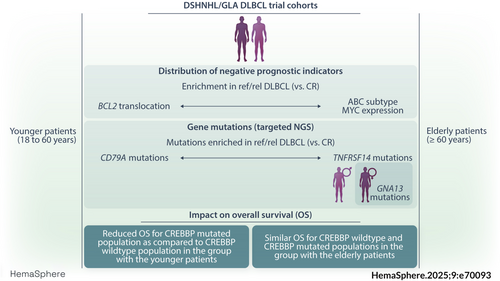
Age- and gender-specific molecular characteristics of diffuse large B-cell lymphoma: Results from clinical trials of the DSHNHL/GLA
Graphical Abstract
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma. Despite a high cure rate, too many patients show refractory (ref) or relapsed (rel) disease. This study examines the frequency of recurring gene mutations and their interplay with well-known biomarkers in female and male patients between 18 and 80 years with ref/rel DLBCL compared to patients with complete remission (CR) to identify biological risk factors associated with treatment response, using cohorts of R-CHOP-like treated DLBCL enrolled in clinical trials of the DSHNHL. The biomarker profile of patients differed between younger and elderly patients with ref/rel DLBCL, with a higher frequency of BCL2 translocations in younger patients, and higher numbers of ABC subtypes and MYC protein expression in the elderly. Amplicon sequencing revealed generally higher mutation frequencies in the younger cohort. Mutations in CREBBP and TNFRSF14 were associated with shorter overall survival (OS) only in younger patients. A higher proportion of GNA13 mutations was detected in female patients of the elderly DLBCL patient cohort, clearly emphasizing the striking differences in biomarker distribution between younger and elderly as well as female and male patients.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common non Hodgkin lymphoma. To date, in spite of numerous attempts to improve outcome over those reported for standard R-CHOP and the emerging knowledge of different molecular subgroups,1 DLBCL still shows high relapse rates in around 30% of patients.2 These are typically associated with resistance to chemotherapy and ultimately, 80% of patients with refractory (ref) or relapsed (rel) disease succumb to their lymphoma.3 Despite remarkable clinical efficacy, new therapeutic approaches like CAR T-cell therapy also are associated with toxicity4-6 and a substantial number of patients experience relapse after CAR T therapy.7, 8 Molecular classifiers based on mutational profiling and patterns of genetic alterations9-11 have not yet led to new therapeutic concepts, although these approaches may foster new individualized therapeutic (targeted) strategies in the future.12, 13 Due to the lack of consensus on platforms and algorithms and their complex and cost-intensive nature many molecular markers are not (yet) ready for application in the routine diagnostic setting. Several biomarkers such as the GCB/ABC cell of origin (COO) classification, translocations of MYC, double hit status of MYC and BCL2 or BCL6 (DH/TH), mutations in CREBBP, or the dual expresser status of MYC and BCL2 proteins (DE), however, are being used in clinical trials and partly became part of the most recent lymphoma classifications.14-18 To date, numerous publications have investigated the impact of these risk facors in patients with rel/ref DLBCL yielding different results regarding the presence or absence of these markers and their clinical impact.19-28 Of note, in many of these reports, only single markers and their possible correlation with treatment response have been studied and their interplay with clinical risk factors has not been fully elucidated. One important clinical parameter in the evaluation of biological parameters is the age of the patients given their different molecular profiles and the significance of biological prognostic markers in elderly and younger patients.29 Here, we have systematically analyzed profiles of recurrently altered genes in younger and elderly patients with DLBCL, also taking into account their gender, and re-investigated the crosstalk of mutations and well-known biomarkers, such as translocations of MYC and BCL2, DH and DE status and COO in patients with well-annotated clinical records treated with various, mostly R-CHOP-based regimens enrolled in prospective clinical trials of the German High Grade Lymphoma Study Group (DSHNHL)/German Lymphoma Alliance (GLA). In addition, we analyzed ATP-binding cassette (ABC) transporter expression involved in the cellular metabolism of vincristine and doxorubicin, and their potential effect on varying treatment responses.
