Diagnosis and treatment of AML in the context of WHO and ICC 2022 classifications: Divergent nomenclature converges on common therapies
Abstract
As a consequence of rapidly developing genetic technologies and advances in the understanding of the pathogenesis of acute myeloid leukemia (AML), the classification of AML has moved gradually from a morphologic and cytochemical-based system to one that is genetically defined. Recent molecular and genetic developments have been integrated into the diagnostic criteria for AML in the fifth edition of the World Health Organization (WHO) Classification of Haematolymphoid Tumours and the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemias, expanding the list of genetically defined entities. In this review article, we use a case-based format describing the diagnostic workup, risk stratification, and possible treatment options to highlight the impact of the 2022 WHO and ICC classifications on clinical practice. We show that despite much commentary and anguish, there is a significant overlap between the two classifications. We further highlight the fact that even for entities with divergent nomenclature, such as TP53-mutated AML, the actual genetic lesion leads to convergent therapy.
INTRODUCTION
Acute myeloid leukemia (AML) is increasingly recognized as a spectrum of heterogeneous malignancies with similar presentation.1 Major advances in the understanding and management of AML have led to the continual evolution of classification and treatment guidelines.2 The updated fifth edition of the World Health Organization (WHO) Classification of Haematolymphoid Tumours and the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemias, both published in 2022, have expanded the list of genetically defined entities for the diagnosis of AML while reducing entities defined by morphology alone.3, 4 Understandably, much has been said about the confusion created by two competing classifications.5 Nonetheless, a careful review of the two classifications reveals many similarities, including an appropriately increased emphasis on genetically rather than morphologically defined entities. Furthermore, both classifications revisit the blast threshold for a diagnosis of AML and recognize that a rigid cutoff of 20% blasts may not be the most suitable approach for the diagnosis of genetically defined entities. However, the two classifications end up with different resolutions. With some exceptions, the ICC 2022 reduces the blast threshold to ≥10% if a recurring cytogenetic arrangement is present. Whereas the WHO 2022 classification does not provide a specific threshold for blast count and instead recommends, “…correlation between morphologic findings and the molecular genetic studies to ensure that the defining abnormality is driving the disease pathology.” A comparison of the WHO 2016, the updated WHO 2022, and the ICC 2022 AML classification is shown in Table 1.
| WHO 20166 | WHO 20224 | ICC 20223 | |
|---|---|---|---|
| 20% blast threshold | 20% PB/BM blasts define AML | ≥20% blasts define AML for the following only: CEBPAmut; BCR::ABL1, AML-MR | ≥20% blasts for AML with BCR::ABL1 ≥10% for other subtypes (AML or MDS/AML) |
| Recurring cytogenetic abnormalities | Distinct entity | Distinct entity (expanded list) |
Distinct entity (expanded list) |
| Therapy-related myeloid neoplasms (including t-AML) | Distinct entity | Diagnostic qualifier (diagnostic workup required) |
Diagnostic qualifier (diagnostic workup required) |
| AML with multilineage dysplasia | Criterion for AML-MRC Requires exclusion of recurrent genetic abnormalities, NPM1mut and biCEBPA Presence of an MDS-related cytogenetic abnormality with exception of del(9q) |
Not included | Not included |
| History of MDS or MDS/MPN | Criterion for AML-MRC Requires exclusion of recurrent genetic abnormalities, NPM1mut and biCEBPA |
Criterion for AML-MR | Diagnostic qualifier (diagnostic workup required) |
| Complex karyotype | ≥3 cytogenetic abnormalities Sufficient to diagnose AML with myelodysplasia-related changes when ≥20% PB or BM blasts are present and prior therapy has been excluded |
≥3 cytogenetic abnormalities For defining AML, myelodysplasia-related |
≥3 unrelated clonal chromosomal abnormalities in the absence of other class-defining recurring genetic abnormalities AML with myelodysplasia-related cytogenetic abnormalities 10%–19% (MDS/AML) and ≥20% (AML) |
| Secondary AML-type genetic mutations | Not included | Criterion for AML-MR 8 genes: ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2 |
Criterion for AML with myelodysplasia-related gene mutations 9 genes: ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2 Requires exclusion of recurrent genetic abnormalities (including NPM1mut, in frame CEBPAmut), and TP53mut |
| Myelodysplasia-related cytogenetic abnormalities | Criterion for AML-MRC Requires exclusion of recurrent genetic abnormalities NPM1mut and biCEBPA Presence of an MDS-related cytogenetic abnormality with exception of del(9q) |
Criterion for AML-MR | Criterion for AML with myelodysplasia-related cytogenetic abnormalities Requires exclusion of recurrent genetic abnormalities (including NPM1mut, in frame CEBPAmut), and TP53mut and myelodysplasia-related gene mutations |
| AML with TP53 mutation | No distinct entity | No distinct entity | Distinct entity (VAF >10%) |
- Abbreviations: AML, acute myeloid leukemia; AML-MR/MRC, AML with myelodysplasia-related/myelodysplasia-related changes; BM, bone marrow; ICC, International Consensus Classification; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PB, peripheral blood; t-AML, therapy-related AML; VAF, variant allele frequency; WHO, World Health Organization.
