The incidence and clinical significance of monoclonal and oligoclonal protein bands in multiple myeloma patients after BCMA–CAR-T cell therapy: A retrospective study based on LEGEND-2
Abstract
The emergence of abnormal protein bands (APBs), also known as oligoclonal protein bands, has been documented in patients with multiple myeloma (MM) post hematopoietic stem cell transplantation. However, the incidence rate and clinical significance of APBs remain contentious. Few studies have explored the occurrence and prognostic implications of APBs in patients with MM treated with B-cell maturation antigen (BCMA)–specific chimeric antigen receptor (CAR)-T therapy. In this retrospective study, we examined the frequency, isotypes, and duration of APBs, as well as their correlation with MM disease characteristics, treatment response, clinical outcomes, and immune signature in patients with relapsed/refractory MM who had received LCAR-B38M therapy at the Xi'an site of the phase 1 LEGEND-2 trial. Among 47 patients assessed, 23 (48.9%) developed APBs following CAR-T therapy, with IgG being the most common isotype. The median onset and duration of APBs post-CAR-T infusion were 3.6 and 5.8 months, respectively. Patients with APBs demonstrated significantly improved response to LCAR-B38M therapy, along with longer overall and progression-free survival. Furthermore, those with APBs exhibited enhanced recovery rates of immunoglobulins and higher absolute counts of white blood cells, neutrophils, and lymphocytes post-CAR-T treatment compared to those without APBs. However, no significant differences were observed between the two groups in the percentages of various T-cell subsets and natural killer cells. Overall, the presence of APBs in patients with MM following CAR-T treatment was associated with deeper remission and a more favorable prognosis, suggesting a robust humoral response and subsequent immune reconstitution.
BACKGROUND
Multiple myeloma (MM) is a hematologic malignancy characterized by the proliferation of cytogenetically heterogeneous clonal plasma cells in bone marrow, with the overproduction of monoclonal paraprotein (M-protein).1 A traditional approach to evaluating treatment response is to detect and assess serum and urine M-protein levels using protein electrophoresis, immunofixation electrophoresis, and/or serumfree light chain (fLC) values.2 According to the International Myeloma Working Group (IMWG), a complete response (CR) occurs when M-protein is undetectable on immunofixation electrophoresis.3
The appearance of abnormal protein bands (APBs), also referred to as oligoclonal protein bands, clonal isotype switch,4 atypical serum immunofixation patterns,5 secondary monoclonal gammopathy of undetermined significance, and M-protein immune reconstitution,6 has been widely reported in patients with MM after high-dose myeloablation following hematopoietic stem cell transplantation (HSCT)7 and induction treatment with novel agents.5, 8 The phenomenon is not unique to MM but is also seen after HSCT in patients with other hematologic diseases including leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma.9, 10 The incidence rate of APBs is highly variable, ranging from about 6%–73%,11, 12 and the clinical significance of APBs is controversial. Some studies have demonstrated that the presence of APBs is a benign signal for longer overall survival (OS) and progression-free survival (PFS),4, 6, 9, 13 while other studies failed to find any additional survival benefit.12, 14, 15 Although the underlying mechanism of APBs remains elusive, one well-recognized hypothesis is that oligoclonality is caused by the reconstitution of nonmalignant B cells rather than new clones of myeloma cells or other lymphoproliferative disease.5, 16
The LEGEND-2 trial investigated LCAR-B38M, a B-cell maturation antigen (BCMA)–specific chimeric antigen receptor (CAR)-T cell therapy, in patients with relapsed/refractory multiple myeloma (RRMM). Initial findings revealed profound responses, boasting an overall response rate (ORR) of 88%.17 Subsequent 4-year follow-up data underscored sustained antimyeloma activity, with a median PFS of 18 months.18 But a paucity of studies reported the incidence, clinical significance, and prognostic impact of APBs in patients with MM receiving BCMA-specific CAR-T therapy.
In this study, we retrospectively investigated the occurrence, isotypes, and duration of APBs, its correlation with MM disease characteristics, response to treatment, clinical outcomes, and immune reconstitution to advance the understanding of APBs and their clinical relevance, thereby furnishing novel perspectives and insights for CAR-T therapy.