MATERIALS AND METHODS
Patients
All patients had been enrolled in prospective multicenter clinical trials of the DSHNHL/GLA. For the re-evaluation of already established and analyzed biomarkers, cohorts of R-CHOP-like treated DLBCL elderly patients (>60 years of age) [RiCOVER60 (n = 474),30 RiCOVER-noRTh (n = 130),31 DENSE-R-CHOP (n = 98)32 and SMARTE-R-CHOP (n = 156)33] and cohorts of younger patients between 18-60 years [MInT (n = 122),34 MegaCHOEP phase III (n = 202),35 and MegaCHOEP observation (n = 68)36] were included (for details see Supporting Information S1: Figure S1). Biomarkers that had been evaluated in previous publications14, 15, 29, 37 include BCL2 and MYC protein expression, DE status, BCL2-translocation, MYC-translocation, DH status of BCL2 and MYC, as well as COO (according to the Lymph2CX classification) (Supporting Information S1: Figure S1). From the initial 1250 DLBCL samples, a total of 695 of the elderly (≥60 years) and 119 samples of the younger patients (18–60 years) were available for subsequent analysis. For the targeted resequencing approach, only samples from the RiCOVER60 trial30 (all patients nall = 287 and DLBCL patients with Rituximab-treatment nR = 117, Supporting Information S1: Table S1) and the MegaCHOEP phase III35 trial (n = 90, Supporting Information S1: Table S2) were available from elderly (RICOVER60) and younger high-risk patients (MegaCHOEP), respectively. For comparative analyses of CR and ref/rel patients data was available from 169 elderly and 90 younger patients (Supporting Information S1: Figure S2). Diagnostic samples had been reviewed by expert hematopathologists according to the 2008 WHO classification38 with the assessment of underlying morphology (centroblastic, immunoblastic, T-cell rich, NOS) that showed similar distribution in all trial cohorts investigated. The most common variant was DLBCL centroblastic (elderly: 58%, younger: 51%), followed by DLBCL NOS (elderly: 34%, younger: 44%), DLBCL immunoblastic (elderly: 6%, younger: 2%) and DLBCL T-cell rich (elderly: 2%, younger: 3%). Clinical trials were conducted in accordance with the Helsinki declaration, and the protocols had been approved by the ethics review committee of each participating center.
Targeted resequencing
The NGS panel was designed to identify recurring mutations in the hotspots of 18 genes (Supporting Information S1: Figure S2) in DLBCL selected according to previously published whole genome sequencing (WGS) and whole exome sequencing (WES) data.39-42 For details see the Supporting Information.
Immunohistochemical stainings
Diagnostic stainings were performed as previously described.14, 15, 29, 37 MYC and BCL2 stainings were considered positive when more than 40% and 50% cells expressed the protein, respectively.15 Immunohistochemical staining of ABCB1 and ABCC2 is described in the Supporting Information.
Statistical evaluation
Event-free survival (EFS) was the main endpoint of the RiCOVER-60 and the MegaCHOEP trial. EFS, progression-free survival (PFS) and overall survival (OS) were estimated by Kaplan–Meier analysis.43
In univariate outcome analyses, log-rank tests were performed. Proportional hazard models for the mutations were adjusted for the factors of the IPI. Treatment response was categorized into patients with continuous complete remission (CR) and refractory (ref) or relapsed (rel) patients (ref/rel) after 2 months of observation (n = 695 elderly patients and 119 younger patients). A variable number of cases failed to yield reliable results in IHC or genetic analyses and were accordingly excluded from analysis. Thus, the number of evaluable cases varied from one marker to another. For the correlation of the biomarker status with treatment response of the patients, as well as for comparison of mutations frequency and treatment response in the cohort of younger and elderly patients we used Fisher's exact test and Benjamini Hochberg correction for multiple testing.
