Efficacy of anti-PD1 therapy in extranodal NK/T cell lymphoma: A matched cohort analysis from the LYSA
Extranodal NK/T cell lymphoma (ENKTCL) is a mature T/NK-cell malignancy associated with Epstein Barr Virus.1 Although asparaginase-based treatments have improved outcomes, the prognosis remains poor for relapsed or refractory (R/R) patients. Increased expression of PD-L1at tumor cell surface is a frequent mechanism of immune evasion in ENKTCL.2, 3 Consequently, anti-PD1 (aPD1) therapy, either alone4-9 or combined with chemotherapy,10, 11 has been evaluated in patients with R/R ENKTCL, showing promising results. These initial findings were primarily observed in Asian patients where the prevalence of the disease is higher. Due to the limited data from Western countries and the lack of comparative studies, we assessed the efficacy of aPD1 therapy in a large French cohort of ENKTCL patients and compared it with a historical national cohort of R/R ENKTCL patients treated before the introduction of immunotherapies.
This study included 37 patients from 24 French centers treated with at least one cycle of aPD1 therapy for relapse or progression between March 2017 and March 2022. Among them, 12 patients were enrolled in the prospective AcSé Pembrolizumab study (Unicancer), a phase II, open-label, multicentric study investigating pembrolizumab monotherapy in rare cancers (NCT03012620). The remaining 25 patients were treated with aPD1 alone or combined with chemotherapy or targeted therapy (Supporting Information S1: Table 1), following the recommendations issued by the T-cell lymphomas committee (TENOMIC) of the LYmphoma Study Association (LYSA). These patients were designated as “real-life” patients. The inclusion criteria are detailed in the Supporting Information Methods.
The median age was 52 [19–79], with a sex ratio M/F of 2/1. At diagnosis, 21 patients (57%) presented with disseminated disease, and 15 patients (42%) had a high PINK score. Overall, the clinical characteristics of patients included in the AcSé study were comparable to those of the “real-life” patients. Although not reaching statistical significance, the rate of disseminated disease and high PINK score at diagnosis tended to be higher in the “real-life” group (64% vs. 41.7%, p = 0.35, and 50% vs. 25%, p = 0.22, respectively). At relapse, no significant difference was observed except for LDH serum level, which was higher in the “real-life” cohort (p = 0.023) (Supporting Information S1: Table 2).
All patients had previously received frontline chemotherapy containing asparaginase including MOGAD or MGAD (in accordance with current French guidelines) in 17 (46%) and 12 (32%) patients, respectively, resulting in a 70% complete response (CR) rate after first-line therapy. Prior treatments before aPD1 salvage also included autologous (n = 5) and allogenic (n = 1) stem cell transplants and external radiotherapy (n = 21).
Thirty-six patients were treated with Pembrolizumab (200 and 140 mg every 3 weeks for 31 and 5 patients, respectively), while one patient received Nivolumab (180 mg every 14 days). aPD1 therapy was administered intravenously for a fixed duration of 2 years in the AcSé group, whereas in the “real-life” cohort, the treatment duration was not predetermined. Finally, patients received a median number of 4 [1–22] aPD1 cycles, with 4 [1–15] cycles for those included in the Acsé study and 4 [1–22] cycles for “real-life” patients.
Consistent with the favorable safety profile of immunotherapy, this study reported no treatment discontinuations due to adverse events or treatment-related deaths.9 Notably, no immune-related adverse event was reported in the six patients previously treated with stem cell transplants. The complete toxicity profile is outlined in Supporting Information S1: Table 3.
Median follow-up time was 6.3 months [1–62.4] for the whole cohort and 23.4 [4.5–62.4] months for survivors. The ORR was 46% (n = 17) at the first evaluation and 38% (n = 14) at the last follow-up. At first evaluation, 12 patients were in CR, 5 in partial response (PR), 1 remained stable and 19 did not respond to aPD1 therapy (Supporting Information S1: Figure 1). Among the 25 patients who did not achieve CR at the initial evaluation, 18 (72%) received salvage chemotherapy, containing Gemcitabine in 24% of the cases (n = 6). As aPD1 therapy could be continued in combination with salvage therapy at the clinician's discretion, 10 of the 18 patients (55.5%) received a combination of immunotherapy and chemotherapy (Gemcitabine, Asparaginase, Brentuximab).
Overall, 20 patients were still alive at the last follow-up, including 13 patients in CR and 7 with progressive/stable disease. The 2-year progression-free survival (PFS) and overall survival (OS) of the whole aPD1 cohort were 22.4% [95% CI, 11.9–42.5] and 51% [95% CI, 36.4–71.5], respectively (Figure 1). The median PFS was 6.9 months, and the median OS was not reached. The PFS and OS of patients included in the AcSé study were similar to those of “real-life” patients (p = 0.1 and 0.49, respectively) (Supporting Information S1: Figure 2).
