Novel approaches in myelofibrosis
Abstract
Myelofibrosis (MF) is a clonal myeloid neoplasm characterized by bone marrow fibrosis, splenomegaly, and disease-associated symptoms, as well as increased mortality, due to thrombosis, severe bleeding, infections, or progression to acute leukemia. Currently, the management of MF patients is tailored according to risk scores, with higher-risk (intermediate-2 and high-risk) patients being assessed for allogeneic stem cell transplantation, which remains the only potentially curative treatment option. On the other hand, lower risk (low- and intermediate-1 risk) patients who are symptomatic may be treated with JAK inhibitors or other drugs. However, none of these drug treatments have induced relevant rates of durable complete remissions, and, therefore, novel treatments are needed to improve the long-term outcomes of MF patients. This review summarizes current preclinical and clinical approaches to MF therapy, including novel drug combinations involving JAK inhibitors and innovative monotherapies. These drugs target transcription, nuclear export, survival pathways, or various intracellular pathways, ranging from JAK-STAT signaling to PI3-Kinase, TP53, PIM1, or S100A8/A9/toll-like receptor pathways. Also, extracellular targeting using interferon, calreticulin mutant-specific antibodies, and other immunotherapeutic approaches are discussed, as well as various antifibrotic strategies. In addition, preclinical approaches that target individual mutated clones, for example, by mutation-specific JAK2V617F inhibitors or DNA repair pathway inhibitors, are presented. Finally, current efforts of generating novel endpoints for clinical trials aim more at disease modification and overall survival than at improvements of splenomegaly or symptoms. Together, the new generations of clinical trials promise to offer substantial improvements in the management of MF patients and long-term disease control.
INTRODUCTION
Myelofibrosis (MF) is a clonal malignancy of the blood system that is characterized by bone marrow fibrosis, splenomegaly, and a variety of disease-associated symptoms, such as fatigue, abdominal pain, night sweats, and weight loss.1 MF subtypes range from prefibrotic primary myelofibrosis (PMF) to overt PMF and other overt forms, such as MF that has progressed from polycythemia vera (Post-PV-MF) or essential thrombocythemia (Post-ET-MF).2 MF is associated with increased mortality, due to thrombosis, severe bleeding, infections, hematopoietic insufficiency with its hallmarks of anemia and thrombocytopenia, or progression to blast crisis.2
Currently, the management of MF relies on the risk-stratification of the patients. In Figure 1, I have summarized a current algorithm for assessing risk scores in MF patients, such as DIPSS4 for PMF or MYSEC prognostic model5 for Post-PV- and Post-ET-MF patients. In addition, scores incorporating more detailed molecular (Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients [MIPSS70]) and cytogenetic (Dynamic International Prognostic Scoring System-plus [DIPSSplus] and MIPSS70plusV2.0) data are being used. As shown in Figure 1, patients with intermediate-2 or high-risk scores should be assessed for their eligibility to undergo allogeneic stem cell transplantation (allo-SCT), and the myelofibrosis transplant scoring system (MTSS score) is useful in helping to define this eligibility. All patients with MF will be assessed for MF-associated splenomegaly and symptoms. If neither splenomegaly nor symptoms are present, watchful waiting may be warranted, as is currently the case for many patients with prefibrotic PMF. Specific problem-oriented therapy, such as physical exercise for fatigue, may be necessary. Whether early treatment of patients with pre-PMF or oligosymptomatic overt MF is beneficial is currently unclear. The first clinical trial, the RE-THINK trial (NCT02598297) which recruited patients with early MF high molecular risk (HMR) mutations, was closed in October 2017 due to poor accrual (which was due to the low prevalence of these mutations within this patient group).
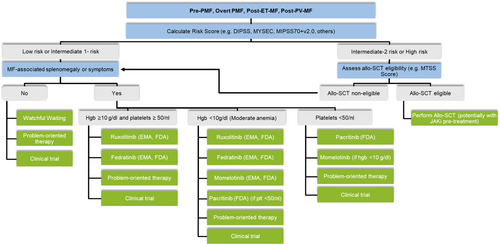
Patients with either MF-related splenomegaly or symptoms should be treated for their disease, and the JAK inhibitors (JAKi), ruxolitinib, and fedratinib, are approved for the treatment of such patients. In addition, for patients with moderate to severe anemia, momelotinib is approved, and for patients with a platelet count of below 50/nL, pacritinib is currently approved in the United States of America. Problem-oriented therapy may include off-label options, such as hydroxyurea for reducing high blood counts or erythropoietin, danazol, or other agents for alleviating anemia. However, a high unmet need for new therapies remains, particularly those that modify the course of the disease and improve event-free and overall survival.
Similar treatment algorithms have been published and updated by the German Society of Hematology and Medical Oncology,3 European LeukemiaNet,6 and National Comprehensive Cancer Network (NCCN).7
- 1.
Several recent clinical trials combining JAKi and other drugs.
- 2.
A selection of novel drugs which are currently being tested clinically as monotherapies.
- 3.
Innovative preclinical experiments targeted at disease-modification.
- 4.
New endpoints for clinical trials in MF.
METHODS
By review of publications (up to June 24, 2024) in PubMed (https://pubmed.ncbi.nlm.nih.gov), using the following keywords: “(Primary myelofibrosis PR post-PV-MF OR post-ET-MF AND clinical trials),” 650 publications were identified. In addition, 75 ongoing clinical trials involving patients with myelofibrosis were identified by using the search criteria: “(Recruiting AND myelofibrosis AND interventional trials)” at https://clinicaltrials.gov/. Additional publications were subsequently selected based on the specific scenarios described in this review.