RESULTS
The risk profile of CR and ref/rel patients is different in younger and elderly DLBCL patients
Of 1250 DLBCL samples, 695 and 119 samples with available biomarker status from elderly and younger patients, respectively, entering continued CR, or relapsing/being refractory to first-line therapy were included in subsequent analyses. Overall, 522 of 695 elderly patients (75%) showed CR, while 173/695 (25%) were refractory to treatment or relapsed. In the cohort of younger patients CR was observed in 88/119 (74%) patients and 31/119 (26%) patients were refractory to treatment or relapsed (Supporting Information S1: Figure S1).
The patterns and frequencies of biomarker expression in CR and ref/rel patients differed in the cohorts of younger and elderly patients. In elderly patients, the frequencies of MYC and BCL2 translocations and of MYC/BCL2 DH was similar between CR and ref/rel patients. (Figure 1A). However, significant differences were observed with regard to enhanced MYC protein expression (CR: 40% vs. ref/rel: 52%, p = 0.02), BCL2 protein expression (CR: 71% vs. ref/rel: 83%, p = 0.004), enrichment of the ABC subtype (CR: 29% vs. ref/rel: 58%, p ≤ 0.0001) and DE status (CR: 31% vs. ref/rel 43%, p = 0.016, Figure 1A, Supporting Information S1: Table S1). In younger patients, no significant differences with respect to MYC protein expression, MYC breaks and ABC subtype were observed between CR and ref/rel patients. In contrast, the number of DLBCL harboring BCL2 breaks and BCL2 protein expression was significantly higher in younger ref/rel compared to CR patients (BCL2 break: 40% vs. 12%, p = 0.0048; BCL2 expression: 89% vs. 65%, p = 0.039). Moreover, tumors from younger ref/rel patients were characterized by higher numbers of DE compared to CR patients (DE: 53% vs. 30%, p = 0.05, Figure 1B, Supporting Information S1: Table S2).
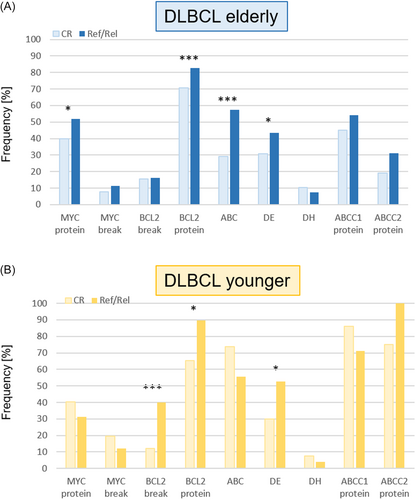
The ABC transporter proteins ABCC1 and ABCC2 are known to be involved in the cellular metabolism of vincristine (ABCC1, ABCC2) and doxorubicin (ABCC1), both of which are components of the R-CHOP regimen (Supporting Information S1: Figure S3A). For staining with ABCC1 and ABCC2, specimens from 51 and 53 CR DLBCL and 26 ref/rel DLBCL were analyzable in the cohort of elderly patients, respectively. In the cohort of younger patients, ABCC1 and ABCC2 stainings were performed in 28 CR DLBCL and in 8 ref/rel DLBCL, respectively.
Both proteins were expressed in significantly more cases in the cohort of younger patients (ABCC1: 30/36 (83%) vs. 37/77 (48%) in the elderly cohort, p = 0.0003; ABCC2: 28/36 (78%) vs. 18/79 (23%) in the elderly patients, p = 2.91E−08, Supporting Information S1: Figure S3B). However, there was no difference in ABCC1 and ABCC2 expression in tumors from CR and ref/rel patients neither in the elderly nor in the younger cohort (Figure 1A,B).
Tumors from patients of the elderly and younger DLBCL cohorts were then grouped according to the absence (no risk factor) or the presence (one or more) of the above mentioned factors (MYC protein, MYC break, BCL2 break, BCL2 protein, COO, DE, DH).1 Samples were exlcuded if information for one biomarker was missing, leaving evaluable data from 89 CR and 66 ref/rel elderly patients and 46 CR and 25 ref/rel younger patients. A significantly higher proportion of elderly CR patients presented without any risk factor compared to the ref/rel patient group (CR: 14/89, 16% vs. ref/rel: 3/66, 5%, p = 0.02 Supporting Information S1: Figure S4A). In contrast, in the cohort of younger patients with ref/rel DLBCL, all patients (n = 25) had at least one risk factor. Patients without any risk factors were exclusively found in the CR cohort (4/46, 6%, Supporting Information S1: Figure S4B), however to a lesser extent as observed in the CR cohort of elderly patients.