Irrespective of classification, the goal of AML treatment is to induce complete remission (CR) with initial therapy, followed by consolidation and/or maintenance therapy. The choice of therapy is determined by a number of factors, including the patient's risk stratification profile and performance status. Mirroring the evolving classifications, risk stratification is also increasingly based on genetic analyses, including next-generation sequencing for a panel of mutations in addition to chromosomal rearrangements by karyotyping malignant cells in metaphase.2, 7 The results of genetic analyses should ideally be rapidly available (3–5 days), and a short delay in starting definitive treatment is recommended to identify the best treatment options.2 The European LeukemiaNet (ELN) guidelines, which provide the framework for diagnostic workup, risk stratification, and management of adult patients with AML, were also revised in 2022.2 The revised ELN risk classification criteria are shown in Table 2. A notable change is the absence of the FLT3-ITD allelic ratio from the risk classification. Rather, patients with FLT3-ITD-mutated AML are considered in the intermediate-risk group, irrespective of their allelic ratio or presence of NPM1 mutation. This change is due in part to the routine addition of novel drugs that inhibit FLT3 tyrosine kinase activity in frontline treatment. It is important to note that the ELN risk classification was developed based on data from intensively treated patients. A recent analysis of the VIALE-A study data set revealed that the ELN 2022 risk stratification does not have the same prognostic impact.8
| Risk category | Genetic abnormality |
|---|---|
| Favorable |
|
| Intermediate |
|
| Adverse |
|
- a Frequencies, response rates, and outcome measures should be reported by risk category, and, if sufficient numbers are available, by specific genetic lesions indicated.
- b Mainly based on results observed in intensively treated patients. Initial risk assignment may change during the treatment course based on the results from analyses of measurable residual disease.
- c Concurrent KIT and/or FLT3 gene mutation does not alter risk categorization.
- d AML with NPM1 mutation and adverse-risk cytogenetic abnormalities are categorized as adverse-risk.
- e Only in-frame mutations affecting the basic leucine zipper (bZIP) region of CEBPA, irrespective of whether they occur as monoallelic or biallelic mutations, have been associated with favorable outcome.
- f The presence of t(9;11)(p21.3;q23.3) takes precedence over rare, concurrent adverse risk gene mutations.
- g Excluding KMT2A partial tandem duplication (PTD).
- h Complex karyotype: ≥3 unrelated chromosome abnormalities in the absence of other class-defining recurring genetic abnormalities; excludes hyperdiploid karyotypes with three or more trisomies (or polysomies) without structural abnormalities.
- i Monosomal karyotype: presence of two or more distinct monosomies (excluding loss of X or Y), or one single autosomal monosomy in combination with at least one structural chromosome abnormality (excluding core-binding factor AML).
- j For the time being, these markers should not be used as an adverse prognostic marker if they co-occur with favorable-risk AML subtypes.
- k TP53 mutation at a variant allele fraction of at least 10%, irrespective of the TP53 allelic status (mono- or biallelic mutation); TP53 mutations are significantly associated with AML with complex and monosomal karyotype. Source: Reprinted from Blood, Vol 140/12, Hartmut Döhner, Andrew H. Wei, Frederick R. Appelbaum, Charles Craddock, Courtney D. DiNardo, Hervé Dombret, Benjamin L. Ebert, Pierre Fenaux, Lucy A. Godley, Robert P. Hasserjian, Richard A. Larson, Ross L. Levine, Yasushi Miyazaki, Dietger Niederwieser, Gert Ossenkoppele, Christoph Röllig, Jorge Sierra, Eytan M. Stein, Martin S. Tallman, Hwei-Fang Tien, Jianxiang Wang, Agnieszka Wierzbowska, Bob Löwenberg, Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN, Page 1359, Copyright 2022, with permission from Elsevier.
