemia, and others.81-83 While these investigations provide helpful information about the expression of metabolic genes across a large number of samples, they reflect mRNA levels in an ill-defined mixture of cells, frequently comprising both malignant and benign cell populations from peripheral blood and/or bone marrow. Advances in the single-cell RNA sequencing (scRNA-seq) field now allow the assessment of metabolic signatures at the single-cell level.84 Individual cells are captured using methods like microfluidics or fluorescence-activated cell sorting. Cells are lysed to capture mRNA, and reverse transcription is performed to generate cDNA using unique molecular identifiers and cell barcodes that later allow the distinguishing of transcripts from different cells. The cDNA is amplified and prepared into sequencing libraries that are subjected to NGS. During data analysis, cells are clustered based on the similarity of their transcriptional profiles. ScRNA-seq offers the advantage of studying the transcriptional metabolic states at a single-cell level and in rare populations that might be too small to be interrogated by other methods. It can also be combined with cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq), a method in which oligonucleotide-labeled antibodies are used to identify surface proteins with sequencing as a read-out.85 Furthermore, there is an increasing number of commercially available platforms for spatial transcriptomics, facilitating a spatially resolved assessment of gene expression.86 Nevertheless, while genomic NGS is broadly established in clinical practice, scRNA-seq and spatial transcriptomics applications are currently limited to research settings due to the required substantial expertise in sample preparation and data analysis, and the relatively high cost.
Taken together, genomic and transcriptomic information delivers the first layer to understanding the biological relevance of metabolism (Figure 3). One major limitation, in particular of gene expression studies, lies in the fact that mRNA levels of a specific transporter or enzyme do not necessarily correlate with the protein expression or actual metabolic activity of a pathway. A significant part of metabolism regulation occurs at the level of enzyme activity and metabolite abundance, making technologies that detect these parameters indispensable.
Protein expression analysis
Metabolism-related protein expression adds another layer to the characterization of cellular metabolic activity. While traditionally, the expression of nutrient transporters and enzymes has been analyzed by immunoblotting, multiple novel techniques have been developed, including mass spectrometry (MS)-based proteomics, flow cytometry, and cytometry by time-of-flight (CyTOF) (Figure 4). These methods are complementary to each other as they all have distinct benefits and limitations concerning coverage, sensitivity, bulk versus single-cell analysis, and spatial resolution.
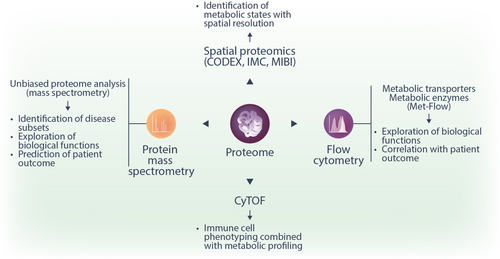
MS and, in particular, tandem mass spectrometry (MS/MS) is an analytical tool that allows the simultaneous identification and quantification of thousands of proteins, metabolites, or lipids in complex biological samples. Inside a mass spectrometer, electrostatic and electromagnetic fields are used to guide and separate analyte molecules. Therefore, these molecules must be electrically charged. Proteins are typically digested to peptides using proteases such as trypsin, whereas metabolites and lipids are mostly analyzed in their native form. For the analysis of biofluids and extracts of blood cells or cultured cells, chromatographic separation before MS is typically used for improved sensitivity and specificity. Liquid chromatography (LC) is a highly versatile technique that can be used for any soluble compound. For studying protein, metabolites, and lipids, electrospray ionization (ESI) is almost exclusively used to couple LC to MS. Spatial separation can be seen as an alternative to chromatographic separation. Matrix-assisted laser desorption ionization (MALDI) and desorption ESI (DESI) are examples of ionization techniques that allow for spatially resolved analysis of proteins or small molecules in tissue slices and are typically coupled to high-resolution mass spectrometers. This technology is also called imaging MS because images of the distribution of metabolites or lipids in tissue can be generated.87, 88 In comparison, chromatography-coupled MS allows higher confidence in the identification of analytes, whereas imaging MS delivers the additional spatial information.
Once inside the mass spectrometer, molecules are separated by their mass-to-charge ratio (m/z), and their relative abundance is counted in a detector. Of note, one molecule usually generates multiple characteristic m/z signals corresponding to different isotopologues (the molecule containing different number of heavy isotopes), different adducts (the molecule forming a transient complex with strong ions such as sodium, potassium, ammonium, chlorine, formate, or acetate), or different fragments (if the molecule breaks during ionization). Correctly assigning the different m/z signals to analytes is one of the challenges of MS-based analysis. Different MS analyzers exist, such as quadrupoles, ion trap, and time-of-flight (TOF) instruments, and their use depends mostly on the specific application.89 Non-targeted MS experiments aim at identifying and (relatively) quantifying all proteins, metabolites, or lipids in a biological sample, while targeted approaches such as selected reaction monitoring quantify pre-defined analytes of specific interest.
While the majority of MS proteomics data to date have been generated from bulk cells, there have been significant developments in the field of single-cell proteomics by MS (scMS) in recent years.90-92 The extent of coverage (about 1500 proteins/cell) and the relatively low throughput speed (up to several hundred cells per day) are major limitations of the current use.90, 93 Nevertheless, we expect that as technologies advance, scMS will gain importance in profiling metabolic states in single cells. Finally, imaging-based spatial proteomics platforms such as co-detection by indexing (CODEX),94 imaging mass cytometry (IMC),95 multiplexed ion beam imaging (MIBI),96 and others, offer first insights into spatially resolved proteomic information within tissues at the single-cell level.
Another technique allowing the quantification of metabolism-related proteins is flow cytometry. Flow cytometry is a standard method in hematological diagnostics laboratories used to analyze the expression of cell surface and intracellular molecules. Single-cell suspensions from cell culture or tissue samples are labeled with fluorescence-coupled antibodies or fluorescent compounds before being injected in the flow cytometry instrument. The cell suspension is focused by the sheath fluid so that one cell at a time passes through a laser beam. Initially, forward scatter (FS) and side scatter (SS) are measured, reflecting cell size and granularity. Subsequently, fluorescence detectors measure the emitted fluorescence from stained cells, thus allowing quantification of the amount of labeling. Classic examples of metabolic markers studied by flow cytometry include the fatty acid transporter CD36, the amino acid transporter CD98, the glucose transporter 1 (GLUT1), and the transferrin receptor 1 (CD71). One particular challenge hampering the use of flow cytometry in metabolic research is the paucity of antibodies able to detect the expression of metabolite transporters and metabolic enzymes reliably. A recent development in this area is Met-Flow, a high-parameter flow cytometry method utilizing antibodies against ten essential metabolic proteins that are critical and rate-limiting in their respective pathways97 (Table 1). Anabolic flux can be analyzed by measuring FA synthesis and levels of an arginine metabolism protein.97 In contrast, catabolic flux analyses include the quantification of proteins involved in glycolysis, the PPP, TCA cycle, OXPHOS, and FAO.97 Advantages of flow cytometry include the broad availability of instruments in clinical diagnostic units, the single-cell resolution with high throughput (up to 10,000–20,000 cells/second), and the relatively simple analysis procedure. We expect further developments to address the scope of antibodies and enable studies of additional pathways.