The mutational profiles of DBLCL differ in younger and elderly patients
For the comparison of mutational patterns in younger and elderly patients, a total of 377 samples were included in a targeted resequencing approach. Of those, 287 samples belonged to the cohort of elderly patients (RiCOVER60) and 90 specimens were from younger patients (MegaCHOEP). For comparative analyses of CR and ref/rel patients clinical data was available from 169 elderly and 90 younger patients (Supporting Information S1: Figure S2, Table S5). Clinical data from both elderly and younger DLBCL cohorts amenable to sequencing analysis closely matched the entire study population (Supporting Information S1: Tables S3 and S4). Apart from age, however, younger and elderly patients differed in their risk profiles as assessed by the international prognostic index (IPI) with significant differences in ECOG performance status (younger: 33% ECOG > 1 vs. elderly: 15%, p < 0.001), lactate dehydrogenase (LDH) levels (younger: 97% LDH > N vs. elderly: 47%, p < 0.001), Ann Arbour stage (younger: 99% stage III/IV vs. elderly: 50%, p < 0.001) and extralymphatic involvement (younger: 42% EXBM > 1 vs. elderly: 15%, p < 0.001, Supporting Information S1: Tables S3 and S4).
Mutations in TP53 (20%, 42/206),44 MYD88 (21%, 35/169), and CREBBP (17%, 28/169) were the most frequently observed alterations in tumors from elderly patients. In contrast, the most prevalent mutations detected in younger patients affected TNFAIP3 (64%, 58/90), EP300 (58%, 52/90) and CREBBP (60%, 54/90, Figure 2A). Accordingly, significant differences in the numbers of mutations in younger and elderly patients were observed for TNFAIP3 (younger: 58/90, 64% vs. elderly: 12/169, 7%, p = 6.3E−22), CREBBP (younger: 54/90, 60% vs. elderly: 28/169, 17%, p = 2.17E−12), EP300 (younger: 52/90, 58% vs. elderly: 20/169, 12%, p = 2.83E−17) and CD79A (younger: 9/90, 10% vs. elderly: 2/169, 1%, p = 0.006, Figure 2A, Supplemental Table S5).
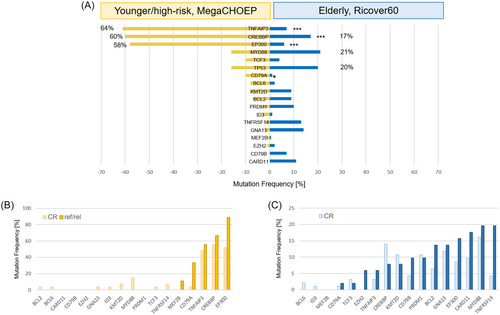
Comparison of mutational profiles in tumors from CR and ref/rel patients in the younger patient cohort indicated a trend toward a higher mutation frequency in CD79A in ref/rel patients (CD79A: 3/9, 33.3% vs. 1/27, 3.7% in the CR patients, p = 0.099; Figure 2B, Supporting Information S1: Table S5). In contrast, a trend toward more mutations in TNFRSF14 was observed in the ref/rel patients of the elderly DLBCL cohort (TNFRSF14: 10/51, 19.6% vs. 4/93, 4.3% in the CR patients, p = 0.076; Figure 2C, Supporting Information S1: Table S5). With regard to ABC and GCB subtype distribution, no significant differences were observed for the number of mutations per sample and the number of mutations per gene, neither in the elderly nor in the younger cohort. In the cohort of the elderly, mutations in BCL2 (p = 0.031), KMT2D (p = 0.017), and TNFRSF14 (p = 0.02) more frequently occurred in the GCB subtype, while CD79B (p = 0.026) and MYD88 (p = 0.0004) mutations were significantly enriched in ABC DLBCL. The number of samples with NGS and COO data in the younger cohort was limited thus hampering stringent evaluation of correlations. In both cohorts, MYD88 L265P mutations were predominantly observed in ABC DLBCL (p = 0.021) and were enriched in cases with extralymphatic involvement in elderly (p = 0.0007), but not in younger patients (p = 0.3441, Supporting Information S1: Figure S5).