In this article, we explore the impact of the WHO and ICC 2022 classifications on clinical practice using illustrative case studies. We describe the diagnostic workup and possible treatment options for each patient as per 2022 ELN guidance and also reflect on recent updates versus past iterations. As there are no generally accepted or validated criteria to consider a patient ineligible for intensive chemotherapy per the ELN 2022 guidelines, all case studies presented here are in patients assumed fit for intensive chemotherapy. Thus, postremission treatment options may include allogeneic transplantation in addition to other consolidation or maintenance therapies.
PATIENT CASE STUDY ONE
A 69-year-old female presented with anemia, thrombocytopenia, and abnormal cells in the peripheral blood (comprising 74% of the total). Her Eastern Cooperative Oncology Group performance status was 1. Her white blood cell (WBC) count was 25,000/μL, with a hemoglobin (Hgb) level of 9.0 g/dL and platelet (Plt) count of 82,000/μL. Analysis of peripheral blood smears showed the presence of large cells with folded nuclei, delicate chromatin, and prominent nucleoli; these were identified as promonocytes (blast equivalents; Figure 1A). Histological analysis of a bone marrow trephine biopsy showed hypercellularity, with no fat cells. Many large cells with folded nuclei, fine chromatin, and prominent nucleoli were present, and there was no residual, normal hematopoiesis (Figure 1B). Focal areas showed dysplastic megakaryocytes and cells with morphology suggestive of monocytic differentiation. Bone marrow aspirate showed sheets of blasts with folded nuclei and no residual hematopoiesis (Figure 1C). Both the core biopsy and bone marrow aspirate showed 70%–80% blasts. Flow cytometry data indicated an immunophenotype suggestive of monocyte differentiation (intermediate CD45/side scatter, CD34 minimal positivity, CD33+, CD117 variable, CD36+, CD64 variable, CD4+, and CD123 dim). Immunohistochemistry showed cytoplasmic NPM1 expression, suggestive of an underlying NPM1 mutation (Figure 1D). Cytogenetics showed a normal female karyotype (46 XX in all 30 metaphase cells). Genetic analyses identified pathogenic variants in NPM1 c.860-863dup, p.W288Cfs*12 (variant allele frequency [VAF] 42%), IDH1 c.394C>T, p.R132C. (VAF 23%), and DNMT3A c.1792C>T, p.R598* (VAF 41%).
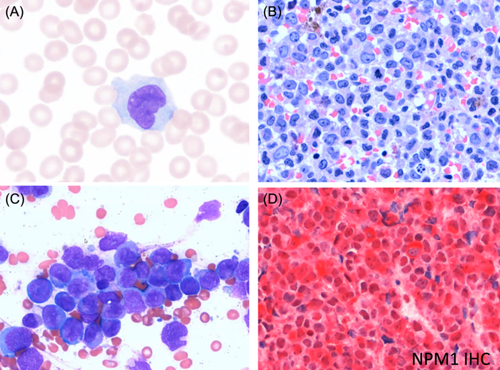
The patient's diagnosis was AML with mutated NPM1, according to the ICC 2022 classification, and AML with NPM1 mutation using either the WHO 2016 or the WHO 2022 classification—a mere difference in syntax. This diagnosis is considered a favorable risk per ELN 2022 guidelines, with no changes to risk stratification from ELN 2017 guidelines.9 There is a high rate of CR achieved with intensive chemotherapy, which may incorporate gemtuzumab ozogamicin (GO), and these remissions are often durable.2, 10 A lower incidence of relapse with fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-Ida) plus GO demonstrates the anti-leukemic efficacy of this treatment in patients with NPM1-mutated AML, suggesting that incorporating FLAG-Ida plus GO into treatment regimens could potentially reduce the need for salvage therapy in these patients.10, 11