| Metabolic pathway | Metabolic enzyme |
|---|---|
| Glucose metabolism |
|
| Pentose phosphate pathway |
|
| Oxidative stress regulation |
|
| Phosphate import |
|
| Fatty acid metabolism |
|
| Urea cycle |
|
| Oxidative phosphorylation |
|
Cytometry by Time-of-Flight (CyTOF) represents another powerful tool in protein expression analysis, enabling high-dimensional single-cell analysis using metal isotope-labeled antibodies to detect proteins. Compared to traditional flow cytometry, the usage of metal isotype-tagged antibodies has only minimal signal overlap and allows the simultaneous measurement of up to 60 markers per cell.98 After being stained with tagged antibodies, single-cell suspensions are introduced into a mass cytometer. They are first nebulized into small droplets containing one cell per droplet. These droplets are then introduced into the inductively coupled plasma where they are vaporized, atomize, and ionized, breaking the cellular contents down into a cloud of metal ions.99 The resulting ion cloud per cell is then passed to the time-of-flight analyzer (TOF), which measures the relative abundance of each metal isotope mass correlating to the expression level of the targeted proteins.100 Similar to flow cytometry, CyTOF is suitable to perform single-cell analyses of patient cells and tissues, including blood. General sample handling as well as staining protocols are comparable. However, CyTOF has the significant benefit that it uses metal isotope-labeled antibodies instead of fluorophore-labeled antibodies, which eliminates the need of fluorescence compensation and the occurrence of spectral overlap.100 However, the throughput with CyTOF is lower compared to flow cytometry with less than 1000 cells/second.
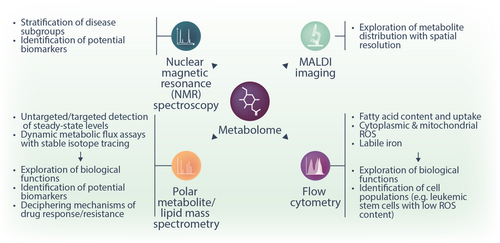
Metabolite quantification
Genomic mutations, transcriptional signatures, and protein expression provide valuable information about the metabolic state of a cell. However, metabolic reactions are highly dynamic, and their reaction rates depend not only on the availability of enzymes but also on their subcellular localization, post-translational modifications, and concentration of substrate metabolites. Critical information is provided by directly quantifying metabolite concentrations in cells and biological fluids. Metabolomics analyses covering a broad range of metabolites are routinely performed with two methods: nuclear magnetic resonance (NMR) spectroscopy and MS. The latter is typically coupled to liquid chromatography (LC-MS) or gas chromatography (GC-MS) for better sensitivity and specificity. Additionally, individual metabolites can be quantified by flow cytometry or enzymatic assays with colorimetric, fluorometric, or bioluminescent read-outs. While NMR, MS, and enzymatic assays can be employed in the analysis of cells/tissues, cell culture supernatants, and biofluids, the use of flow cytometry is limited to cells and tissues.
In NMR spectroscopy, the spectra of active nuclei, very frequently 1H, are recorded to determine intra-molecular proton ratios and infer information about the molecules. NMR spectroscopy has several advantages, such as being a highly reproducible, easily quantifiable method that requires little to no sample treatment or chromatographic separation. These advantages render it a highly suitable technique for large-scale or multi-center studies.101 Nevertheless, significant disadvantages limit the use of NMR spectroscopy compared to MS-based methods. Most importantly, NMR spectroscopy has a lower separation power, leading to a significantly (10–100×) lower sensitivity compared to MS in complex samples such as biofluids or cell extracts. Consequently, it yields information only for a lower number (typically tens) of highly abundant metabolites.102-104 MS can be used not only for the detection of proteins (see “Protein expression analysis” section) but also to detect and quantify small polar and non-polar (lipid) metabolites. In addition to imaging MS and LC-MS, gas chromatography (GC) coupled to MS is suitable for volatile compounds, small polar compounds (up to about 200 Da, such as amino acids, TCA intermediates, glycolytic intermediates), and fatty acids. For GC, electron impact (EI) ionization is commonly used as the ion source for MS coupling. For a detailed overview of MS metabolomics sample preparation, measurement, and data analysis, we refer to the following sources.105-107 The great physiochemical diversity of metabolite classes and their large dynamic range present a significant challenge in fully describing the metabolome in a single sample. Hence, adapted methods for sample extraction, chromatography, and MS have been developed to preferentially capture specific metabolite or lipid classes. Metabolomics analyses can be performed either in an untargeted or a targeted manner. In untargeted metabolomics, all MS signals meeting particular criteria are recorded in an unbiased manner, and annotation to specific molecules is attempted at the analysis stage by matching the exact mass, the chromatography retention time, and potentially other parameters to those of known molecules and/or standard compounds. Untargeted metabolomics is used as a discovery approach as it holds the potential to identify previously unsuspected metabolic changes. However, only a fraction (typically 5%–10%) of the recorded MS signals can indeed be annotated to a known molecule. In targeted metabolomics, a list of metabolites of interest is predefined, and signals matching the characteristics of these metabolites are recorded. This method frequently offers a higher sensitivity and confidence in the identification. However, as the selection of metabolites for analysis is based on prior knowledge, this can introduce a bias and result in overlooking critical metabolic pathways that were previously unknown. Finally, metabolomics analyses can capture not only the steady-state levels of metabolites but also the activity of metabolic pathways. The latter is achieved by stable-isotope tracing. Synthetically produced molecules containing, for instance,13C (instead of 12C) or 15N (instead of 14N) atoms (e.g., glucose, amino acids, and many more) can be fed to cells, and the incorporation of 13C/15N atoms into downstream metabolites provides information about the relative activity of the respective metabolic pathways.108 In a basic stable-isotope tracing experiment, isotope labeling can be analyzed by both NMR and MS.109 Labeling can be performed not only by adding the stable isotope tracers to cell culture media, but also by infusing them to living organisms, which provides information about dynamic metabolic flux rates in vivo.109 While the majority of in vivo stable-isotope tracing studies have been done in animal models, infusion of stable-isotope tracers has also been done in humans since the 1930s, when Hevesy (later awarded the Nobel Prize in Chemistry for the development of radioactive tracers) used deuterium oxide to estimate the size of the whole-body water pool and the rate of water elimination.110
In practice, the acquisition of high-quality MS data remains a challenge for non-expert laboratories. Many metabolic reactions occur very rapidly, making it hard to capture the in vivo metabolic state precisely. Cold and quick handling of the samples is critical to maintaining metabolic states as close as possible to the in vivo situation. Moreover, MS provides information on a bulk cell population and, at least for classical approaches, a relatively high number of cells (>100,000 is required). Nevertheless, new developments in the field allow us to perform metabolomics from rare cell populations, including HSCs, with an input as low as 5000 cells.111, 112
MS imaging and MALDI-TOF, as methods allowing to perform spatial metabolomics, have undergone substantial advances in the last few years, but their application to the hematological field is impeded by several significant obstacles. As metabolites are prone to rapid degradation and transformation, spatial metabolomics relies on obtaining fresh snap-frozen tissue without preceding incubation or washing step. However, classical bone marrow biopsies require decalcification to enable tissue sectioning. Moreover, metabolite identification remains a considerable challenge.87 Further improvements in the MS imaging field will be necessary to allow spatial metabolomics from the bone marrow. Such advances will be highly informative, given the complexity and heterogeneity of cell populations in the bone marrow niche and our current lack of understanding of the metabolic cross-talk between them.