The number of samples with more than one mutation per gene was higher in younger than in elderly patients (39/90, 43.3% vs. 26/169, 15.4%, p = 1.24E−6). Those multiple gene mutations predominantly affected TNFAIP3, EP300, and CREBBP in the cohort of younger patients.
Clinical impact of mutations
The number of mutations varied between 0 and 7 per sample (mean = 1.55 variants/sample) in the cohort of elderly patients and 0–9 per sample (mean = 3.38 variants/sample, p = 2.23E−14) in younger patients (Supporting Information S1: Figure S6A,B).
When compared with patients without any mutations, elderly patients whose tumors harbored at least one or more mutations had inferior overall survival (p = 0.017, Supporting Information S1: Figure S6C). This effect was not evident in the cohort of younger patients (p = 0.404, Supporting Information S1: Figure S6D).
TP53 mutations were of prognostic impact in the cohort of eldery patients with DLBCL predicting inferior EFS, PFS and OS44 in the entire cohort, but not in the cohort of patients treated with rituximab. In contrast, TP53 mutations did not predict EFS (p = 0.224), PFS (p = 0.527), or OS (p = 0.774) in the younger patient cohort.
Mutations in BCL2 (p = 0.039 and p = 0.045) and TNFRSF14 (p = 0.034 and p = 0.004) predicted shorter progression free survival (PFS) in the entire cohort of elderly patients with DLBCL in univariate and multivariate analyses, respectively, but the difference was not significant for OS (BCL2: p = 0.084; TNFRSF14: p = 0.183) and EFS (BCL2: p = 0.259; TNFRSF14: p = 0.092, data not shown). However, this effect was not observed in patients treated with rituximab. A trend toward a higher number of TNFRSF14 mutations was detected in the entire cohort of elderly ref/rel patients (19.6%) compared to patients in CR (4.3%, p = 0.0763, Figure 2C, Supporting Information S1: Table S5).
In contrast, TNFRSF14 mutations were associated with inferior PFS and OS in univariate (p = 0.019 and p = 0.003, respectively) and multivariate analyses (p = 0.028 and p = 0.006, respectively) in this group, although only a small number of cases showing a mutation were available (data not shown). Mutations in CD79A were found to be enriched in ref/rel patients (33.3% vs. 3.7% in CR patients, p = 0.0995) in the younger DLBCL cohort (Figure 2B, Supporting Information S1: Table S5), however, no significant impact on EFS, PFS or OS was observed.
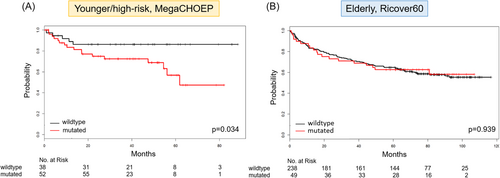
Of interest, the presence of mutations in CREBBP was not correlated with inferior outcome in the entire cohort (EFS: p = 0.719; PFS: p = 0.998; OS: p = 0.939), nor in rituximab-treated elderly patients (EFS: p = 0.991; PFS: 0.510; OS: p = 0.491). However, OS was significantly shorter in the younger patient cohort with CREBBP mutations in univariate (p = 0.034) and multivariate analyses (p = 0.050, Figure 3A,B). No difference was observed concerning the frequency of CREBBP mutations in CR and ref/rel patients in either cohort.