As the patient was eligible for intensive therapy, treatment options from ELN 2022 consist of induction therapy with daunorubicin 60 mg/m2, idarubicin 12 mg/m2, or mitoxantrone 12 mg/m2 (all IV on Days 1–3), plus cytarabine 100–200 mg/m2/d continuous IV (CIV) on Days 1–7 (7 + 3 regimen). For patients with no response to 7 + 3, re-induction with 7 + 3 (or 5 + 2) or a regimen containing higher doses of cytarabine is recommended, preferably the latter. Older patients (aged ≥75 years) who are unable to tolerate a second course with anthracycline may benefit from azacitidine plus venetoclax at this point based on the results of the VIALE-A trial in previously untreated older adults.12 Alternatives to azacitidine include decitabine or low-dose cytarabine, each given with venetoclax. After achievement of a CR, consolidation therapy may be appropriate, consisting of 3–4 cycles of intermediate-dose cytarabine (IDAC) 1000–1500 mg/m2 IV (500–1000 mg/m2 if ≥60 years old) over 3 h every 12 h on Days 1–3. The recommended maintenance therapy consists of oral azacitidine (CC-486) 300 mg daily on Days 1–14 every 4 weeks until disease progression. Based on current evidence, the choice of postremission therapy should be guided by MRD, particularly with regard to decisions concerning transplant. Prospective data from the UK NCRI AML17 and AML19 trials have shown that molecular MRD postinduction chemotherapy may identify patients with NPM1-mutated AML and who may benefit from transplant in first remission. Overall survival (OS) outcomes varied significantly by MRD status; MRD-positive patients showed an OS benefit following transplant in first remission (3-year OS with vs. without transplant: 61% vs. 24%; HR: 0.39, 95% CI: 0.24–0.64, p < 0.001), while MRD-negative patients experienced no benefit (3-year OS with vs. without transplant: 79% vs. 82%; HR: 0.82, 95% CI: 0.50–1.33, p = 0.4).13
If this patient experiences a relapse of AML that still demonstrates the mutation in IDH1, then treatment with ivosidenib or olutasidenib, either alone or in combination with other agents, should be considered.14 Additionally, low-intensity chemotherapy combined with venetoclax has shown potential as an effective treatment for relapse in NPM1-mutated AML, either as a bridge to transplant or as a stand-alone treatment.15 Menin inhibitors also show promise as a treatment for AML, especially in subtypes with genetic abnormalities such as NPM1 mutations, with early clinical trial data indicating favorable response rates and safety in heavily pretreated patients. However, further studies are needed to confirm efficacy, identify optimal patient subgroups, and determine the best timing and dosage.16
PATIENT CASE STUDY TWO
A 66-year-old male originally presented with myelodysplastic syndrome (MDS; 8% blasts in bone marrow) 6 months earlier. The patient received four cycles of decitabine and had a partial response. He subsequently presented with blasts in the peripheral blood (3%). His WBC count was 2500/μL, with an Hgb level of 8.0 g/dL, mean corpuscular volume (MCV) of 107 fL, and a Plt count of 57,000/μL. The patient's peripheral blood smear showed macrocytic red cells, basophilic stippling, and the presence of dysplastic neutrophils with nuclear abnormalities (hypolobulation and hyperlobation) (Figure 2A). Bone marrow biopsy showed increased blasts (approximately 20%), evidence of reduced granulocytic maturation, and the presence of megaloblastoid erythroid precursors and dysplastic megakaryocytes (Figure 2B). Findings from the bone marrow aspirate were similar, showing increased blasts (22%), and the presence of dysplastic erythroid precursors and megakaryocytes (Figure 2C). Immunohistochemistry showed TP53 protein expression (Figure 2D). Cytogenetic analysis identified a sole deletion in chromosome 5 [46,XY,del(5)(q13q35)]. This was also observed in the majority of cells by fluorescence in situ hybridization (FISH; chromosome 5 deletion-positive 2G1R, 80%). Genetic analyses indicated a mutation in TP53 (c.537T>G, p.H179Q; VAF 58%).