Drug combinations including JAK inhibitors (JAKi)
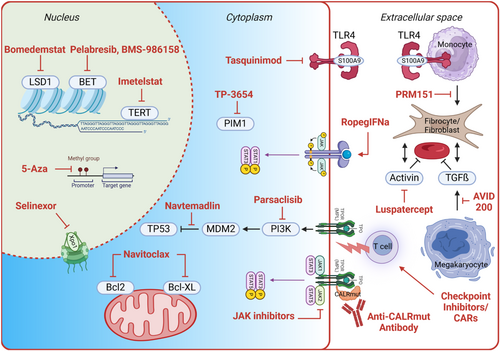
Figure 2 shows a selection of drugs and their targets which are being addressed in clinical trials in patients with MF or are being developed preclinically (Figure 2 and Table 1). These include drugs that inhibit transcription and nuclear export, mitochondrial activity, and various intracellular pathways, ranging from JAK-STAT signaling to PI3 kinase and TP53 activity, as well as PIM1 and Toll-like receptor pathways. Also, extracellular targeting using interferon-alpha, CALR mutant-specific antibodies, and other immunotherapy approaches, as well as various antifibrotic strategies, will be discussed.
| NCT identifier | Trial name | Phase | 1st drug | 2nd drug | 1° EP |
|---|---|---|---|---|---|
| NCT04472598 | TRANSFORM-1 | 3 | Navitoclax or placebo | Ruxolitinib | Percentage of Participants who achieve Spleen Volume Reduction of at least 35% at Week 24 (SVR35) |
| NCT04603495 | MANIFEST-2 | 3 | Pelabresib or placebo | Ruxolitinib | Splenic response at Week 24 |
| NCT05569538 | IMG-7289-IIS001 | 2 | Bomedemstat | Ruxolitinib | Safety (AEs, DLTs, SAEs) |
| NCT03194542 | ACE-536-MF-001 | 2 | Luspatercept | NA | The number of participants with anemia responses over any 84-day period during the primary treatment period |
| NCT04717414 | INDEPENDENCE | 3 | Luspatercept or placebo | JAKi | Red blood cell-transfusion independence (RBC-TI) ≥12 weeks (RBC-TI 12) |
| NCT04817007 | CA011-023 | 1b/2 | BMS-986 158 | None or ruxolitinib or fedratinib | Safety |
| NCT02742324 | RUXOPEG | 1/2 | Peg-IFNalpha-2a | Ruxolitinib | Phase 1 and 2: Safety; Phase 2: Occurrence of at least 50% reduction in spleen length as measured by palpation within the first 6 months after randomization |
| NCT04562389 | XPORT-MF-34 | 1-3 | Selinexor or placebo (phase 3) | Ruxolitinib | Phase 3: Proportion of participants with Spleen Volume Reduction (SVR) of ≥35% (SVR35) at week 24; and proportion of participants with Total Symptom Score (TSS) reduction of ≥50% (TSS50) at Week 24 |
| NCT04485260 | KRT-232-109 | 1b/2 | Navtemadlin ( = KRT232) | Ruxolitinib | Phase 1: To determine the KRT-232 RP2D in combination with ruxolitinib; Phase 2: To determine the spleen volume reduction (SVR) at Week 24 |
| NCT01644110 | POMINC | 1b/2 | Pomalidomide | Ruxolitinib | Best response rate within 12 treatment cycles according to the IWG-MRT criteria (including CR, PR, CI) and red cell transfusion (RCT) independency according to Gale et al. 2010 and 2011 |
| NCT04551066 | LIMBER-313 | 3 | Parsaclisib or placebo | Ruxolitinib | Proportion of participants achieving a targeted reduction in spleen volume at Week 24 |
| NCT06245941 | TQ05105-TQB3909-Ib/II-01 | 1b/2 | TQB3909 (bcl2 inhibitor) | TQ05105 (JAK1/2/ROCK inhibitor) | Phase 1: MTD, RP2D; Phase 2: ≥35% reduction in spleen volume (SVR35) at Week 24 |
| NCT06122831 | TQ05105-TQB3617-Ib/II-01 | 1b/2 | TQB3617 (BET inhibitor) | TQ05105 (JAK1/2/ROCK inhibitor) | Phase 1: MTD, RP2D; Phase 2: ≥35% reduction in spleen volume (SVR35) at Week 24 |
| NCT05280509 | TL-895-209 | 1b/2 | TL-895 (BTK inhibitor) | Ruxolitinib | Phase 1: RP2D; Phase 2: Spleen Volume Reduction (SVR) at Week 24 |
| NCT04640532 | KRT-232-113 | 1b/2 | TL-895 (BTK inhibitor) | Navtemadlin (=KRT232) | Phase 1b: MTD/MAD and RP2D; Phase 2: ≥35% reduction in spleen volume (SVR35) at Week 24 |
| NCT05714072 | 22-075 | 1 | Abemaciclib (CDK4/6 inhibitor) | Ruxolitinib | Proportion of patients with dose-limiting toxicity (DLT) |
| NCT05371964 | MYF1001 | 1/1b | Imetelstat | Ruxolitinib | Phase 1: DLTs; Phase 1b: AEs, symptom response rate at Week 24 |
| EudraCT-Nr. 2021-003 650-23 | FAMy (Accelerated phase MF) | 1/2 | CC-486 (oral azacytidine) | Fedratinib | Phase I: DLTs, MTD, RP2D; Phase II: Best response at week 24 according to the IWG-MRT and the ELN consensus report. This includes clinical improvement (CI), partial remission (PR), and complete remission (CR) |
| NCT04282187 | RG1006644 (Accelerated/blast phase MF) | 2 | Decitabine | JAKi (ruxolitinib, fedratinib, or pacritinib) | Proportion of patients enrolled who receive hematopoietic stem cell transplantation (HCT) |