Besides the high-resolution technologies of NMR spectroscopy and MS, flow cytometry-based methods for the detection of some metabolite classes have been developed. For instance, ROS can be detected by incubating cells with cell-permeant dyes (such as CM-H2DCFDA, CellROX [ThermoFisher Scientific], and others) that are weakly fluorescent while in a reduced state and exhibit bright, photostable fluorescence upon oxidation by ROS.113 Another example of probes enabling metabolite quantification by flow cytometry are BODIPY dyes—chemicals containing a boron difluoride group and a dipyrromethene group, which strongly absorb UV light and re-emit it in very narrow frequency spreads.114 BODIPY derivatives have been used in the context of leukemia for the quantification of neutral lipids, fatty acids, and lipid peroxidation.115 Some ions can also be quantified by flow cytometry, such as iron, calcium, and others.
Finally, the abundance of individual metabolites can be quantified in enzymatic assays coupled to a colorimetric, fluorometric, or bioluminescent read-out. In these assays, the concentration of a metabolite of interest is quantified by a chemical reaction yielding a product for which absorption, fluorescence, or bioluminescence signal intensity can be measured and is proportional to the concentration of the metabolite of interest. Such assays exist for some common metabolites, such as glucose, ATP, lactate, glutamine, glutamate, glutathione, creatine, creatinine, and others. While the implementation is simple and requires only standard laboratory equipment, a relatively high amount of input material is necessary due to the comparatively low sensitivity of these assays. Another disadvantage is that the majority of these assays can measure the abundance of only one to two metabolites per sample.
In conclusion, measuring metabolite abundance is now possible via NMR spectroscopy, MS, flow cytometry, and enzymatic assays (Figure 5). While MS-based bulk and spatial metabolomics is the most sensitive method, it requires specialized equipment and expertise in sample preparation and data interpretation. For this reason, MS metabolomics is predominantly used in basic research. Flow cytometry and enzymatic assays are easier to implement but cover a much narrower range of metabolites. A combination of these technologies delivers broad information on steady-state metabolite content, metabolic flux, and spatial distribution.
Functional read-outs
Functional read-outs provide information about dynamic cellular metabolic processes and build the final dimension of metabolism studies. Enzymatic assays are one classical approach in which the activity of an enzyme can be assessed by measuring the conversion of a specific substrate, for instance, using a spectrophotometric or a fluorometric assay. More recent technologies allow the monitoring of the activity of whole metabolic pathways, such as glycolysis or OXPHOS. Here, we discuss the use of extracellular flux assessments and flow cytometry for functional metabolic characterization (Figure 6). Both of these technologies are suited to exploring the metabolic activity of cells or cell suspensions obtained from tissues. Furthermore, we discuss the possibility of measuring metabolism using the OmniLog analyzer and tracking metabolic functions on the organismal level in mice using metabolic cages.
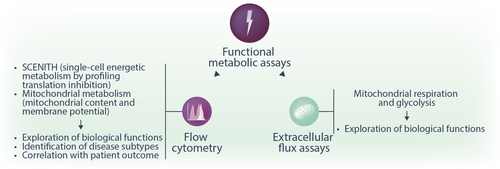
The extracellular flux assay is now a standard method for evaluating cellular metabolic states, both in vitro and ex vivo. Seahorse real-time cell metabolic analysis (Seahorse XF Analyzer, Agilent) provides two key real-time measurements: glycolytic activity, assessed by the extracellular acidification rate (ECAR), which reflects lactic acid and bicarbonate accumulation in the extracellular environment, and mitochondrial respiration, measured by the oxygen consumption rate (OCR), which indicates extracellular oxygen levels.116 To perform a typical extracellular flux assay, cells are plated in a monolayer onto specialized cell culture microplates. The other integral part of the assay is the proprietary solid-state biosensor cartridge. The biosensor cartridge contains two fluorophores: one is quenched by oxygen, and the second one is sensitive to protons (hydrogen protons H+, providing information on glycolysis). The biosensor cartridge plate is placed onto the cell culture microplates containing the cells to be analyzed. Inside the instrument, the fiber optics of the instrument emit light, which can excite the embedded fluorophores. The sensors can then detect the changes in fluorescence, which are a result of the change in the total levels of oxygen and hydrogen protons. During the measurement cycle, the sensor cartridge creates a transient microchamber in each well of the cell microplate. Through this process, the Seahorse XF analyzer can detect real-time changes in oxygen consumption or hydrogen production by the analyzed cells within minutes.117 The Seahorse assay further offers the opportunity to measure the dynamic metabolic changes in cells upon drug challenge. The cartridge contains four compound injection ports, and drugs plated in these ports can be added to the cells at selected time points.118 In the case of a classical Mitochondrial Stress test, four drugs are injected: oligomycin, which inhibits the ATP synthase (ETC complex V), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), a proton gradient uncoupler, and a combination of rotenone and antimycin A, which inhibit ETC complex I and III, respectively. By observing the changes in OCR, parameters such as basal respiration, maximal oxygen consumption, spare respiratory capacity, and non-mitochondrial OCR can be calculated and give information about the mitochondrial respiration activity and capacity of cells.117, 118 Similarly, the Glycolysis Stress test uses the addition of glucose, oligomycin, and the glycolysis inhibitor 2-deoxyglucose (2-DG) to investigate the glycolytic rate of the cell.118 With this assay, key parameters such as the basal glycolytic rate, the glycolytic capacity (ECAR, following oligomycin treatment), glycolytic reserve (as the difference between basal glycolysis and maximal glycolytic capacity), and non-glycolytic acidification (following 2-DG, injection, indicative of proton production from non-glycolytic sources) can be quantified. These quantifications give important information regarding the cells' capacity to fulfill energy requirements via glycolysis and their metabolic flexibility in response to stress.118
Besides determining rates of respiration and glycolysis, bioenergetic profiling of cells with the Seahorse XF analyzer can also be utilized to probe cells for their specific preference of different fuels or substrates in the Mito Fuel Flex assay. It is designed to measure how cells utilize key mitochondrial fuels—glucose (pyruvate), glutamine (glutamate), and long-chain fatty acids—by measuring the OCR in response to specific inhibitors. These include UK5099 (which inhibits glucose oxidation by blocking the mitochondrial pyruvate carrier MPC), BPTES (glutamine oxidation inhibitor), and etomoxir (FAO inhibitor). By sequentially blocking these pathways, the assay determines fuel dependency (how reliant cells are on a specific fuel), capacity (the ability to use a fuel when alternatives are inhibited), and flexibility (the ability to switch between fuels). A lack of flexibility indicates a strong reliance on a particular pathway to sustain basal mitochondrial respiration.119
Additionally, it is also possible to analyze respiration in isolated mitochondria from different cells and tissues, such a brain, heart, liver, and others.120, 121 Apart from investigating overall respiration in isolated mitochondria, one can also assess the activity of single ETC complexes by providing specific substrates for these complexes, even on frozen tissue material.122 As rates of oxygen consumption by cells can be influenced by oxygen diffusion into and out of the transient microchamber, it is furthermore possible to conduct the Seahorse XF assays in hypoxia chambers (with ≥3% oxygen).121, 123 Extracellular flux assays provide a dynamic insight into the real-time metabolism of cells with a moderately high cell input and can be flexibly adjusted to address different scientific questions by varying the chemical compounds the cells are treated with. Disadvantages of the technology include the limited insight into individual metabolic pathways, the necessity to obtain a homogenous cell population, and the lack of single-cell resolution.