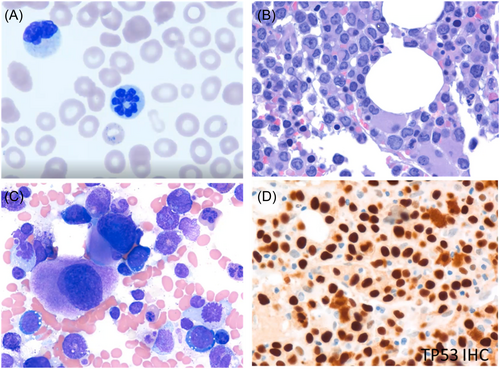
Based on ICC 2022, this patient's diagnosis was AML with mutated TP53 progressing from MDS, whereas, using WHO criteria, the diagnosis would be AML, myelodysplasia-related (AML-MR). While this highlights a difference between systems, irrespective of the nomenclature, the risk stratification is adverse according to ELN 2022 guidelines, unchanged from the ELN 2017 guidelines.2, 9
Furthermore, irrespective of the nomenclature, the treatment strategy suggested for this patient would be induction therapy with CPX-351, that is, liposomal daunorubicin and cytarabine, 100 U/m2 (daunorubicin 44 mg/cytarabine 100 mg) IV on Days 1, 3, and 5. For a second induction course (if not in CR/CR with hematological recovery [CRh], or CR with incomplete hematological recovery [CRi]), CPX-351 100 U/m2 IV is given on Days 1 and 3 only. The recommended consolidation regimen is 1–2 cycles of CPX-351 65 U/m2 (daunorubicin 29 mg/cytarabine 65 mg) IV on Days 1 and 3. It should be noted that ELN 2022 treatment recommendations for CPX-351 refer to the previous disease nomenclature (AML-MRC, therapy-related AML [t-AML]). Additionally, there is generally a poor response and short OS with CPX-351 in TP53-mutated AML.17 However, the presence of a TP53 mutation alone should not be a reason to avoid CPX-351 treatment, as disease progression will be worse without chemotherapy.18 An alternative, lower intensity regimen to consider would be azacitidine plus venetoclax for patients who are ineligible for intensive chemotherapy, as given in the VIALE-A trial. It should be noted that prior HMA, as given to this patient, was an exclusion criterion for VIALE-A, and an OS benefit with azacitidine plus venetoclax was not observed in TP53 patients in a subgroup analysis.12 However, there was a significant improvement in the incidence of composite remission compared to azacitidine plus placebo, making this a reasonable choice.12
For patients with AML with mutated TP53 progressing from MDS, myeloablative conditioning followed by early allogeneic transplantation while in first CR is commonly performed. Investigational therapies with anti-CD47 monoclonal antibodies may be a potential option in the future. While magrolimab in combination with azacitidine with or without venetoclax showed initial promise in phase 1b/2 trials,19, 20 subsequent randomized phase 3 trials (ENHANCE, ENHANCE-2, and ENHANCE-3) were prematurely terminated and further development of magrolimab in hematologic cancers is not being pursed. Other CD47-targeting antibodies sparing red blood cells continue development, with maplirpacept being furthest along.21, 22
Of note, another area of discordance between the ICC and WHO 2022 classifications is the classification of patients with TP53-mutated MDS and blast counts >10% but less than 20%. In the ICC 2022, these patients would get classified as MDS/AML with mutated TP53 irrespective of the number of TP53 mutations, provided the TP53 mutation is present at a VAF of >10%. However, per WHO 2022, classification of these patients is dependent on the number of TP53 mutations: patients with multi-hit TP53 mutations would be classified as MDS with biallelic TP53 inactivation (MDS-biTP53). This discordance is reflective of real-world differences in the approach to treating these myeloid malignancies with complex pathologies. Furthermore, it should be noted that the WHO acknowledges that MDS-IB2 may be regarded as AML-equivalent for therapy as well as clinical trial design when appropriate. Therefore, once again, irrespective of the nomenclature, it is likely that the therapeutic decisions would converge on very comparable therapies.
PATIENT CASE STUDY THREE
A 65-year-old male was treated for Hodgkin lymphoma 5 years ago and presented with pancytopenia and 50% peripheral blasts. His WBC count was 3000/μL with an Hgb level of 9.0 g/dL, MCV of 104 fL, and Plt count of 12,000/μL. Histologic assessment of bone marrow biopsy showed a blast count of >20%, with reduced granulocytic maturation and presence of megaloblastoid erythroid precursors and dysplastic megakaryocytes (Figure 3A). Cytogenetic analysis revealed a complex karyotype (Figure 3B). Next-generation sequencing molecular studies identified no pathogenic variants.