| NCT03878199 | OSU-20 393 (Accelerated/blast phase MF) | 1/2 | CPX-351 | Ruxolitinib | Phase 1: DLTs; Phase 2: Proportion of participants that achieve at least an Acute Leukemia Response-Partial response (>=ALR-P, per 2012 myeloproliferative neoplasm-blast phase [MPN-BP] criteria) |
| NCT04576156 | MYF3001 | 3 | Imetelstat or best available therapy (not JAKi) | NA | Overall survival |
| NCT05936359 | INCA 33 989-101 | 1 | INCA33989 (Anti-mutCALR antibody) | NA | DLTs, TEAEs |
| NCT06034002 | INCA33989-102 | 1 | INCA33989 (Anti-mutCALR antibody) | NA | DLTs, AEs |
| NCT06150157 | 88549968MPN1001 | 1 | JNJ-88 549 968 (bispecific anti-mutCALR and anti-CD3 antibody) | NA | DLTs, AEs |
| NCT05025488 | GCO 17-2449 | 1 | mutated-CALR vaccine | NA | DLTs |
| NCT05393674 | FRACTION | 2 | Nivolumab | Fedratinib | Best response rate within 12 treatment cycles |
| NCT06327100 | 2023-0934 | 2 | Tasquinimod (S100A9 inhibitor) | None or ruxolitinib | AEs |
| NCT04176198 | BBI-TP-3654-102 | 1/2 | Nuvisertib (TP-3654) | None or ruxolitinib or Momelotinib | DLTs, AEs, and assess patients for any evidence of preliminary activity by determining the number of patients with ≥35% spleen volume reduction (SVR35) |
| NCT05731245 | 202208080MIPC | 2 | Ropeginterferon alpha-2b | NA | Clinicohematological responses at 24 weeks |
| NCT01981850 | BO42355 | 2 | Zinpentraxin (PRM-151) | NA | Overall response, bone marrow response rate |
| NCT03895112 | GCO 07-0548-01 401 | 1 | AVID-200 (TGFbeta inhibitor) | NA | MTD. Subjects attaining at least a CI (clinical improvement) by IWG/ELN criteria, or a decrease in bone marrow fibrosis by ≥1 grade with otherwise stable disease will be allowed to continue to the extension phase |
| NCT05279001 | ZGJAKUS001 | 1 | Jaktinib (JAKi) | NA | DLTs, AEs |
| NCT05037760 | KER050-MF-301 | 2 | KER-050 (TGFbeta pathway inhibitor) | NA | AEs, SAEs |
| NCT04676529 | PXS5505-MF-101 | 1/2 | PXS-5505 (pan-lysyl oxidase inhibitor) | NA | AEs, SAEs |
| NCT05835466 | STUDY-22-01 764 | 2 | Reparixin (CXCR1/2 inhibitor reparixin blocking IL-8 signaling) | NA | Efficacy of reparixin treatment per IWG/ELN criteria |
| NCT04279847 | INCB 57 643-103 | 1 | INCB 57 643 (BET inhibitor) | None or ruxolitinib | TEAEs |
| NCT04455841 | INCB000928 | 1/2 | INCB000928 (ALK2 inhibitor) | None or ruxolitinib | TEAEs |
| NCT05153343 | FM-21-001 | 1/2 | Flunotinib (JAK2/FLT3 inhibitor) | NA | Pharmacokinetics |
| NCT05467800 | MPN-RC 122 | 2 | Canakinumab (IL1beta monoclonal antibody) | NA | Number of participants with response (24 weeks), based on the IWG-MRT criteria |
| NCT04517851 | 2020-0522 | 2 | Elotuzumab (anti-SLAM7 monoclonal antibody) | NA | Overall response (up to cycle 36) |
| NCT05320198 | DISC-0974-102 | 1/2 | DISC-0974 (monoclonal antibody that binds to hemojuvelin and inhibits bone morphogenetic protein signaling) | NA | Phase 1 and 2: TEAEs; Phase 2: Anemia response, defined per IWG-MRT criteria |
| NCT06343805 | AJX-101 | 1 | AJ1-11095 (Type II JAK2 inhibitor) | NA | TEAEs, DLTs, MTD, RP2D |
| NCT06313593 | INCB160058-101 | 1 | INCB160058 (selective JAK2 pseudokinase domain binder) | NA | DLTs, TEAEs |
| NCT06218628 | 23-1048 | 1 | Talazoparib (PARP inhibitor) | Pacritinib | MTD |
- Note: Trials can be found online at clinicaltrials.gov/study/NCT… [insert the number of the respective trial] or https://www.clinicaltrialsregister.eu/ctr-search/search?query=2021-003650-23.
- Abbreviations: 1° EP, primary endpoint or outcome measure; AE, adverse event; DLT, dose-limiting toxicity; IWG-MRT, International Working Group for Myelofibrosis Research and Treatment; JAKi, JAK inhibitor; MTD, maximum tolerated dose; NA, not applicable; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Randomized phase 3 trials combining JAK inhibitors with navitoclax or pelabresib
The introduction of ruxolitinib monotherapy has led to considerable improvements in patients' disease-related splenomegaly, disease-related symptoms, and, arguably, overall survival compared with previous therapies.9-11 Aiming at improving these results, combination trials using novel drugs in combination with ruxolitinib have been performed. The first results of two phase 3 combination trials are presented here.