Mutational profiles and treatment response of younger and elderly male and female patients
Gender differences in the frequency and the pattern of somatic mutations are known to occur and to possibly impact treatment response in lymphoma patients.45, 46 We therefore also analyzed the landscape of biomarker expression and mutations taking into account the gender of the patients. When treatment responses in cohorts of both younger and elderly patients were stratified according to gender, no differences were noted. The distribution of biomarkers and mutations in younger female and male patients failed to show any significant differences. In contrast, a significantly higher number of GNA13 mutations was detected in female patients in the elderly patient cohort (19.5% vs. 8.8%) in comparison with male patients, p = 0.031) In addition, tumors from female patients showed higher BCL2 expression frequencies (75.9% vs. 65.2% in male patients, p = 0.021; data not shown).
DISCUSSION
In spite of the continuing evolution in the molecular subtyping of patients with DLBCL and encouraging data toward optimization of risk-adapted therapeutic inventions,12, 13 the proportion of DLBCL patients failing first- and later-line therapies remains high (70%–80%).1 Although age influences the survival of patients diagnosed with lymphoma and is represented in all International Prognostic Indices (IPI),2, 47 biological differences between younger and elderly patients have only rarely been investigated. Moreover, important gender differences have been reported for efficacy and toxicity in many solid tumors and hematologic neoplasms. Intriguingly, more than 50% of the genes targeted by FDA-approved cancer drugs show sex-biased molecular signatures, including gender differences in somatic mutations.45, 46 However, most treatment strategies and the investigation of underlying molecular features do not take into account differences in gender and age. Previously published data from our group suggested that DLBCL with immunoblastic morphology – an adverse risk factor - more frequently occur in elderly patients.48 Klapper et al. reported an enrichment of the activated B-cell (ABC)-like DLBCL subtype as well as increased genetic complexity in patients with higher age at diagnosis.49 While BCL2 expression is associated with higher age at diagnosis,49 BCL2 translocations emerged as a major risk factor in younger DLBCL patients.29 Numerous studies have been published correlating known risk factors like COO, MYC translocations, MYC-BCL2 DH or MYC-BCL2 DE with treatment response in patients with ref/rel DLBCL. Results differ considerably, however. While COO was of prognostic impact in the CORAL study for ref/rel DLBCL19, 28 as well as in high-risk DLBCL patients treated with autologous stem cell transplantation consolidating first-line therapy,20 an association of COO with EFS, PFS or OS was not observed in other studies.15, 21-23 The same finding was evident for MYC translocations, DH or DE status, being significantly associated with lower overall response rates (ORR) and CR rates in some,19, 25, 26, 28 but not in other studies.24, 27 In spite of large data sets, a systematic and comprehensive analysis comprising all above-mentioned biomarkers, has not been performed yet. The herein presented evaluation of established and novel risk factors in younger and elderly DLBCL cohorts with respect to treatment response enables a more detailed insight into lymphoma age- and gender-associated disease biology. In the present study, the risk profiles associated with DLBCL recurrence or relapse in younger patients was significantly different from that of elderly patients. BCL2 translocations were more frequently observed in younger ref/rel patients. The BCL2 translocation occurs as an early initiating event promoting tumor development via driving anti-apoptotic mechanisms. Assuming that BCL2 translocation is one of the driving events in both younger and elderly patients, it could be argued that secondary genetic hits might differ in these patient groups, allowing a more tumor-supporting climate in the younger patients developing overt lymphomas earlier than elderly patients. A comparison of the mutational profile of BCL2 translocation-positive DLBCL in younger and elderly patients, however, did not reveal any significant differences on the basis of the 18 genes analyzed. Since only a small number of genes was investigated a validation of this hypothesis needs to be further elucidated.While BCL2 translocations were significantly associated with ref/rel DLBCL in younger patients exclusively, an increased BCL2 expression more frequently occured in ref/rel patients in both younger and elderly patients, as well as DE of BCL2 and MYC protein. This is well in line with a recent report from Prochatzka and colleagues who reported that DE status correlates with an inferior treatment response to first-line immunochemotherapy characterized by lower disease control rate.25 Besides established biomarkers obviously influencing the treatment response of patients suffering from DLBCL, transporter proteins of the ABC transporter family have been identified to confer drug resistance in lymphoma (reviewed in Wang et al.50). Doxorubicin and vincristine, components of the multi-agent regimen R-CHOP, are substrates for the ABC transporter proteins ABCC1 and ABCC2.51, 52 In patients with DLBCL, high expression of ABCC1 and ABCC2 was previously shown to correlate with inferior clinical outcome.53-55 Within the present study, the number of tumors with high expression of ABCC1 and ABCC2 did not differ in ref/rel and CR patients. Intriguingly, however, DLBCL from younger and elderly patients had different ABC expression patterns with significantly increased numbers of tumors expressing ABCC1 and ABCC2 in the cohort of younger DLBCL.