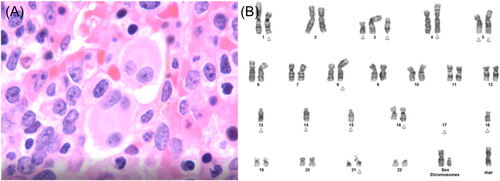
This patient was diagnosed with AML with myelodysplasia-related cytogenetic abnormality, therapy-related, according to ICC 2022 classification criteria. Using WHO 2022 criteria, the diagnosis would be AML, myelodysplasia-related, postcytotoxic therapy (postchemotherapy). Based on the WHO 2016 classification, the patient's diagnosis would have been t-AML. Due to the complex karyotype, this diagnosis is classified as adverse risk, per ELN 2022 guidelines (no change from ELN 2017 guidelines).2, 9
As in the previous case with adverse risk AML due to the TP53 mutation, ELN 2022 guidelines recommend induction therapy for t-AML with CPX-351. Because of prior chemoradiotherapy for Hodgkin lymphoma, treatment may be complicated by poor tolerance due to organ toxicity and slow recovery of normal hematopoiesis.
It is noteworthy that ELN 2022 treatment recommendations for CPX-351 use previous disease nomenclature (AML-MRC and t-AML). Furthermore, the indications in the approved label for CPX-351 no longer match the descriptors used by the WHO or the ICC. The registrational trial for CPX-351 did not include patients with AML-MRC based on morphology alone or genetics.23 Instead, patients were required to have a pathologic diagnosis of AML according to the WHO 2008 criteria (≥20% blasts in peripheral blood or bone marrow)24 including t-AML or AML-MRC (history of MDS [with and without prior hypomethylating agents], chronic myelomonocytic leukemia, or de novo AML with MDS-related cytogenetic abnormalities).
PATIENT CASE STUDY FOUR
A 59-year-old male with no medically significant prior history was evaluated for weakness and malaise. His WBC count was 3800/μL, with Hgb level of 9.0 g/dL, MCV of 100 fL, and Plt count of 60,000/μL. Differential blood count showed 8% blasts, 7% myelocytes, 36% segmented neutrophils, and 3% monocytes. Histological assessment of bone marrow aspirate smears showed that most blasts had a distinctive morphology, including the presence of Auer rods (Figure 4A,B). Immunophenotyping indicated dim CD45 expression with intermediate side scatter, positivity for precursor cell markers CD34 and CD117, and a CD33+/CD13− profile, reminiscent of granulocytic maturation. Samples were CD15 negative and showed aberrant expression of CD56 and CD19. Cytogenetic analysis revealed a 46,X,-Y,+8,t(8;21)(q21.3;q22.1) karyotype with a RUNX1::RUNX1T1 fusion (Figure 4C). This fusion leads to the disruption of the normal function of the core-binding factor, namely, its role in hematopoietic differentiation and maturation.25 Genetic analyses indicated the presence of a KIT mutation (c.2447A>T, p.D816V; VAF 10%). Although this patient would be considered to have a complex karyotype by both criteria, the presence of additional cytogenetic abnormalities has not been shown to impact clinical outcomes in t(8;21) AML.26
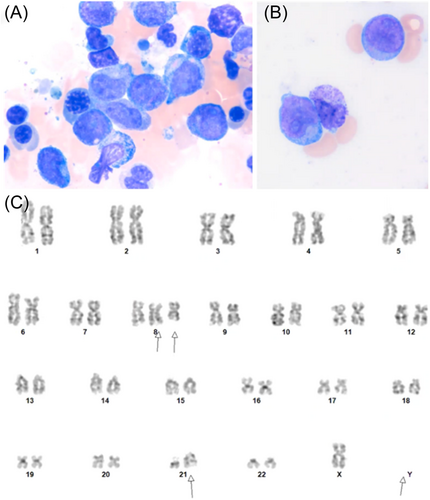
Based on ICC 2022 classification criteria, the patient's diagnosis was AML with t(8,21)(q22;q22.1)/RUNX1::RUNX1T1; however, using WHO 2022 criteria, the diagnosis would be AML with RUNX1::RUNX1T1 fusion (as in the WHO 2016 iteration). This diagnosis is considered to be of favorable risk per ELN 2022 guidelines (no change from ELN 2017 guidelines).2, 9
Based on ELN 2022 guidelines, the recommended induction therapy for this patient is daunorubicin 60 mg/m2 IV on Days 1–3 and cytarabine 100–200 mg/m2/d CIV on Days 1–7; plus GO 3 mg/m2 (maximum dose 5 mg) IV on Days 1, 4, and 7. GO is also widely administered on Day 1 of induction only, based on evidence that there is no difference in outcomes according to the number of GO doses, and patients treated with one dose of GO along with the standard of care had 3-year OS of 96%.11 If not in CR/CRh/CRi, the re-induction regimen is often daunorubicin 60 mg/m2 IV on Days 1–2 and cytarabine 1000 mg/m2 IV (500–1000 mg/m2 if ≥60 years old) over 3 h every 12 h on Days 1–3 without GO. An alternative regimen would be high-dose cytarabine alone (i.e., without an anthracycline). Consolidation with 2–4 cycles of IDAC 1000–1500 mg/m2 IV (500–1000 mg/m2 if ≥60 years old) over 3 h every 12 h on Days 1–3 is recommended; GO 3 mg/m2 may be added on Day 1 (up to 2 cycles). Clinicians may