In the TRANSFORM-1 phase 3 trial (NCT04472598), patients with untreated MF were randomized to either the combination of ruxolitinib and the Bcl2 and BclXL inhibitor navitoclax versus ruxolitinib plus placebo. The primary endpoint of the trial was a spleen volume response of ≥35% at 24 weeks (SVR35@24wk), and one of the secondary endpoints was the symptom response at Week 24. As presented by Pemmaraju and colleagues at the ASH 2023 meeting,12 the primary endpoint was met, with a statistically significant doubling of SVR35@wk24, increasing from 31.5% with ruxolitinib plus placebo to 63.2% with the combination. However, symptom response at 24 weeks, one of the secondary endpoints of the trial, was not different between the two arms. Adverse events (AEs) of thrombocytopenia, anemia, and neutropenia were common and managed by dose reduction or interruption. However, 33% of patients discontinued study treatment.
In a second phase 3 trial, the MANIFEST-2 trial (NCT04603495), as presented by Rampal et al. at ASH 2023,13 patients with untreated MF were randomized to receive either the combination of the bromodomain and extraterminal motif protein (BET) inhibitor pelabresib and ruxolitinib or ruxolitinib plus placebo. Preclinical studies in MPN mouse models had shown that BET/NF-κB-dependent signaling sustained the inflammatory phenotype of the mice and that a combination treatment of ruxolitinib and a BET inhibitor in vivo was able to reduce the disease burden and BM fibrosis of the mice.14 Primary and secondary endpoints of the MANIFEST-2 trial included SVR35@wk24 and symptom response, respectively. Also in this trial, there was a statistically significant doubling of the spleen response at Week 24, from 35.2% with ruxolitinib and placebo to 65.9% with the combination of pelabresib and ruxolitinib. But, again, symptom response at Week 24, the key secondary endpoint of the trial, was not significantly different between the two treatment arms, even though TSS change showed a positive trend for significance. The combination of these two drugs was rather well tolerated. Both of these upfront JAKi combination trials show that it is difficult to improve the symptom response over that achieved with ruxolitinib alone, given the already very strong positive impact of ruxolitinib monotherapy on the symptoms.9, 15 It will be interesting to see whether future trials will either need to show significant improvements of novel drug combinations in both spleen and symptom response, or whether we need novel endpoints of disease modification for clinical trials in MF.
Combination with LSD-1 inhibition using bomedemstat
Preclinical evidence had demonstrated (a) that Lysine-specific demethylase-1 (LSD-1) is crucial for physiologic differentiation of hematopoietic stem and progenitor cells in mice,16 (b) that the combination of ruxolitinib and the LSD-1 inhibitor bomedemstat (also termed IMG-7289) reduced MPN-associated leukocytosis and thrombocytosis as well as splenomegaly in a JAK2V617F transgenic mouse model,17 and (c) that bomedemstat monotherapy led to increased survival of the mice (100% vs. 0% survival at Day 75 with IMG-7289 and vehicle treatment, resp. [p < 0.01]).17 The combination of bomedemstat and ruxolitinib was assessed in a phase 2 trial of patients with either relapsed/refractory JAKi-pretreated or JAKi-naive MF patients (NCT05569538).18 The primary endpoint was safety, and the secondary endpoints were spleen and symptom responses. The combination proved to be safe, with the most prominent grade 3 or 4 AE's being anemia and thrombocytopenia, occurring in approximately 20% of patients. Spleen and symptom responses at Week 24 were seen in 23% and 22% of pts, respectively.18
Combination with drugs targeting MF-associated anemia
In addition to MF-related splenomegaly and MF-associated inflammatory symptoms, patients with MF often suffer from dizziness, fatigue, and other anemia-induced symptoms.1 In addition to its impact on the quality of life of patients, anemia has also been associated with decreased survival in MF.19 Further work has shown that in patients with MF, mild, moderate, and severe anemia (corresponding to hemoglobin levels of Hb ≥10, 8–10, and <8 g/dL, resp.) were correlated with a decreasing median survival of 4.9, 3.4, and 2.1 years, respectively.20 And, in line with these data, MF patients commencing red blood cell transfusions, comprising 36% in one analysis,21 showed a significantly inferior survival as compared to patients who did not receive transfusions (hazard ratio: 7.8, 95% confidence interval [CI]: 5.1–11.9; p < 0.001) (median follow-up of 3.1 years, range 0–17.9).21
A number of causes have been elucidated for MF-associated anemia, including activation of the activin A receptor type 1 (ACVR1)/activin receptor-like kinase 2 (ALK2) axis and thereby induction of hepcidin expression.22 Momelotinib, in addition to its properties as a JAK1- and JAK2-inhibitor, acts as an inhibitor of ALK222 and is thought to exert its beneficial effects on transfusion burden and hemoglobin levels through inhibition of increased hepcidin.22 Momelotinib has been approved for the treatment of MF patients with moderate and severe anemia. However, not all patients respond to momelotinib (25% and 45% TSS response of the remaining patients at Weeks 2423 and 48,24 resp.), and only 12% remained on momelotinib therapy for ≥5 years, as reported in a combined analysis of the MOMENTUM (NCT04173494), SIMPLIFY-1 (NCT01969838), and SIMPLIFY-2 trials (NCT02101268).25 Thus, further improvements are desirable.