METHODS
Study design
We conducted a retrospective analysis of patients enrolled at the Xi'an site of the LEGEND-2 trial (NCT03090659), which was a phase 1, single-arm, open-label study conducted in China. The study included patients aged between 18 and 80 years who were diagnosed with RRMM. Diagnosis of MM was based on the criteria established by the IMWG.2 All patients analyzed in this study had RRMM and had undergone autologous BCMA-specific CAR-T therapy (LCAR-B38M). The study excluded patients who did not undergo regular serum immunofixation electrophoresis, those who died during LCAR-B38M therapy, and those lost to follow-up (Supporting Information S1: Figure 1). Response to treatment and outcomes were evaluated based on guidelines provided by the IMWG.3, 19
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional independent ethics committee of the Second Affiliated Hospital of Xi'an Jiaotong University (Approval No. 2016002). Written informed consent was obtained from all patients. The study commenced on March 30, 2016, and survival data reported here were collected up to December 10, 2023.
Clinical data collection
We collected demographic information and baseline disease characteristics, including MM paraprotein subtypes, Durie-Salmon staging system stage, International Staging System stage, Eastern Cooperative Oncology Group performance status, cytogenetic abnormalities, presence of extramedullary disease, transplantation history, pre-CAR-T treatment regimens and levels of β2-microglobulin, free calcium, lactic dehydrogenase, serum creatinine, and albumin. Additionally, serum immunoglobulin and fLC levels, blood profile parameters (white cell count, platelet count, neutrophil count, monocyte count, and lymphocyte count), percentage of bone marrow myeloma cells, and minimal residual disease (MRD; assessed via flow cytometry with a sensitivity of 10−5) were recorded. Furthermore, data on response to LCAR-B38M CAR-T therapy, grades of cytokine release syndrome, and response and survival outcomes were collected.
Serum immunofixation electrophoresis and protein electrophoresis
IFE technical procedures, migration, and staining programs were performed according to the manufacturer's instructions, and run on the standard Sebia, Hydrasys 2 platform, with the HYDRAGEL 9 IF kit. Briefly, 10 μL of diluted serum samples were added to a sampling comb and placed in a wet box for 5 min. After selecting the electrophoresis procedure, the buffer strip was fixed, and the gel was placed on the temperature control board without bubbles. The comb was moved to the electrophoresis stand, and electrophoresis was started. Afterward, 10 μL of antibodies were added to the gel for immunofixation. Following a 10-min incubation, excess antibodies were absorbed with filter paper, and the gel was dried for 3 min. After another 6 min, the gel was moved to the staining area, and staining was initiated. Once staining, decolorization, and drying were completed, the dried gel sheet was collected. The same platforms and operators were used consistently throughout the study to minimize technical bias.
We used serum protein electrophoresis to differentiate monoclonal immunoglobulins from polyclonal immunoglobulins, with the M spike percentage displayed on the electrophoretic map. Multiplying the M spike percentage by the total serum protein content provided the quantitative measurement of M protein. The lower limit of M protein quantification was 19.0 mg/dL.
Definition of APBs
APBs were defined as the appearance of (1) new monoclonal or oligoclonal immunoglobulin protein bands with different light chain and/or heavy chain components compared to the primary diagnostic M-protein or (2) the same type of monoclonal or oligoclonal immunoglobulin protein bands as the initial diagnosis but with a different migration pattern and absence of disease relapse. Oligoclonal bands were characterized by the presence of two or more concurrent distinct bands of the same isotype.4-6, 12, 20
Flow cytometry
Peripheral blood samples from patients who received LCAR-B38M infusion were collected for flow cytometry. All human antibodies were purchased from BD Biosciences, including CD3 (AmCyan), CD4 (PerCP-Cy5.5), CD8 (FITC), granzyme B (PE), IFN-γ (FITC), IL-17A (PE), IL-4 (APC), CD127 (PE), CD25 (APC), CD56 (PE), and CD16 (APC). Different immune cells were identified as CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), type 1 T-helper (Th1) cells (CD3+CD4+IFN-γ+), type 2 T-helper (Th2) cells (CD3+CD4+IL-4+), type 17 T-helper (Th17) cells (CD3+CD4+IL-17A+), regulatory T (Treg) cells (CD3+CD4+CD25+CD127-), cytotoxic T cells (CD3+CD8+granzyme B+), and total natural killer (NK) cells (CD3−CD56+; further divided by CD56bright and CD56dimCD16+).