Another assay type focuses on mitochondrial metabolism by employing fluorescent dyes, which can be visualized by flow cytometry or microscopy. Fluorescently labeled positively charged molecules (for instance, MitoTrackerTM, ThermoFisher Scientific) are attracted to the negative charge of the mitochondria and covalently bind to healthy mitochondria components in living cells. Such fluorescent probes can be used to investigate mitochondrial mass, distribution, and morphology by flow cytometry or microscopy. In contrast to this covalent binding, another fluorescent dye based on the tetramethylrhodamine methyl ester (TMRM) can be used to investigate changes in the mitochondrial membrane potential specifically. This cationic dye binds in a reversible and directly proportional to the mitochondrial membrane potential manner. Therefore, it can be used as a dynamic real-time indicator of mitochondria health and function (e.g., in drug response treatments).124 Besides functional readouts concerning overall mitochondrial health, another crucial aspect of mitochondrial metabolism is the production of mitochondrial superoxide generated as a byproduct of OXPHOS. With the help of cell-permeable fluorescent dyes, which are designed to accumulate in the mitochondria selectively and to react there with the produced superoxide to produce a fluorescent signal, oxidative stress can be monitored.113, 125, 126 The advantages of these technologies include that they require a low cell number, offer a single-cell resolution, and allow to study heterogeneous cell mixtures when cell type-specific antibodies are added.
Single-cell energetic metabolism by profiling translation inhibition (SCENITH) is an emerging flow cytometry-based assessment of metabolic activity with single-cell resolution.127 This technology is based on the fact that the energy state of a cell is closely reflected by its protein synthesis rate. By measuring protein translation (via quantifying the incorporation of puromycin), researchers can gain information about the metabolic state of a cell. In the original study, the authors treated cell suspensions with 2-deoxyglucose (2-DG) to inhibit glycolysis, oligomycin to inhibit the ATP synthase, or a combination of both.127 By quantifying protein synthesis via flow cytometry, four functional metabolic parameters were calculated.127 Glucose dependence was estimated as the proportion of protein synthesis, and thus energy production, dependent on glucose oxidation. Mitochondrial dependence was quantified as the proportion of protein synthesis dependent on OXPHOS activity. Glycolytic capacity was defined as the maximum capacity to sustain protein synthesis when mitochondrial OXPHOS is inhibited. Fatty acid and amino acid oxidation capacity was defined as the capacity to use fatty acids and amino acids as sources for ATP production when glucose oxidation is completely inhibited.127 By combining protein translation measurement with a panel of flow cytometry antibodies, SCENITH allows gaining information about the metabolic state at the single-cell level even of rare cell subpopulations in a heterogeneous suspension.127 Another advantage is that only a relatively small cell number is required and that the read-out can be done on any flow cytometer. Nevertheless, similar to extracellular flux assays, SCENITH provides an overview of the energetic capacities and dependencies of a cell without offering an insight into individual metabolic pathways. More recently, CENCAT (cellular energetics through noncanonical amino acid tagging) was introduced128 as an alternative to SCENITH. While SCENITH measures protein translation by quantifying puromycin incorporation,127 puromycin can be toxic via the induction of ER stress.129 In CENCAT, the authors proposed using click labeling of alkyne-bearing noncanonical amino acids to quantify protein synthesis without potential cellular toxicity.128
The Biolog OmniLog phenotype microarray analyzer is a fully automated high-throughput microarray system that is designed to perform metabolic profiling mainly in microorganisms. Using a 96-well plate-based colorimetric detection system, it can assess the utilization of different sources, including carbon and nitrogen, and, therefore, measure the cellular respiration and metabolic activity. This technology detects changes in cellular respiration by measuring the reduction of a tetrazolium-based dye, which generates a colorimetric signal corresponding to metabolic activity.130 So far, this technique has been used to study antibiotic susceptibility or nutrient utilization in microorganisms,130-132 and has not been employed for functional metabolic investigations of hematopoietic and leukemic cells.
While these aforementioned techniques are suited to study metabolism ex vivo, metabolic cages can provide information about in vivo metabolic functions of rodents at the broader organismal level. Metabolic cages are designed in a way that food and water intake are measured, and urine and feces samples are collected separately. Additional parameters, such as O2 consumption and CO2 production, can also be recorded in some systems. While metabolic cages are used in pharmacodynamic and pharmacokinetic studies (such as Brunner et al.133), the specifics of this tool have been shown to elicit stress and alter physiological metabolism.134, 135 Animal welfare considerations further limit the use of metabolic cages.
Altogether, extracellular flux assays and flow cytometry-based metabolic analyses are common approaches to probe cell metabolism upon metabolic stress. By measuring the cellular responses to metabolic inhibition, we can obtain information about the native metabolic cell state.
APPLICATIONS OF METABOLISM RESEARCH OPEN NEW PERSPECTIVES ON THE BIOLOGY OF HEALTHY AND DISEASED HEMATOPOIETIC AND IMMUNE CELLS
In this section, we discuss examples of metabolic studies in hematopoietic and immune cells that have uncovered novel aspects of biology, disease stratification, outcome prediction, or therapeutic targeting.
Functional, proteomic, and genomic metabolism studies identify mitochondrial metabolism as a vulnerability in AML
Mitochondrial metabolism represents one of the best-characterized metabolic vulnerabilities in AML, as evidenced by multiple studies combining different technologies. Lagadinou et al. found that human primary leukemic stem cells (LSCs)-enriched populations were metabolically dormant with low levels of OCR and ECAR compared to AML blasts from the same patients, as assessed by Seahorse extracellular flux assays.136 Interestingly, LSC-enriched populations also had a significantly decreased capacity to upregulate the glycolytic machinery when mitochondrial respiration was blocked with oligomycin.136 Moreover, the authors showed that LSCs expressed high levels of BCL-2 and that BCL-2 inhibition decreased OCR and induced cell death.136 These data are in line with an earlier work by Skrtic et al., in which tigecycline, an inhibitor of mitochondrial translation, reduced OCR in the human AML cell line TEX, and selectively killed LSCs compared to their nonmalignant counterparts.137 In another study, Sriskanthadevan et al. showed that AML cells had low spare respiratory capacity (by Seahorse extracellular flux assays), in particular with a low reserve in ETC complexes I, III, IV, and V.138 These studies represent early examples of the utilization of the extracellular flux assay technology to discover metabolic profiles specific to AML cells. OXPHOS upregulation has also been described as a mechanism of chemotherapy resistance. Farge et al. utilized 25 patient-derived xenografts (PDX) to study the metabolic profile of residual leukemic cells (RLCs) after cytarabine treatment.57 Interestingly, RLCs had an increased mitochondrial mass (estimated by MitoTracker flow cytometry staining) and mitochondrial membrane potential (by TMRM labeling).57 Transcriptome analysis confirmed a high OXPHOS signature in RLCs recovered from cytarabine-treated compared to vehicle-treated PDX mice.57 Furthermore, leukemic cell lines with low and high mitochondrial ATP production and OCR were injected in immunodeficient mice, which were treated with cytarabine. While low OXPHOS cell lines had a high sensitivity to cytarabine, high OXPHOS cell lines showed resistance.57 These and other studies paved the way for multiple translational efforts aiming at targeting mitochondrial metabolism in AML.139, 140
Interestingly, AML patients show heterogeneity in their mitochondrial metabolism, which might be related to disease outcome. Jayavelu et al. performed proteogenomic analyses of 252 AML samples, in which they integrated information from protein expression, gene expression, mutation, and cytogenetic profiles.141 They identified five proteomic AML subtypes, including the high-risk Mito-AML, which was only detectable in the proteome but not by analyzing genomic mutations or transcriptional signatures.141 Characterized by high expression of mitochondrial proteins, this subtype conferred poor patient outcomes, reduced remission rate, and shorter overall survival upon treatment with induction chemotherapy.141 The authors could demonstrate that this Mito-AML subtype was hypersensitive to mitochondrial complex I inhibitors, highlighting a potential therapeutic target.141 In another study, quantitative proteomics revealed a higher expression of ETC complex I and V proteins in human LSCs compared to AML blasts and healthy hematopoietic stem and progenitor cells.142 More recently, a proteomic analysis of 47 adult and 22 pediatric patients with AML at serial time points during disease progression found higher expression of mitochondrial ribosomal proteins and ETC complex subunits at relapse compared to primary diagnosis.143 These studies give examples of the use of proteomics to define metabolic features and subtypes of disease and support the notion that mitochondrial metabolism defines aggressive AML subgroups.