In addition to ALK2 and hepcidin, another factor suppressing erythropoiesis is transforming growth factor-beta (TGF-beta). Early experimental data had shown that chronic TGF-beta-1 treatment of mice resulted in suppression of erythropoietic cell proliferation and differentiation.26 And given that TGF-beta serum levels have been demonstrated to be elevated in patients with MF,27 inhibition of TGF-beta signaling, including activin signaling, in MF has been pursued. Luspatercept binds several TGF-beta superfamily ligands, including activin ligands, and thus antagonizes Smad2/3 signaling and improves late-stage erythropoiesis.28 The efficacy of this pathway inhibition to rescue suppressed erythropoiesis in MF was addressed in the phase 2 ACE-536-MF-001 trial (NCT03194542), investigating the combination of the activin receptor ligand trap, luspatercept, alone or in combination with ruxolitinib in anemic MF patients who were or were not transfusion-dependent (TD).29 The primary endpoint was anemia response, as defined as 12 weeks without transfusion in the TD pts and a hemoglobin increase of at least 1.5 g/dL without the need for transfusions in non-TD patients. The data showed that the best responses were seen in cohort 3B, representing TD patients receiving the combination of luspatercept and ruxolitinib. In this cohort, the primary endpoint of transfusion independence was achieved in 26.3% of patients, and 50% of patients had at least a 50% reduction of red blood cell transfusions after Week 24.29 Currently, a randomized phase 3 trial, the INDEPENDENCE trial (NCT04717414), is assessing the efficacy of the luspatercept and ruxolitinib combination as compared to ruxolitinib alone in patients with MF and anemia.
Further trials of drug combinations including JAK inhibitors
-
the phase 1/2 BMS-986158 study (NCT04817007), assessing the combination of the BET inhibitor BMS-986158 and ruxolitinib (1st line) or fedratinib (2nd line) in MF, reporting SVR35@wk24 in 90% (n = 16) of 1st line- and 43% (n = 24) of 2nd line-treated patients30;
-
the phase 1/2 RUXOPEG study in MF (NCT02742324), which assessed the combination of pegylated interferon-alpha 2a (pegIFNa-2a) and ruxolitinib in JAKi and IFNa treatment-naïve MF patients, reported a 50% decrease of spleen length (as measured by palpation) in 70% of patients31;
-
the phase 1 XPORT-MF-34 study (NCT04562389), evaluating the safety and efficacy of the XPO1 inhibitor, selinexor, plus ruxolitinib, in JAKi-naïve MF patients, which found a high rate of responders (SVR35@wk24 and TSS50@wk24 in 79% and 58% of patients, respectively), albeit in a small cohort of patients treated with the 60 mg dose (n = 14)32;
-
the phase 1b/2 study of the safety and efficacy of the MDM2 inhibitor, navtemadlin (KRT-232) (NCT04485260), added-on to ruxolitinib in patients with MF who have a suboptimal response to ruxolitinib, showing SVR35@wk24 and TSS50@wk24 in 31% and 38% of patients, respectively33;
-
the phase 2 POMINC trial in MF with anemia (NCT01644110), reporting at least a clinical benefit (hemoglobin increase <2 g/dL or prolongation of RBC transfusion intervals and/or improvement of symptoms) in 29% of patients treated with the combination of pomalidomide with ruxolitinib34;
-
the phase 2 study of parsaclisib (NCT04551066), a PI3K-delta inhibitor, added on to ruxolitinib in patients with MF who responded suboptimally to ruxolitinib, showing SVR35@wk24 and TSS50@wk24 responses in 5% and 36% of patients.35
Further ongoing trials in MF (Table 1) are assessing the safety and efficacy of the combination of a JAK1/2 inhibitor with a Bcl2 inhibitor (NCT06245941) a BET inhibitor (NCT06122831), the BTK inhibitor TL-895 (NCT05280509), the CDK4/6 inhibitor abemaciclib (NCT05714072), or imetelstat (NCT05371964), as well as of the combination of KRT-232 and TL-895 (NCT04640532).
Drug combination trials in accelerated phase MF
There are not many clinical trials assessing the efficacy of drug treatments in patients with accelerated phase MF, that is, patients with 10%–19% of blasts in the blood or BM. One such study is the phase 1/2 FAMy study led by Al-Ali and colleagues, investigating the safety and efficacy of a combination of oral 5-azacytidine (CC-486) and fedratinib (EudraCT-Nr. 2021-003650-23). In addition, the combination of decitabine in combination with a JAKi as a bridge to allo-SCT is being assessed in a phase 2 trial in patients with accelerated or blast phase MPN (NCT04282187). Finally, the safety and efficacy of combined treatment of ruxolitinib and CPX-351 is being addressed in a phase 1/2 trial in patients with accelerated or blast phase MPN (NCT03878199). These clinical trials are desperately needed, since the outcome of patients with accelerated phase MF is still dismal (median survival of 16.7 months).36
Novel drug monotherapies in MF and immunotherapeutic approaches
This section presents a selection of monotherapy approaches to treat patients with myelofibrosis (see also Table 1).
Targeting telomerase using imetelstat
The first approach to be presented is the inhibition of telomerase. This strategy relies on data demonstrating that telomerase is aberrantly upregulated in MPN cells,37 including BM-derived CD34+ cells,38 likely leading to increased proliferation, which is reflected by the shortened telomere length of these cells.38 The telomerase inhibitor imetelstat binds to the RNA template of the telomerase enzyme and thus prevents telomere maintenance, inducing senescence and, ultimately, cell death of the malignant cells, including PMF cells in vitro and in vivo,39 possibly by targeting both telomerase39 and JAK-STAT signaling.40 It is still debated to which extent the efficacy of imetelstat is due to canonical versus noncanonical telmerase inhibition pathways.40 Tefferi et al. were able to show in a pivotal phase 1 trial that imetelstat treatment led to clinically meaningful responses in 21% of progressed MF patients, including complete remissions and partial remissions.41 In a follow-up phase 2 trial, Mascarenhas and colleagues showed that, while formal spleen and symptom responses were comparable to other drugs in MF trials, a remarkably long overall survival of 30 months was noted in this very high-risk population of patients.42 This beneficial effect on survival was dose-dependent and was significantly enhanced over that of a propensity-matched cohort.43 Interestingly, spleen and symptom responses correlated closely with the reduction of telomerase activity by imetelstat,42 suggesting a direct effect of the drug on the clinical outcome.