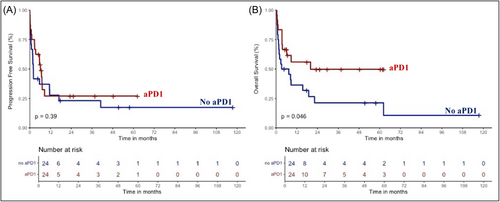
Notably, the overall survival of patients treated with aPD1 tended to be higher when aPD1 was combined with chemotherapy compared to aPD1 monotherapy (p = 0.16) (Supporting Information S1: Figure 3). We also observed that continuing aPD1 therapy in combination with chemotherapy may improve the survival of patients with relapsed or refractory disease already on aPD1 treatment (p = 0.06) (Supporting Information S1: Figure 4).
Regarding prognostic factors, we identified that patients with performance status (PS) ≥2, B symptoms and/or ≥2 extranodal sites involved at the time of aPD1 immunotherapy initiation had significantly worse OS in univariate analysis (Supporting Information S1: Table 4). However, none of them remained significant in multivariate analysis (Supporting Information S1: Figure 5).
To further compare the efficacy of aPD1 to those of other salvage regimens, we analyzed a historical cohort of 38R/R ENKTCL who received at least one cycle of salvage chemotherapy regimen without aPD1 between April 2006 and December 2018. Indeed, since 2019, aPD1 has increasingly been used as the first-line salvage treatment.
Median age of the patients from the historical cohort was 49 [19–82], with 52.6% male. At diagnosis, 58% (n = 22) had disseminated disease, and 42% (n = 15) presented with a high PINK score (Supporting Information S1: Table 5).
All patients in the historical cohort had received frontline chemotherapy with asparaginase prior to relapse; however, only 18% (n = 7) and 13% (n = 5) received MGAD and MOGAD, respectively. This may explain the lower CR rate after first-line therapy (42% in the historical cohort vs. 70% in the aPD1 group, p = 0.001) (Supporting Information S1: Table 5). Salvage therapy primarily consisted of chemotherapy, with Gemcitabine used in 39.5% of cases (n = 15) and Asparaginase re-challenge in 26.3% (n = 10). Details of other salvage regimens are provided in Supporting Information S1: Table 1.
Among the 38 patients in the historical cohort, 13 patients achieved CR or PR (23.7% and 10.5%, respectively), while 25 (65.8%) experienced disease progression following initial salvage therapy (Supporting Information S1: Figure 1).
We then performed a propensity score matching 1:1 using the greedy nearest neighbor method to mitigate differences between cohorts regarding frontline therapy (type of regimen and response). Twenty-four patients with well-balanced confounding factors were ultimately included in each group (Table 1). No significant difference in PFS was observed between aPD1 and historical cohorts (p = 0.39) whereas 2-year OS was significantly improved in patients treated with aPD1 (49.7% vs. 21.2%, p = 0.046) (Figure 1).
| Characteristic | No aPD1 N = 24a | aPD1 N = 24a | p Valueb |
|---|---|---|---|
| Sex = Male (%) | 13 (54%) | 14 (58%) | NS |
| Age ≤ 60 years (%) | 18 (75%) | 17 (71%) | NS |
| Disseminated stage (Ann Arbor) at diagnosis (%) | 10 (42%) | 13 (54%) | NS |
| PINK score at diagnosis (%) | NS | ||
| Low | 11 (46%) | 6 (25%) | |
| Intermediate | 5 (21%) | 7 (29%) | |
| High | 8 (33%) | 11 (46%) | |
| PINK-E score at diagnosis (%) | NS | ||
| Low | 11 (48%) | 6 (27%) | |
| Intermediate | 5 (22%) | 7 (32%) | |
| High | 7 (30%) | 9 (41%) | |
| Unknown | 1 | 2 | |
| Frontline chemotherapy (%) | NS | ||
| MGAD | 7 (29%) | 7 (29%) | |
| MOGAD | 5 (21%) | 10 (42%) | |
| Other Asparaginase-based regimen | 12 (50%) | 7 (29%) | |
| Frontline RTE (%) | 15 (63%) | 14 (58%) | NS |
| Frontline ASCT or HSCT (%) | 4 (17%) | 3 (13%) | |
| Response to frontline therapy (%) | NS | ||
| CR | 16 (67%) | 16 (67%) | |
| PR | 2 (8.3%) | 6 (25%) | |
| PD | 6 (25%) | 2 (8.3%) | |
| Disseminated stage (Ann Arbor) at relapse (%) | NS | ||
| Disseminated | 15 (71%) | 12 (60%) | |
| Unknown | 3 | 4 | |
| Number of previous lines of therapy (n) | NS | ||
| 1 | 24 (100%) | 17 (71%) | |
| >1 [2–4] | 0 (0%) | 7 (29%) | |
- Abbreviations: ASCT, Autologous Stem Cell Transplantation; CR, complete response; HSCT, Allogeneic Hematopoietic Stem Cell Transplantation; MGAD, methotrexate, gemcitabine, l-asparaginase, dexamethasone; MOGAD, methotrexate, oxaliplatine, gemcitabine, l-asparaginase, dexamethasone; p, p-value; PD, progressive disease; PR, partial response; RTE, radiotherapy.