Molecular biomarkers indicating high risk of failure in patients suffering from DLBCL are of critical importance in choosing the appropriate therapeutic management. Although single markers might be able to provide meaningful information for robust risk stratification, it might be helpful to consider various combinations of established risk factors including the mutational status of selected genes.9-11 In our study, the occurrence of adverse risk factors was more frequently seen in ref/rel patients. However, a significant proportion of CR patients were also shown to harbor one or more adverse factors. Only a minority of patients achieving CR were not affected by any inferior event. These findings emphasize the challenge of molecular stratification and indicate the necessity also to more precisely define the interplay of individual biological risk factors. An impressive example of molecular complexity within single risk factors in DLBCL was recently reported in the context of MYC translocations.56 Zhang and colleagues demonstrated that the basically known wide spectrum of underlying fusion constellations in MYC translocations is associated with differential MYC protein expression and clinical impact, directly guiding to an adapted routine diagnostic work-up and perspectively also to therapeutic implications.
Until now, only few data are available concerning mutation patterns in relation with patients' age.9, 10 Thus, the significantly differing mutation patterns in younger and elderly patients is remarkable, as well as the differences in clinical outcome associated with the mutational landscape. The higher frequencies of TNFAIP3, EP300 and CREBBP gene mutations in the cohort of younger patients was unexpected, in particular considering the age evolution model as postulated by Klapper and colleagues, indicating an increase of genomic aberrations with higher age although the mutational profiles, were not investigated.49 It is well-known that normal B-lymphocytes are among those cells loaded with the highest number of mutations per cell.57 Moreover, B cells from younger individuals show a trend toward an increased proliferation rate,58 which might possibly explain the finding of enhanced mutation rates in DLBCL from younger patients. One caveat in our study was a selection bias in the DLBCL cohorts investigated: apart from age, the two trial cohorts significantly differed in their inclusion criteria: while intermediate-risk elderly patients were recruited into the RiCOVER60-trial, the MegaCHOEP trial was designed to address younger high-risk patients. It thus cannot be entirely excluded that mutational differences might possibly also emerge as a result of the underlying risk group. A similar phenomenon has already been described in follicular lymphoma (FL), indicating that high-risk FL more frequently harbor somatic hypermutations.59 However, more recently it has been shown that DLBCL patients with high tumor mutational burden (TMB) did not differ in clinical features (e.g. age, IPI category) when compared to patients with low TMB.60
Intriguingly, inactivating mutations in the histone acetyltransferase CREBBP were significantly associated with inferior OS exclusively in the cohort of younger patients, while no difference was observed in the frequency of CREBBP alterations in CR and ref/rel patients underscoring the pivotal importance of a thorough work-up of individual risk factors, as mentioned before. A prognostic impact of CREBBP gene mutations had already been reported within patients of the SAKK 38/07 trial.16 These patients had an average age of 58.5 years intermediate between the average age of 48 years for MegaCHOEP patients35 and that of the patients within the Ricover60-trial (68 years).30 Although it is not surprising that the genetic composition of DLBCL varies with age,29, 49 the striking difference in the prognostic relevance of CREBBP mutations was unexpected. A critical role for CREBBP mutations and their adverse impact on clinical outcome had already been reported in younger patients with hematological malignancies,61 but also within solid tumors.62
In line with enhanced mutation frequencies of BCL2 and TNFRSF14 in elderly patients with ref/rel DLBCL, both gene mutations predicted inferior PFS in the cohort of elderly patients with DLBCL in univariate and multivariate analysis. However, the predictive effect was no longer present when patients treated without rituximab were excluded. Whether these genes might be candidates of interest in the risk stratification aiming at patients with early relapses needs to be confirmed in larger R-CHOP-like treated cohorts.