This concept of telomerase inhibition is currently being assessed in a phase 3 clinical trial of imetelstat versus best available therapy excluding JAKi, in MF patients who have failed JAKi therapy (NCT04576156).44 Of note, this is one of the few clinical MF trials with overall survival as the primary endpoint, and it will be very interesting to see the results of this trial when they are available. Also, as stated above, in addition to imetelstat monotherapy, a phase 1/1b combination trial of imetelstat and ruxolitinib is ongoing (NCT05371964).
Monoclonal antibodies against mutant calreticulin
In addition to these anti-inflammatory and antifibrotic approaches, mutation-specific targeting is an important aim, in order to develop strategies that inhibit the disease-driving oncogenes in MF. One such approach is the specific targeting of the mutant CALR protein on the surface of MPL-expressing cells.45
Preclinical experiments using CALR-mutant cells have shown that the CALR-mutant-specific antibody, INCA33989, inhibits JAK-STAT signaling, here shown as STAT5 phosphorylation in CD34+ cells from MPN patients.46 This effect was not seen in JAK2V617F-expressing cells, confirming the high selectivity of the antibody for mutant CALR. Further in vitro experiments showed that both immature progenitor and megakaryocytic cells expressing mutant CALR but not control cells were specifically reduced by incubation with the CALR antibody. In vivo experiments in a CALR-mutant mouse model showed that weekly administration of this antibody led to a normalization of platelets in the blood and megakaryocytes in the bone marrow as well as reversal of splenomegaly in these mice, as performed by Dr. Reis in collaboration with Dr. Plo's group.46
These results have prompted two clinical phase 1 trials of the INCA33989 antibody in patients with CALR-mutant MF or ET (NCT05936359 and NCT06034002). In these trials, patients undergo dose escalation and dose expansion of the antibody alone or in combination with ruxolitinib. The primary endpoint is safety, and the trials are currently recruiting patients.
A similar trial has been started, assessing the safety of the bispecific CALR-mutant antibody JNJ-88549968, recognizing mutant CALR and the T cell epitope CD3, also in CALR-mutant MF and ET patients (NCT06150157). The major effect of this vaccine is thought to be mediated through CALRmut-selective T-cell activation and direct cytotoxicity.47
Furthermore, another ongoing clinical trial is assessing the safety and efficacy of a CALR-mutant-based vaccine (NCT05025488).
It will be very interesting to see the first results of these mutation-specific approaches in MF, as many aspects are still unknown, including potential cytopenias and optimal treatment scheduling.
Immunotherapeutic approaches using checkpoint inhibitors
In addition to the presented approaches, immunotherapy has become an important focus of MF treatment strategies. Support for this strategy comes from various pre-clinical studies. In one of these studies, Zeiser and colleagues have shown that programmed death-ligand 1 (PD-L1) expression is increased in JAK2V617F-positive cells from mice and patients with MPN disease.48 Importantly, administration of PD-L1 antibodies to the mice led to increased CD8 positive effector T cells as well as increased survival of the mice.48
A first small clinical trial of nivolumab monotherapy in eight patients with MF was terminated early due to failure to reach the primary endpoint (objective response rate, defined as complete remission, partial remission, and clinical improvement after eight doses of therapy).49 Therefore, combination treatments of checkpoint inhibitors and JAKi were pursued. However, this group and the group of Ahsan et al also showed that, in vitro, ruxolitinib led to a decrease of PD-L1 expression on the malignant cells, potentially hampering their amenability to checkpoint inhibitors.48, 50 This suppressive effect was significantly less pronounced with the JAKi fedratinib.50 This has led Heidel and colleagues to design the FRACTION trial, a phase 2 combination study of fedratinib and the checkpoint inhibitor nivolumab in resistant or suboptimally responding MF patients (NCT05393674).51 The primary endpoint is the clinical response after 12 months. This trial is currently recruiting patients, and recruitment is expected to be completed by the end of 2024.51
Targeting S100A8/A9 alarmins using tasquinimod
The next approach relies on the inhibition of the S100A8/A9 alarmin axis in MF. In elegant preclinical studies using single cell RNAseq (scRNAseq) technologies of bone marrow cells, the team of Dr. Schneider in Aachen has done pioneering work in separating different bone marrow (BM) stroma cell populations. They were able to show that mesenchymal stromal progenitor cells drive BM fibrosis in a JAK2V617F-positive retroviral mouse model of MPN and in patients and that inflammation in the BM stroma via the alarmins S100A8/A9 may be an attractive target for early fibrosis monitoring and targeted therapy.52 The scRNAseq data showed that S100A8/A9 transcripts were specifically upregulated in the BM microenvironment and in megakaryocytes of patients with PMF but not controls.52 Further results, using enzyme-linked immunosorbent assay of the peripheral blood serum and immunohistochemistry of the BM, showed that S100A8/A9 alarmin proteins were increased in the serum and BM of MPN patients and that their concentration correlated with the grade of bone marrow fibrosis, thus representing an early marker of myelofibrosis.
To investigate the functional role of these alarmins, the TLR4 inhibitor tasquinimod, which acts as an inhibitor of S100A8/A9 signaling, was used as an oral treatment in a retroviral transplant mouse model of JAK2V617F-driven MPN disease.52 In this mouse model, tasquinimod treatment led to remission of disease-associated leukocytosis and splenomegaly as well as significant reduction of fibrosis in the BM, confirming its potential disease-modifying properties. These results have led to the development of a phase 2 clinical trial of tasquinimod in pre-treated MF patients, the TasqForce trial led by Drs. Crysandt and Schneider in Aachen, and Dr. Te Boekhorst in Rotterdam.53 A similar clinical trial is planned in the United States (NCT06327100).