- a n (%).
- b Pearson's Chi-squared test; Fisher's exact test.
Overall, this retrospective-matched cohort study provided evidence for the efficacy of aPD1 in a European series of R/R ENKTCL. Our propensity-score-based comparative analysis also suggested its superiority over other salvage regimens used before the era of immunotherapy.
However, we observed a discrepancy between PFS and OS: the aPD1 cohort showed improved OS, while PFS was similar in both the aPD1 and historical cohorts.
The variability in the timing of the first PET-CT after treatment initiation may lead to some patients being misclassified as stable or progressing, due to a delayed response to immunotherapy, as seen in other malignancies. In our cohort, 2 patients were initially classified with PR and 4 with PD later subsequently achieved CR during immunotherapy. Similarly, first-line sintilimab, anlotinib, and pegaspargase combined with radiotherapy showed a 55% complete response rate after two cycles and 87.8% after six cycles,12, 13 indicating that responses to immunotherapy may be delayed. Alternatively, subsequent salvage therapy might account for this difference, as the next treatment strategy could be sensitized by prior aPD1. In addition, combining chemotherapy with immune checkpoint blockade (ICB) in tumors insensitive to ICB monotherapy may improve outcomes by enhancing tumor antigen release and immunogenicity. In our study, subsequent salvage chemotherapy, particularly while pursuing aPD1 immunotherapy, could indeed improve survival in patients experiencing on-treatment relapse.
Recently, innovative therapeutic approaches combining aPD1 with chemotherapeutic agents to bolster the anti-tumor immune response have been evaluated as first-line treatments of ENKTCL. In the phase II clinical trial SPIRIT, evaluating the efficacy of first-line sintilimab alongside pegaspargase, gemcitabine, and oxaliplatin in 34 patients with high-risk advanced-stage ENKTCL, the overall response rate (ORR) was an impressive 100%, with a remarkable CR rate of 85%, following a median follow-up period of 21 months.14
Although no randomized controlled trials have assessed the efficacy of aPD1 in R/R ENKTCL, aPD1 blockade has emerged as the standard of care in this setting. The presence of 3-UTR structural variants of the PD-L1 gene15 and high levels of PD-L1 expression in tumor tissue7 have been associated with better responses to aPD1. However, biomarkers that can predict response or resistance to aPD1 blockade have yet to be determined.
A deeper understanding of the response to immunotherapy in ENKTCL could pave the way for the design of more targeted frontline randomized clinical trials incorporating immune checkpoint blockade.
ACKNOWLEDGMENTS
The authors thank the treating physicians from the TENOMIC network for enrolling their patients in this study and all the patients for their participation.
AUTHOR CONTRIBUTIONS
Amira Marouf, Sammara Chaubard, Lucile Couronné, Olivier Hermine, and Arnaud Jaccard designed and supervised the research study. Amira Marouf, Sammara Chaubard, Raphaël Liévin, and Lucile Couronné collected the clinical data. Sammara Chaubard, Jean-Marie Michot, Julien Rossignol, Doriane Cavalieri, Camille Golfier, Olivier Allangba, Laure Philippe, Benoît Tessoulin, Adrien Chauchet, Bénédicte Deau, Lucie Oberic, Jacques Vargaftig, Aline Moignet, Aline Clavert, Rémy Dulery, Gabriel Brisou, Stéphanie Tardy, Virginie Fataccioli, Roch Houot, René O. Casasnovas, Catherine Thieblemont, Hervé Ghesquières, Sylvain Carras, Steven Le Gouill, Guillaume Cartron, Aurélien Marabelle, Olivier Tournilhac, Gandhi Damaj, Philippe Gaulard, Laurence De Leval, François Lemonnier, Emmanuel Bachy, Olivier Hermine, and Arnaud Jaccard included patients in this study. Amira Marouf, Sylvie Chevret, and Nicolas Molinari performed the statistical analyses. Amira Marouf, Olivier Hermine, Lucile Couronné, and Arnaud Jaccard wrote the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING
Amira Marouf was supported by a PhD grant from the French Institute of Health and Medical Research (poste d'accueil INSERM) and Imagine Institute. Lucile Couronné and Olivier Hermine received financial support from the CALYM Carnot Institute. Lucile Couronné was supported by the ATIP-Avenir program.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