Strikingly, in the cohort of elderly patients, mutations in the GNA13 gene were significantly enriched in female DLBCL patients. It has recently been reported that GNA13 mutated DLBCL express high levels of BCL2, since GNA13-deficiency negatively regulates BCL2 expression.63 This is well in line with our findings that tumors from female patients also show significantly enhanced expression of BCL2 protein. Although the tumor suppressor gene GNA13 is widely considered as not druggable, BCL2 inhibitors have been found to be effective in GNA13-deficient cells.63 Given the promising results of adding venetoclax to R-CHOP in DLBCL patients with BCL2 overexpression,64 it might be of interest to obtain further results regarding GNA13 and BCL2 testing particularly in female patients.
In conclusion, prognostic biomarkers occur in both CR and ref/rel patients with DLBCL and show overlapping features but also significant differences, both in younger and elderly patients. While the number of mutations per sample was associated with clinical outcome only in elderly patients, younger patients showed significantly more mutations. Intriguingly, mutations in the CREBBP gene predict overall survival in the younger cohort exclusively. Furthermore, female patients have higher numbers of GNA13 mutations that possibly might guide to an enhanced BCL2 expression. Whether such patients might benefit from additional treatment with venetoclax needs to be further evaluated.
ACKNOWLEDGMENTS
We would like to thank Katja Bräutigam (Stuttgart), Petra Hitschke (Stuttgart) Hannah-Lena Armbruster (Stuttgart), Emma Attwood (Stuttgart) and Theodora Nedeva (Würzburg) for excellent technical assistance. This work was supported by the Robert Bosch Stiftung (project O3), Stuttgart, Germany.
AUTHOR CONTRIBUTIONS
Sophia Steinlein was responsible for collection and interpretation of the data. She contributed to writing of the manuscript. Markus Kreuz, Marita Ziepert, Markus Loeffler, and Heike Horn performed data analysis and contributed to manuscript writing. Annette M. Staiger and Julia Richter collected and assembled data. Thomas F. E. Barth, Heinz-Wolfram Bernd, Alfred C. Feller, Sylvia Hartmann, Wolfram Klapper, Harald Stein, Sylvia Hartmann, Martin-Leo Hansmann, Peter Möller, Andreas Rosenwald, and German Ott were part of the pathology reference panel and provided study material of the patients. Norbert Schmitz and Lorenz Trümper collected the clinical data of patients and provided study materials of the patients. Katrin S. Kurz, German Ott, and Heike Horn were responsible for the conception and design of the study, collected and interpreted data and wrote the manuscript. All listed authors have contributed to the manuscript substantially and have agreed to the final submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING
Robert Bosch Stiftung : O3.
GERMAN HIGH GRADE NON-HODGKIN LYMPHOMA STUDY GROUP (DSHNHL)/GERMAN LYMPHOMA ALLIANCE (GLA)
Marita Ziepert (Institute for Medical Informatics, Statistics, and Epidemiology, Universität Leipzig, Leipzig, Germany), Markus Kreuz (Institute for Medical Informatics, Statistics, and Epidemiology, Universität Leipzig, Leipzig, Germany and Department of Diagnostics, Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany), Wolfram Klapper (Institute of Pathology, Hematopathology Section and Lymph Node Registry, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel, Germany), Lorenz Trümper (Department of Hematology and Oncology, Georg-August Universität, Göttingen, Germany), Markus Loeffler (Institute for Medical Informatics, Statistics, and Epidemiology, Universität Leipzig, Leipzig, Germany), Norbert Schmitz (Department of Medicine A for Hematology, Oncology, and Pneumology, University Hospital Münster, Münster, Germany), Andreas Rosenwald (Institute of Pathology, Universität Würzburg, Würzburg, Germany), and German Ott (Department of Clinical Pathology, Robert-Bosch-Krankenhaus, Stuttgart, Germany).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.