-
TP-3654 (PIM inhibitor) in MF (phase 1 trial) (NCT04176198), reporting SVR35@wk24 and TSS50@wk24 in 23% and 54% of patients, respectively54;
-
RopegIFNa in pre-MF and low-risk/int1-MF (phase 2 trial) (NCT05731245), demonstrating blood count responses at Week 24 in 76.3 to 83.1% of patients, TSS50@wk24 in 43% of patients, and JAK2V617F partial molecular responses @wk24 in 26% of patients55;
-
Zinpentraxin-alfa (PRM151) in MF (phase 2 trial) (NCT01981850), with a 33% overall response @wk24 (CR, PR, or CI)56; and
-
AVID200 (TGFbeta 1/3 inhibitor) in advanced MF (phase 1b trial) (NCT03895112), reporting an encouraging platelet increase in 81% of patients and significant decreases of SMAD2 phosphorylation were observed as an indicator of effective TGFbeta pathway inhibition.57
Additionally, ongoing clinical trials assessing monotherapies in MF (Table 1) include other JAK inhibitors (NCT05279001), the TGFbeta pathway inhibitor KER-50 (NCT05037760), the pan-lysyl oxidase inhibitor PXS5505 (NCT04676529), the CXCR1/2 inhibitor reparixin blocking IL-8 signaling (NCT05835466), the BET inhibitor INCB057643 (NCT04279847), the ALK2 inhibitor INCB000928 (NCT04455841), the JAK2/FLT3 inhibitor flunotinib (NCT05153343), the anti-IL1beta monoclonal antibody canakinumab (NCT05467800), the anti-SLAM7 monoclonal antibody elotuzumab (NCT04517851), or the monoclonal antibody DISC-0974, which binds to hemojuvelin and inhibits bone morphogenetic protein signaling (NCT05320198).
Innovative preclinical experiments targeted at disease-modification
JAK2V617F-mutant specific inhibitors
More recent preclinical approaches have increasingly been directed against genetically defined malignant clones. In one such approach, Levine and colleagues have spear-headed a pre-clinical study in which they have addressed the question of whether, in a JAK2V617F mouse model, oncogenic JAK2 remains a crucial driver or not.58 This is important for the decision of whether mutation-specific approaches make sense in patients with JAK2V617F-mutated MPN, including MF. In transgenic mice with JAK2mutant-Cre-induced disease, abrogation of JAK2V617F expression by Dre recombination led to a reversal of leukocytosis and erythrocytosis, as well as splenomegaly and bone marrow fibrosis, and to enhance the survival of the mice.58 These results suggest that continuous expression of JAK2V617F is necessary for the maintenance of the MPN phenotype and that the MF phenotype is potentially reversible. Currently, JAK2V617F mutations-specific inhibitors are being developed and preclinically and clinically tested (NCT06343805 and NCT06313593).59, 60
Targeting the DNA repair pathway
Another approach to target specific clones in any given MF patients has been proposed by Skorski and team.61 They have shown that MPN cells carrying different MF-associated mutations, for example, in JAK2, TET2, or EZH2, are differentially sensitive to DNA repair pathway inhibitors such as PARP inhibitors, ATM inhibitors, or ATR inhibitors61: in an example of an AML case, they showed the reduction of specific mutant subclones after in vitro treatment with a combination of ATR and PARP inhibitors, suggesting preferential sensitivities toward ATR inhibition of the EZH2-mutant clone and PARP inhibition of the TET2-mutant clone.61 Subsequently, the authors confirmed eradication of the malignant cells in vivo after xenotransplantation of cells from the same AML patient into mice and treatment of the mice with the combination of an ATR and a PARP inhibitor, resulting in complete eradication of the malignant cells in the mice.61
Along these lines, Elbracht et al found a high incidence of germline mutations in DNA repair genes, such as BRCA, ATM, and CHEK2 mutations, in patients with familial MPNs, including MFs.62 Interestingly, in vitro experiments showed that 32D cells harboring both the JAK2V617F oncogene and a heterozygous BRCA1 mutation displayed increased sensitivity toward PARP inhibitors, both alone or in combination with interferon-alpha treatment.63 These results pave the way for more individualized therapeutic approaches and genetic counseling in MPN patients, and the concept of combined JAK and PARP inhibition is currently being evaluated in a phase 1 trial of pacritinib and talazoparib in patients with MPNs, including myelofibrosis, who are unresponsive to JAK2 Inhibition (NCT06218628).
New endpoints for clinical trials in MF
In the final section of this review, some of the approaches to generate novel endpoints for future clinical trials in MF will be discussed. Until recently, primary endpoints of clinical trials in MF have mostly included spleen and symptom response. This was reasonable, given the paucity of approved therapies before the advent of JAKi. However, given the encouraging long-term results of allogeneic stem cell transplantation, novel drug approaches in MF should also aim at improving event-free or overall survival and thus at disease modification (see also Ross et al.64).
Correlation of spleen volume reduction with overall survival
The use of JAKi in MF has resulted in meaningful reductions of spleen volume, and two studies have found evidence for a positive correlation of spleen response after 6 months and improved overall survival,65, 66 although this positive correlation lost significance in one study after adjustment for IPSS risk.65 Additionally, there was a correlation between better survival and spleen response at 3 or 6 months, maintenance of a sufficient ruxolitinib dose of at least 20 mg BID, and achievement of transfusion independence during 3 and 6 months of ruxolitinib therapy.66 These results suggest that clinical responses during therapy can be a biomarker for overall survival.