Statistical analysis
Continuous variables are presented as medians (ranges) and categorical variables as counts (percentages). Differences in categorical variables were analyzed using the chi-square statistic or Fisher's exact test, while differences in continuous variables were assessed using the Mann–Whitney test or Kruskal–Wallis test. Kaplan–Meier survival analysis with log-rank testing was employed to estimate OS and PFS probabilities. PFS after LCAR-B38M therapy was calculated from the time of LCAR-B38M infusion to the first instance of progressive disease post-CAR-T therapy or death for any reason, while OS was calculated from the time of LCAR-B38M infusion to death or the cut-off date. All reported p values were two-sided, and values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 22 software (IBM).
RESULTS
Frequency and characteristics of APB after LCAR-B38M treatment
A total of 57 patients with RRMM were enrolled at the Xi'an site for LEGEND-2, with 47 of them eligible for this retrospective study. Ten patients were excluded due to insufficient serum immunofixation electrophoresis results (N = 8), loss to follow-up (N = 1), or mortality during CAR-T cell infusion (N = 1).
Of the 47 patients evaluated, 23 (48.9%) developed APBs after LCAR-B38M therapy. The demographic and clinical characteristics of patients, categorized based on the presence or absence of APBs, are delineated in Table 1. Although a higher percentage of males was observed in the APB group (74%) compared to the no-APB group (46%, p = 0.05), no significant difference was found between the two groups in age, primary paraprotein type, Durie-Salmon (DS) staging system stage, International Staging System (ISS) stage, cytogenetic abnormalities, transplantation history, prior therapy lines, presence of extramedullary disease, grade of cytokine release syndrome (CRS), and various biological parameters including platelet, β2-microglobulin, albumin, serum creatinine, lactate dehydrogenase, and free calcium level.
| Characteristics | With APBs (n = 23) | Without APBs (n = 24) | p Value |
|---|---|---|---|
| Sex | 0.050 | ||
| Male, n (%) | 17 (74) | 11 (46) | |
| Female, n (%) | 6 (26) | 13 (54) | |
| Age, median (range), y | 58.5 (46–67) | 54.0 (27–68) | 0.280 |
| Paraproteins, n (%) | 0.162 | ||
| IgG | 14 (61) | 8 (33) | |
| IgA | 4 (17) | 8 (33) | |
| Light chain only | 5 (22) | 8 (33) | |
| Light chain, n (%) | 0.109 | ||
| κ | 9 (39) | 15 (62) | |
| λ | 14 (61) | 9 (38) | |
| DS stage, n (%) | 0.313 | ||
| I | 3 (13) | 0 | |
| II | 1 (4) | 2 (8) | |
| III | 19 (83) | 22 (92) | |
| ISS stage, n (%) | 0.699 | ||
| I | 5 (22) | 4 (17) | |
| II | 2 (9) | 3 (12) | |
| III | 14 (61) | 12 (50) | |
| Unknown | 2 (9) | 5 (21) | |
| Cytogenetic abnormalities,a n | 11 of 12 | 11 of 13 | 1.000 |
| p53 deletion | 4 | 7 | 0.201 |
| RB1 deletion | 6 | 8 | 0.659 |
| 1q21 amplification | 8 | 7 | 1 |
| Del13q | 6 | 10 | 0.149 |
| IgH rearrangement | 5 | 6 | 1 |
| Transplant history, n (%) | 5 (22) | 5 (21) | 1 |
| Prior therapy lines, median (range) | 2 (1–9) | 3 (1–6) | 0.813 |
| PI | 10 (43) | 10 (42) | |
| IMiD | 17 (74) | 15 (62) | |
| PI + IMiD | 8 (35) | 7 (28) | |
| Extramedullary disease, n (%) | 3 (13) | 6 (25) | 0.461 |
| CRS grade, n (%) | 1 | ||
| 0 | 2 (9) | 2 (8) | |
| 1 | 12 (53) | 12 (50) | |
| 2 | 7 (30) | 8 (33) | |
| 3 | 2 (9) | 2 (8) | |
| Platelet, ×109/L | 139.74 ± 90.90 | 123.83 ± 81.72 | 0.531 |
| β2-microglobulin, mg/L | 5.94 ± 6.77 | 5.20 ± 3.37 | 0.705 |
| Albumin, g/L | 37.65 ± 5.70 | 36.21 ± 8.02 | 0.484 |
| Serum creatinine, mmol/L | 88.69 ± 116.21 | 74.10 ± 38.65 | 0.563 |
| Lactate dehydrogenase, U/L | 181.00 ± 52.82 | 332.26 ± 537.67 | 0.482 |
| Calcium, mmol/L | 2.25 ± 0.44 | 2.18 ± 0.18 | 0.478 |
- Abbreviations: APB, abnormal protein band; CRS, cytokine release syndrome; DS, Durie-Salmon staging system; Ig, immunoglobulin, IMiD, immunomodulatory drug; ISS, International Staging System; PI, proteasome inhibitor.