The quantification of mtDNA has been proposed as a relatively easy-to-measure proxy of mitochondrial activity. Elevated mtDNA content was first reported by Boultwood et al. in patients with AML (n = 25) and chronic myeloid leukemia (n = 9).78 More recently, in a cohort of n = 502 de novo AML patients, the authors identified a subset of patients with increased mtDNA content (n = 163) compared to an age- and sex-matched healthy control cohort.144 Notably, high mtDNA was associated with worse clinical outcomes and reduced sensitivity to cytarabine treatment ex vivo.144 Mechanistically, AML blasts with increased mtDNA content displayed increased mitochondrial mass and membrane potential measured by flow cytometry along a proteomic signature indicative of a higher ETC complex I activity. Blocking complex I activity with metformin reduced the mtDNA content of AML blasts in vitro (n = 40), synergized with cytarabine, venetoclax, and FLT3 inhibitors in vitro, and prolonged the survival of K562-bearing leukemic mice.144 While high mtDNA content appeared detrimental in this setting, the opposing effects have been observed in acute promyelocytic leukemia (APL).79 Overall, these studies suggest that the quantification of mtDNA might allow the identification of specific biological disease subtypes. Further studies will be required to disentangle the biological importance and therapeutic consequences of high mtDNA in distinct disease types.
Identification of metabolic vulnerabilities in AML by metabolomics
The advances in MS-based metabolomics have opened up significant opportunities in translational medicine, including the characterization of disease states, the identification of new biomarkers, and targeted drug development. While we discuss only few examples of studies based on metabolome analysis of primary human AML cells here, a detailed summary of published datasets is provided in Table S1.
Several landmark studies uncovering metabolic vulnerabilities in AML with the use of LC-MS have been published by the Craig Jordan lab. For instance, global metabolic profiling from 15 primary AML patients found that 16 amino acids were enriched in LSCs compared to blasts.145 LC-MS tracing experiments with stable isotope-labeled amino acids revealed significantly faster uptake, in particular of glutamine and glutamate, in LSCs.145 Functional experiments showed that limiting amino acid uptake selectively targeted LSCs by impairing their OXPHOS.145 In another study, primary AML cells were cultured ex vivo in media depleted of individual amino acids.146 Depletion of arginine, glutamine, and especially cysteine reduced cell viability of both blasts and LSCs.146 Cysteine depletion led to impaired glutathione synthesis with subsequently reduced glutathionylation of succinate dehydrogenase A (SDHA), a key component of ETC complex II.146 As a consequence, cysteine-starved AML cells had reduced OXPHOS activity, lower ATP rates, and higher cell death.146 Characterizing changes in metabolite abundance also offers insights into the therapeutic mechanisms of action of anti-leukemic drugs. For instance, MS analysis of AML patient samples collected pre- and 24 h post-treatment with azacitidine and venetoclax showed significant decreases in glutathione and the TCA intermediates αKG, malate, and fumarate, and an increase in succinate.147 These metabolic perturbations suppressed OXPHOS and specifically targeted LSCs, building the foundation for the clinical efficacy of azacitidine and venetoclax.147 Further studies investigated the metabolic profiles of refractory or relapsed AML. Global metabolomics analysis of LSCs isolated from n = 6 patients with de novo AML and n = 6 patients with relapsed/refractory (R/R) AML after treatment with venetoclax/azacitidine showed distinct metabolic profiles in LSCs from patients with R/R AML.148 In particular, 10 amino acids had significantly higher abundance in R/R samples compared to de novo AML, supporting the hypothesis that venetoclax/azacitidine acts by disrupting amino acid metabolism, and elevating amino acid levels might contribute to therapy resistance.148 Interestingly, LSCs from R/R AML patients also had higher levels of the NAD+ precursor nicotinamide.148 Targeting nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in nicotinamide metabolism, selectively eradicated R/R LSCs.148 Similarly, the upregulation of FAO induced resistance to azacitidine/venetoclax.149 Inhibition of FAO by deletion or pharmacological blockade of the fatty acid transporter CPT1A reduced viability and OXPHOS of LSCs from patients with venetoclax/azacitidine-resistant primary AML.149 Interestingly, high FAO rates were correlated with RAS mutations in some patients, underscoring the link between oncogenic mutations and metabolic profiles.149 Another such example is ceramide metabolism. Dany et al. found higher levels of ceramides in human AML blasts from patients with FLT3-ITD mutations compared to patients with wild type FLT3.68 Furthermore, multiple other studies have also demonstrated that recurrent oncogenic mutations and karyotype aberrations elicit specific metabolic profiles in leukemic cells or patient plasma, which might be exploited as therapeutic vulnerabilities.150-152 These are examples of how MS metabolomics contributes to a better understanding of disease biology and treatment responses.
While the vast majority of studies has been performed ex vivo, evaluating dynamic metabolic functions by stable-isotope tracing (see “Metabolite quantification” section) in vivo offers the advantage of obtaining information about metabolic processes as they occur in the complex physiological or pathophysiological environment of the living organism. Stable-isotope tracing studies for cancer have mostly been performed in patients with solid tumors and were recently reviewed.109 Notably, one study performed intravenous infusion of 13C glutamine into healthy volunteers (n = 7), patients with monoclonal gammopathy of undetermined significance (n = 11), and patients with multiple myeloma (n = 12).153 The participants underwent bone marrow biopsy 60 minutes after the infusion, and CD138+ (plasma) cells as well as CD138- cells (remainder of bone marrow mononuclear cells) were sorted and analyzed by GC-MS or LC-MS.153 The authors observed a higher 13C fractional enrichment of TCA cycle metabolites in malignant CD138+ (relative to CD138− cells from the same patient) compared to premalignant CD138+ plasma cells.153 While stable-isotope tracing studies provide very valuable information, several caveats and challenges need to be accounted for.109 For instance, tracer infusions can measure nutrient contributions to metabolites but usually rely on a single biopsy time point, making it impossible to quantify actual metabolic fluxes. Accounting for tumor heterogeneity, potentially by implementing technologies with spatial resolution, and the development of computational frameworks to integrate the generated data are further priorities for the field.109
Inferring metabolic states from transcriptional data
While metabolomics analyses are frequently possible only for expert laboratories, platforms for transcriptome profiling, as well as extensive public datasets, are more readily available. Thus, it is of significant interest whether metabolic states can be inferred from transcriptional profiles. While the activity of metabolic pathways cannot be measured by the expression of individual genes, computational and machine-learning methods are being developed.