consider omitting GO if allogeneic hematopoietic cell transplantation is planned within 3 months to reduce the risk of veno-occlusive disease. In general, patients with a core-binding factor AML, such as those with t(8;21) or inv(16), are not recommended to undergo allogeneic transplantation in first remission because they have a favorable outcome following intensive higher dose cytarabine consolidation. However, disease relapse remains the most important single cause of treatment failure, occurring in up to 35% of patients, and monitoring of minimal residual disease is an important diagnostic tool that permits the assessment of response to therapy and can detect early relapse during remission and guide therapeutic decisions.27
SUMMARY AND CONCLUSION
Expert hematopathologists, laboratory scientists, cytogeneticists, and clinicians are using rapidly developing genetic technologies to identify biologically homogeneous subsets of AML and to better predict clinical course and treatment response. There are now two classification systems using slightly different terminology and thresholds to describe the heterogeneous syndrome of AML. Fortunately, both are increasingly anchored by objective measurements of recurring gene mutations and chromosomal rearrangements. These genetic features, rather than clinical history or percentages of blasts, are the real determinants of response to treatment and ultimate outcomes. Both classifications have expanded the category of AML with genetic abnormalities and recognize that a minimum blast count of 20% may not be required to support a diagnosis of AML depending on the genetic abnormalities. Even though WHO has retained the diagnosis of MDS-IB2 (increased blasts 2), there is agreement and precedent that some patients with MDS-IB2 should be treated with AML-like therapies based on the clinical context.
Eligibility for clinical trials depends upon a validated and uniformly applied classification of subtypes of disease. Many AML clinical trials have included patients with high-risk MDS (10%–19% marrow blasts) and their outcomes appear equivalent to those who have >20% blasts.28 Both of the new classification systems emphasize the integration of clinical, molecular/genetic, morphologic, and immunophenotypic parameters to facilitate precision diagnosis and risk stratification of AML cases. This integration can then guide treatment decisions and provide access for patients who may qualify for novel first-line treatment options rather than conventional chemotherapy.
ACKNOWLEDGMENTS
Medical writing support, under the direction of the authors, was provided by Paul O'Neill, PhD, and Trina Soluta of CMC Affinity, a division of IPG Health Medical Communications, in accordance with Good Publication Practice (GPP 2022) guidelines.
AUTHOR CONTRIBUTIONS
All authors were involved in study concept and design and have contributed to the case studies and discussions around the manuscript content. All authors critically reviewed the manuscript and approved the final version for submission. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work.
CONFLICT OF INTEREST STATEMENT
Uwe Platzbecker has acted as a consultant or advisor to AbbVie, Takeda, Celgene/BMS, Curis, Jazz Pharmaceuticals, Novartis, and Servier; has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi-Sankyo, FortySeven/Gilead, Novartis, and Rafael Pharmaceuticals; and has received royalties from UpToDate. Richard A. Larson has acted as a consultant or advisor to AbbVie, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, Curis, CVS/Caremark, Epizyme, Immunogen, Jazz Pharmaceuticals, Kling Biotherapeutics, MedPace, MorphoSys, Novartis, and Servier; has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi-Sankyo, FortySeven/Gilead, Novartis, and Rafael Pharmaceuticals; and has received royalties from UpToDate. Sandeep Gurbuxani has served as a consultant to AbbVie; has received honoraria from Jazz Pharmaceuticals; and collects royalties from UpToDate.
ETHICS STATEMENT
Patient consent was not applicable as the case studies were deidentified and anonymized data were used in an illustrative manner.
FUNDING
This article was supported by Jazz Pharmaceuticals.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