Correlation of transfusion independence with overall survival
Recently, novel endpoints of clinical MF trials have been generated, including overall survival44 and transfusion independence.29 Transfusion independence was shown to correlate with survival in a combined analysis of the SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2 (NCT02101268) clinical trials.67 Specifically, transfusion independence at baseline was identified by multivariate analysis as significantly correlated with improved survival in both trials (hazard ratios of 0.474 and 0.226, resp.), and response at Week 24 of momelotinib therapy was correlated with improved survival in the SIMPLIFY-1 trial.67 Thus, transfusion independence may be an important surrogate marker for survival in future clinical trials for MF. In order to assess transfusion independence and other anemia response parameters, revised International Working Group-European LeukemiaNet criteria for anemia response in myelofibrosis have recently been published.68
Correlation of bone marrow fibrosis reduction with survival
Other endpoints such as changes in BM fibrosis69 or variant allele frequency (VAF) have been considered but have not yet been validated. For example, improvement in BM fibrosis has been shown to occur after allogeneic stem cell transplantation and to be associated with improved survival.70 However, it is unclear whether BM fibrosis improvement can serve as a valid biomarker for survival for medical drug treatments as well. Recent data from the SIMPLIFY-1 clinical trial (NCT01969838) of momelotinib versus ruxolitinib treatment showed that, while both momelotinib and ruxolitinib were able to lead to an improved BM fibrosis grade, this improvement was not associated with an improved survival.69 However, several limitations of this analysis were pointed out by the authors, including a lack of central histology review and rather early assessment during the study (week 24).69 Also, patients receiving imetelstat on the MYF2001 trial (NCT02426086) who achieved an improvement in BM fibrosis of at least 1 grade had longer overall survival than those without such improvement,42 and the same beneficial effect was seen for patients receiving add-on navitoclax to ruxolitinib treatment.71 Taken together, the clinical relevance of BM fibrosis improvement in MF and its usefulness as a biomarker have been demonstrated most clearly in the allo-SCT setting, but there are encouraging first results from drug treatment trials as well.
Correlation of variant allele frequency reduction with overall survival
JAK2V617F- and CALR-mutant VAF in ET72, 73 and JAK2V617F-mtuant VAF in PV72 have been shown to be correlated with MF-free survival. In MF, positive correlations of VAF reduction have been described for patients treated with imetelstat, where patients achieving at least a 20% reduction in JAK2V617F VAF showed longer median overall survival than those without such a VAF reduction.42 Likewise, MF patients treated with add-on navitoclax to ongoing ruxolitinib therapy within the REFINE trial (NCT03222609) who showed a least 20% VAF reduction showed a prolonged median survival (not reached vs. 28.5 months of those without such a VAF response).71
Finally, criteria for the combined endpoint of spleen and symptom response,13 progression-free and event-free survival in MF clinical trials are being discussed.74 It will be crucial to select from the above-described novel endpoints the ones that best describe an advancement in the treatment of patients with MF. Likely, these will include survival-related endpoints (OS, PFS, and/or EFS), biomarkers (spleen volume response [SVR], transfusion independence, VAF), or combinations of these. Renewed efforts will be required to reach consensus for these endpoints, in order to design successful clinical trials that will improve long-term outcomes of MF patients.
CONCLUSION
In conclusion, there are numerous novel drugs being tested clinically, either alone or in combination. However, we need better synergism of these drugs and better disease-modifying activities. Second, there are interesting novel approaches addressing the cause of MF, and the results from the first clinical trials are pending. Third, new endpoints for clinical MF trials have been generated, but they now need to be tested prospectively. And finally, since allogeneic SCT remains the only potential curative option in MF so far, this approach should be learned from, for example by using combinations of active anti-MPN drugs and immunotherapy strategies, in order to improve not only spleen and symptom responses but also long-term quality of life and survival of patients with MF.
ACKNOWLEDGMENTS
The author wishes to thank all colleagues who have supported him with helpful discussions. He would also like to apologize for any work that could not be cited due to the space limitations of this manuscript. The manuscript is based on an invited oral presentation in the MPN Educational Session at the 29th EHA 2024 Congress in Madrid, Spain.
AUTHOR CONTRIBUTIONS
Steffen Koschmieder conceptualized and designed this review manuscript, analyzed and critically assessed the relevant publications, drafted the original manuscript, generated the Table and Figures, and performed the revisions of the manuscript.
CONFLICT OF INTEREST STATEMENT
Steffen Koschmieder is a HemaSphere editor; reports research grant/funding from Novartis, Bristol-Myers Squibb, AOP Pharma, Imago Biosciences, Janssen, Geron; advisory board honoraria and consulting fees from Pfizer, Incyte, Ariad, Novartis, AOP Pharma, BMS, Celgene, Geron, Janssen, CTI, Roche, Baxalta, Sanofi, Sierra Oncology, GSK, Abbvie, MSD; patent for a BET inhibitor at RWTH Aachen University; honoraria from Novartis, BMS, Celgene, Geron, Janssen, Pfizer, Incyte, Ariad, Shire, Roche, AOP Pharma, GSK, Abbvie, PharmaEssentia, iOMEDICO, MPN Hub; and other financial support (e.g., travel support) from Alexion, Novartis, BMS, Incyte, Ariad, AOP Pharma, Baxalta, CTI, Pfizer, Sanofi, Celgene, Shire, Janssen, Geron, Abbvie, Kartos, Sierra Oncology, Imago Biosciences, GSK, Abbvie, PharmaEssentia, MSD, iOMEDICO.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
This work emerged as part of the research unit CRU344 (KO 2155/7-2 and KO 2155/9-2), funded by the Deutsche Forschungsgemeinschaft (German Research Foundation), and the German Cancer Aid (Deutsche Krebshilfe; DKH) funding to S.K. (70114726).