One excellent example is a recent study on sphingolipid metabolism in AML.154 Sphingolipid metabolism has been recognized as a metabolic vulnerability in AML (see “Metabolic regulation in healthy and diseased hematopoietic cells” section). In this study, Paudel et al. quantified 33 sphingolipid metabolites in 213 primary AML samples, 30 human AML cell lines, and 6 healthy CD34+ bone marrow samples by MS.154 Within AML samples, the authors found two clusters: cluster 1 with proportionally less hexosylceramides (Hex) and more sphingomyelins (SM) and cluster 2 with proportionally more Hex and less SM. Patients with a HexlowSMhigh profile had twice the failure rate after induction therapy of patients with a HexhighSMlow profile. Transcriptomic analysis by bulkRNA-sequencing was performed for 33 primary AML samples and 30 cell lines. Based on 60% this cohort, the authors developed a vector machine stratifier of Hex-SM profiles using the 284 most variable and differential genes. The classifier showed a predictive performance on the remaining 40% of the cohort with an AUC of 0.91. Furthermore, when patients from the TCGA72 and BeatAML2.074 cohorts were stratified for their Hex-SM profiles based only on transcriptional data, patients inferred as HexlowSMhigh had significantly worse outcomes than those predicted to be HexhighSMlow. Interestingly, Hex-SM profiles were not predictable by genetic mutations.154
Several studies highlight possibilities for defining metabolic states of immune cells from single-cell transcriptome analyses.155, 156 For instance, Fernandez-Garcia et al. could define metabolic states of CD8+ T cells as cells transitioned through the activation/differentiation cascade by single-cell RNA-seq.155 The authors used gene set variation analysis (GSVA) to determine per-cell pathway activity scores in the transition from an unactivated to an activated state.155 This approach identified several well-known hallmarks of T cell metabolism, such as a substantial increase in aerobic glycolysis157 and amino acid158 uptake upon activation. Importantly, the study also uncovered novel aspects of T cell metabolism by recognizing a role for asparagine synthetase expression in effector T cell differentiation, function, and anti-tumor responses.155 Moreover, Wagner et al. developed Compass, an algorithm that characterizes cellular metabolic states based on single-cell transcriptomics and employed it as a tool to uncover metabolic signatures in non-pathogenic and pathogenic Th17 cells.156 Efforts to model metabolic states from single-cell transcriptomics have been recently reviewed by Hrovatin et al.159
Flow cytometry-based assessments of metabolism and their application in hematology
Flow cytometry has versatile applications for the assessment of metabolic profiles. It can be used to assess the expression of metabolic proteins (see “Protein expression analysis” section), to measure metabolites, such as fatty acids or ROS (see “Metabolite quantification” section), and to evaluate functional metabolic properties, such as mitochondrial membrane potential and glucose/mitochondrial metabolism via SCENITH (see “Functional read-outs” section). The broad availability of flow cytometers and the necessary reagents as well as the relative simplicity of the analysis and the high throughput, make these techniques widely used tools in hematological and immunological research.
For instance, primary AML samples showed substantial heterogeneity in their redox staining profile, with a ROS-low fraction characterized by quiescence, lower cell cycle activity, and higher engraftment capacity in mouse xenograft models compared to a ROS-high AML cell fraction from the same patients.136 ROS-low AML cells also maintained engraftment capacity in serial transplantation experiments, identifying them as LSC, compared to the blast-enriched ROS-high population.136 This observation allowed a relatively simple identification and isolation strategy for the LSC-enriched fraction in primary patient samples. Furthermore, SCENITH was utilized to describe the metabolic signature of LSCs in AML.160 In an analysis of CD34+ samples from n = 29 AML patients, Forte et al. observed that CD34+ cells relied predominantly on glucose oxidation as measured by high glycolytic capacity and glycolytic dependence, and low mitochondrial dependence and FAO/amino acid oxidation capacity.160 However, the metabolism of the more immature CD34+CD38low/− fraction was marked by an increase in mitochondrial dependence, in line with the notion that mitochondrial metabolism represents a therapeutic vulnerability in AML.160 SCENITH-derived metabolic profiles also showed a correlation with risk groups according to the ELN 2022 classification.161 For example, samples from the favorable group had a lower mitochondrial dependence (8.2%) compared to samples from the intermediate and adverse risk groups (22.3% and 23.5%, respectively).160 Altogether, these studies demonstrate how flow cytometry-based assessments of metabolism can be implemented to define hematopoietic cell subset biology but also as a non-invasive, easy-to-implement approach for metabolic risk stratification in hematological cancers. We expect that the development of new antibodies and compounds will facilitate the studies of individual metabolic pathways and increase the scope of these studies.
Definition of disease and risk prognosis by biofluid metabolomics
Several studies have attempted to characterize the serum metabolome of patients with AML and other hematological diseases (Table S1). Both NMR spectroscopy and LC-MS have been utilized in these studies. NMR spectroscopy was used to measure metabolites in 116 human serum samples, including patients with AML, non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and healthy controls.162 A total of n = 50 metabolites were assigned, and their concentrations in the respective disease group were used to infer the metabolic profiles associated with a specific disease type. Interestingly, more aggressive malignancies (such as AML) caused more profound changes in the serum metabolome.162 Significant differences in the serum metabolome were also found in a study of 183 patients with de novo AML and 232 age- and sex-matched healthy controls.163 In particular, differences in glycolysis/gluconeogenesis, TCA cycle, biosynthesis of proteins and lipoproteins, and metabolism of fatty acids and cell membrane components were observed.163 Similar results were obtained in an NMR spectroscopy analysis of serum samples from n = 32 patients each with AML, ALL, aplastic anemia, and healthy controls.164 Simonetti et al. investigated the serum and urine metabolome from >100 patients with AML and healthy controls.165 They found that both the serum and the urine metabolome (assessed by NMR spectroscopy) provided efficient discrimination between patients and healthy controls with an accuracy of 83% and 85%, respectively, and the integration of serum and urine data yielded an even higher (90%) accuracy. Thirteen metabolites were significantly altered in the biofluids of AML patients, compared to controls, including elevated levels of lactate, polyunsaturated fatty acids, pyruvate and succinate in AML patients and reduced glutamine, citrate, glycine, and creatinine. Importantly, the authors also leveraged analysis of intracellular metabolites in AML cells (CD34+ and CD33+), normal umbilical cord blood CD34+, and normal peripheral blood CD33+ cells by LC-MS. By integrating intracellular and biofluid metabolomics, they observed particularly prominent aberrations in polyamine, purine, ketone bodies, and polyunsaturated fatty acid metabolism in AML. Moreover, metabolome-based clusters showed enrichment for specific mutations, such as NPM1. Within NPM1-mutated samples, two subgroups were identified. One was enriched for mutations in cohesin/DNA damage-related genes and had increased serum choline + trimethylamine-N-oxide and leucine. This study highlights how the integration of metabolome data from cells and biofluids with genomic and transcriptomic analyses can define disease subgroups that might be particularly susceptible to metabolic pathway targeting.165 Additional studies exploring the metabolome of biofluids of patients with hematological diseases are referenced in Table S1.
CyTOF and spatial proteomics uncover immune cell metabolic profiles based on protein expression
Similar to flow cytometry, CyTOF allows the quantification of the expression of proteins related to metabolism in combination with analysis of phenotypic identity at the single-cell level. CyTOF offers the advantage of minimal signal overlap, generally allowing for a higher number of analyzed parameters within the same panel. Hartmann et al. combined CyTOF with spatial analysis by multiplexed ion beam imaging by time-of-flight (MIBI-TOF) to establish single-cell metabolic regulome profiling (scMEP) with the analysis of metabolic phenotypes in conjunction with spatial organization.166 Initially, the authors assessed the performance of over 110 commercially available antibodies by immunohistochemistry, CyTOF, and MIBI-TOF. Of these, they selected a panel of 41 antibodies covering glycolysis, amino acid metabolism, fatty acid metabolism, the PPP, the TCA cycle and ETC, mitochondrial dynamics, biosynthesis, signaling, and transcription. Next, they studied the scMEP of human CD4+ and CD8+ T cells upon in vitro activation and benchmarked those against classical read-outs such as extracellular flux assays. Mass cytometry-based scMEP recapitulated multiple hallmarks of T cell metabolic remodeling, such as increased glycolysis upon activation (as measured by increased expression of the glucose transporter GLUT1, hexokinase 2, phosphofructokinase 2, and lactate dehydrogenase A). Simultaneously, TCA/ETC proteins such as citrate synthase and ATP synthase were elevated, and this was in line with increased levels of basal OXPHOS measured using the Seahorse analyzed. Notably, the authors could transfer the technology to spatial assessments with MIBI-TOF and analyzed the spatial architecture and heterogeneity of immune cells in colon tissue from patients with colorectal carcinoma (n = 4) and non-malignant control sections from independent healthy donors (n = 3) using a panel of 36 antibodies.166 Similarly, Levine et al. employed a metabolic CyTOF panel covering T cell phenotypic markers, signaling molecules, nutrient transporters, and enzymes involved in the TCA cycle, ETC, and FAO to study the metabolic profiles of mouse CD8+ T cells during activation and memory development.167 Effector T cells showed an elevated expression of GLUT1, GAPDH, and the amino acid transporter CD98, compatible with an activated glycolytic signature, while memory T cells upregulated the expression of the fatty acid transporter CPT1A. Furthermore, using L. monocytogenes infection as a model of in vivo T cell activation, the authors identified a group of early activated T cells that comprised up to 20% of the total T cell population and had simultaneously the highest expression of GLUT1 and GAPDH, but also of oxidative markers such as CTP1A and ATP5a.167 Taken together, these technologies illustrate that CyTOF-based metabolic profiling recapitulates known metabolic processes in activated immune cells and allows to address questions such as population heterogeneity, which cannot be captured by extracellular flux assays, bulk proteomics, or metabolomics. The combination with imaging-based technologies also paves the way to a better understanding of immune cell metabolism heterogeneity in complex tissues.
Metabolic signatures in allogeneic HSC transplantation
While the majority of studies we have discussed so far concern hematological diseases outside of an allogeneic hematopoietic stem cell transplantation (allo-HCT) setting, several studies could demonstrate the translational relevance of metabolic profiles in shaping and predicting patient transplantation outcomes. A recent study was performed on pre-transplantation serum samples derived from 92 consecutive allo-HCT recipients with AML or MDS.168 Using LC-MS non-targeted metabolomics, the authors could identify significant differences in metabolites in the recipients, which could be correlated to the occurrence of severe transplantation-related mortality (TRM). Comparing recipients who suffered from TRM-associated factors (early extensive fluid retention, pre-transplantation inflammation, and aGVHD) to those without, they could identify significant differences in overall metabolism. Specific metabolic changes could be associated with each of the three TRM-associated factors mentioned earlier, as evidenced by increased levels of amino acid metabolites, altered levels of xenobiotics and lipid metabolites, specifically in the group of patients who developed aGVHD. The authors could further identify patient subsets with an increased risk of 2-year TRM, using unsupervised hierarchical cluster analyses to subclassify allotransplant recipients based on their metabolomic profiles.168 These findings suggest that integrating metabolomic analysis into clinical practice could enhance patient monitoring and improve long-term transplant success rates.
Other examples concerning the role of metabolomics (also as potential predictive biomarkers) in transplantation outcomes include studies on the occurrence of chronic GVHD (cGVHD) after allo-HCT. Research on serum and plasma metabolites comparing samples from patients with or without cGVHD could demonstrate significantly different levels of metabolites, mainly belonging to amino acid and lipid metabolism, establishing associations between the patients' metabolome and post-transplant outcome.169-171 A similar association could be seen investigating serum pre-transplant profiles of patients with or without post-transplant capillary leak syndrome.172 These studies collectively suggest a potential for using distinct metabolic signatures as predictive biomarkers for early diagnosis, risk assessment, and treatment monitoring. More studies and detailed information on this topic can be obtained from Table S1.
Besides using systemic metabolomics as predictive biomarkers, recent studies also highlight how immune-related metabolic alterations can influence post-transplant outcome. Studying fecal samples after allo-HCT and early administration of azithromycin (an antibiotic shown to increase relapse risk), a complex network of bacterial taxa and metabolites, among others, associated with relapse occurrence and remission status could be identified.173 Furthermore, distinct enterotypes could be associated with the status of exhausted T cells, showing how an antibiotic can potentially influence the microbiome and modify immune cells, and thereby promote disease relapse.173 Similarly, the accumulation of oxidative DNA damage measured by increased concentrations of 8-hydroxydeoxyguanosine (8-OHdG) in serum and reconstituting T cells after allo-HSCT was linked to increased metabolic stress, leading to premature exhaustion of the T cells and impaired anti-leukemic activity, which ultimately increased the risk of relapse and reduces survival.174 Taken together, these findings highlight the role of both systemic and immune-related metabolic alterations in post-transplant outcomes and how they can potentially be used as biomarkers or therapeutic targets for improving the outcomes of allo-HCT recipients.
CONCLUSIONS AND OUTLOOK
Metabolism is tightly linked to cell phenotype and function in health and disease. Recent technological advances allow an in-depth characterization of metabolism at distinct levels. In this study, we have discussed how metabolism can be studied at the levels of genes, transcripts, protein expression, metabolite abundance and flux, and the function of metabolic pathways. We have summarized the broad range of technologies with their individual benefits and disadvantages in Table 2. Currently, these techniques are predominantly employed in research laboratories rather than in clinical applications and practice. They inform experimental research and are instrumental in the identification of novel therapy targets. Furthermore, specific metabolic profiles (e.g., mitochondrial DNA content, proteomic signatures, and expression of individual proteins) have been linked to disease outcomes and therapy responses.
| Technology | Input material | Output | Sample destruction | Required input (range) | Single-cell resolution | Advantages | Limitations | # of features |
|---|---|---|---|---|---|---|---|---|
| Gene and transcript-based methods | ||||||||
| Gene sequencing | Cells Tissues (DNA) |
Gene mutational status | Yes | 100 ng to 1 µg DNA |
|
|
|
>10,000 |
| Mitochondrial DNA analysis | Cells Tissues (DNA) |
Quantification and ratio of mitochondrial (e.g., CYTB) and nuclear genes (e.g., PKLR, HBB) | Yes | 20 ng | No |
|
|
N/A |
| RNA sequencing | Cells Tissues (RNA) |
Gene expression | Yes | 100 ng to 1 µg RNA for bulk RNA-sequencing | Bulk and single-cell analysis possible |
|
|
>10,000 |
| Protein-based methods | ||||||||
| Proteome MS | Cells Tissues Biofluids Tissue culture supernatants (Protein lysate) |
Protein abundance | Yes | 1–100 µg >100 µg for PTM analysis |
Mostly bulk analysis, but single-cell resolution is also possible |
|
|
Hundreds to thousands |
| Proteome MS with spatial resolution (e.g., MALDI-MS) | Tissue slices | Protein abundance with spatial resolution | Yes | Approx. 10 µm thickness | Near-single-cell |
|
|
Hundreds to thousands |
| Cytometry time-of-flight (CyTOF) | Single-cell suspensions | Protein abundance | Yes | 104–106 cells | Yes |
|
|
Tens |
| Imaging-based spatial single-cell proteomic assays (CODEX, IMC, MIBI) | Tissue slices | Protein abundance with spatial resolution | Yes | Depending on application | Yes |
|
|
Tens |
| MetFlow | Single-cell suspensions | Fluorescence intensity corresponding to protein abundance | Yes | 104–106 cells | Yes |
|
|
Max. 10 |
| Metabolite-based methods | ||||||||
| NMR spectroscopy | Cells Tissues Biofluids Tissue culture supernatants |
Metabolite abundance | No | 10–1000 µg | No |
|
|
Tens |
| LC/GC-MS metabolomics | Cells Tissues Biofluids Tissue culture supernatants (Metabolite/lipid extract) |
Metabolite/lipid abundance | Yes |
|
No |
|
|
Tens to hundreds |
| Metabolomics and lipidomics with spatial resolution (e.g., MALDI-MS) | Tissue slices | Metabolite/lipid abundance with spatial resolution | Yes |
|
Near-single-cell |
|
|
Tens to hundreds |
| Enzymatic assays | Cells Tissues Biofluids |
Metabolite abundance | Yes | 106 cells appr. 10–100 µL biofluids | No |
|
|
One-two |
| Flow cytometry metabolite detection | Single-cell suspensions | Fluorescence intensity corresponding to metabolite abundance | No | 104–106 cells | Yes |
|
|
Ones to tens |
| Functional assays | ||||||||
| SCENITH | Single-cell suspensions | Fluorescence intensity of puromycin as a measure of protein synthesis under metabolic stress | Yes | 104–106 cells | Yes |
|
|
Calculation of four metabolic parameters based on protein synthesis activity |
| Mitochondrial mass and mitochondrial membrane potential measurement | Single-cell suspensions | Fluorescence intensity of mitochondrial probes | No | 104–106 cells | Yes |
|
|
One-two |
| Extracellular flux assays | Single cell suspensions, isolated mitochondria |
|
No | 1–2 × 105 cells | No |
|
|
Two direct measures—additional parameters can be calculated |
As technologies have developed in the past decade, more and more studies combine several approaches to explore metabolism in patient samples. Simonetti et al. combined serum, urine, and intracellular metabolomics with genomic profiling to delineate metabolic profiles and their correlation with genetic subgroups165 (see “Definition of disease and risk prognosis by biofluid metabolomics” section). This study demonstrated that subgroups with different metabolic profiles and, potentially, different drug sensitivity exist within the same genetic subgroup. The study by Jayavelu et al. combining genomic, transcriptomic, and proteomic information revealed that proteomic subtypes—in particular, the Mito-AML subtype—can only be captured in the proteome and not by current genome-based risk stratifications141 (see “Functional, proteomic, and genomic metabolism studies identify mitochondrial metabolism as a vulnerability in AML” section). Vallet et al. combined microbiome and metabolome studies with immune profiling to dissect how early post-transplant azithromycin administration increased malignancy relapse risk173 (see “Metabolic signatures in allogeneic HSC transplantation” section). With the development of more and more assays to probe metabolism by flow cytometry,97, 127 it becomes easier to obtain information about the metabolic state at the single-cell level and combine these analyses with immune phenotyping, genomic and transcriptional studies.
We expect to observe developments in several areas in the coming years. For instance, we hope that the development of new antibodies and metabolic probes will allow us to study more metabolic characteristics by flow cytometry and CyTOF. Since flow cytometry analyses are routinely performed in hematological diagnostics, such advances could bring metabolism examination closer to clinical practice. Furthermore, we expect advances in the field of MS imaging, allowing the broader application of spatial proteomics and metabolomics to specimens from the bone marrow. Third, we believe that more dynamic studies of metabolism, such as through metabolic flux analysis or flow cytometry, will be pivotal to understanding the contribution of metabolic processes to the physiology and pathophysiology of the hematopoietic system. Finally, optimizing the integration of such multi-modal data will be essential to unravel the complex dynamic metabolic processes at cellular and systemic levels. Several such software solutions have been proposed, such as Ingenuity Pathway Analysis (IPA; Qiagen), Compass,156 or Metaboverse.175
One key question for the field is whether metabolic signatures can be implemented in clinical practice to stratify disease risk or predict patients who are likely to have poor responses to standard treatment. While LC-MS-based technologies are likely to remain limited to experimental research laboratories due to the complexity of the workflows and data interpretation, we believe that advances in flow cytometry and genome-based techniques hold the potential for clinical translation. The presence of high-risk metabolic features (e.g., high mitochondrial activity) might provide hints that certain patients are likely to have refractory disease or relapse after standard treatment. As venetoclax/azacitidine treatment is tightly linked to cellular metabolism,147 we can envision a prediction of therapy-refractory patients by assessment of metabolic signatures. Of note, a “Mediators of apoptosis combinatorial score” (MAC-Score) based on flow cytometry assessment of BCL2, BCL-xL, and MCL1 expression in LSCs had a positive predictive value for increased event-free survival after azacitidine/venetoclax treatment of more than 97%.176 While the MAC score is not based on classical metabolic pathways, we believe that similar metabolic parameters exist and will be the focus of future studies.
While our understanding of the role of metabolism in hematological diseases has advanced, developing therapeutic strategies based on metabolic targets remains a considerable challenge. One classical compound in the treatment of acute lymphoblastic leukemia (ALL) is asparaginase, an enzyme that breaks down asparagine. As asparagine is an essential amino acid for ALL cells (but not for other tissues), depletion of asparagine affects protein synthesis in ALL blasts and induces cell death.177 However, the identification of metabolic vulnerabilities that can be selectively targeted in malignant cells while sparing healthy tissues has proven challenging. Recently, IACS-010759, a highly potent and selective small-molecule complex I inhibitor, was tested in two dose-escalation phase I trials in patients with relapsed/refractory AML (NCT02882321, n = 17) and advanced solid tumors (NCT03291938, n = 23). Despite encouraging preclinical data, the trials were terminated due to a narrow therapeutic window with emerging dose-limiting toxicities, such as elevated lactate concentrations and neurotoxicity.178 Nevertheless, we expect that technological advances in metabolism studies will allow us to identify cancer-specific metabolic vulnerabilities or develop therapeutic approaches to target malignant cells selectively, thus opening new avenues for translation.
ACKNOWLEDGMENTS
The authors would like to thank Cécile Demarez for help with reference management.
AUTHOR CONTRIBUTIONS
All authors performed literature research and wrote the manuscript. All authors read and agreed to the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
P. A. has received travel support from Pfizer, outside of the scope of the current work. E. H. and J. M. B. declare no relevant conflicts of interest.
FUNDING
P. A. receives financial support by the Swiss National Science Foundation (grant number 10.001.317), the European Hematology Association (Bilateral Collaborative Research Grant 2024), and the Novartis Foundation for Biomedical Research (grant number 24C246).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