- a N = 25 with available data.
The detailed clinical profiles of the 23 patients with APBs are summarized in Table 2. Predominantly, the most prevalent isotype among these cases was IgG (21/23, 91.3%), with almost equal distribution of κ/λ light chains. Oligoclonality was found in two patients (Cases #3 and #17), and four patients (17.4%) showed biclonality of IgGλ and IgGκ; one patient had APBs of IgGλ and free κ light chain. Furthermore, three patients (13.0%) developed distinct types of APBs; seven patients (30.4%) had APBs that were the same as the original types but with different migration patterns or absence of recurrences. The median time of onset and duration of APBs after CAR-T infusion was 3.6 months (range, 1.2–13.7) and 5.8 months (range, 1.6–21.1), respectively. Notably, abnormal serum free κ light chain to free λ light chain ratio (fLC normal range, 0.26–1.65) was noted in nine patients with APBs (39.1%), all surpassing the upper limit of normal. Moreover, only two patients (12.5%) in the non-APB cohort exhibited aberrantly elevated fLC ratios (p = 0.013). Moreover, we found that six patients had serum protein electrophoresis results during APB persistence, and these values were below the detection threshold, with no detectable M spike.
| No. | Age, y | Sex | ASCT | Original M-protein |
APB type | Onset time, d | Duration, d | fLC ratio | ifLC, (mg/L) | dfLC (ifLC-ufLC) | Efficacy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | N | IgGλ | IgGλ | 112 | 618 | 0.691 | 9.898 | 3.059 | CR |
| 2 | 56 | M | N | λ | IgGλ | 410 | 194 | 1.394 | 18.400 | −7.200 | CR |
| 3a | 61 | F | N | λ light chain | IgGλ + IgGκ | 218 | 424 | 0.482 | 15.767 | 8.167 | CR |
| 4 | 46 | M | Y | IgGκ | IgGκ → polyclonal → free λ |
183 | 554 | 2.43b | 6.270 | 3.690 | CR |
| 5 | 44 | M | N | IgAκ | IgAκ → IgGκ |
70 | 306 | 0.29 | 16.950 | 12.034 | CR |
| 6 | 66 | M | N | λ light chain | IgGλ + IgGκ | 220 | 145 | 0.897 | 18.800 | 1.936 | CR |
| 7 | 51 | M | Y | IgGλ | IgGλ | 129 | 63 | 1.805b | 7.370 | −5.930 | CR |
| 8 | 61 | F | N | IgGλ | IgGλ + IgGκ | 112 | 251 | 0.585 | 26.633 | 11.053 | CR |
| 9 | 68 | M | N | IgGκ | IgGκ | 100 | 90 | 0.516 | 5.534 | −5.190 | CR |
| 10 | 52 | M | N | IgGκ | IgGλ | 100 | 635 | 5.213b | 110.00 | 88.900 | CR |
| 11 | 49 | F | Y | IgAκ | IgGκ | 137 | 63 | 1.098 | 10.687 | 0.954 | CR |
| 12 | 49 | M | N | IgGλ | IgGλ | 63 | 189 | 1.869b | 7.010 | −6.090 | CR |
| 13 | 63 | F | N | IgGκ | IgGκ | 129 | 245 | 0.969 | 10.735 | −0.343 | CR |
| 14 | 65 | M | Y | κ light chain | IgGκ | 42 | 114 | 2.549b | 11.965 | 7.270 | CR |
| 15 | 60 | F | N | IgGλ | IgGλ | 114 | 175 | 0.999 | 11.133 | 0.012 | CR |
| 16 | 51 | M | N | IgGλ | IgGλ | 65 | 175 | 0.762 | 11.605 | 2.762 | VGPR |
| 17a | 48 | M | N | IgGλ | IgGλ → IgGλ + free λ |
48 | 147 | 0.574 | 19.567 | 8.336 | CR |
| 18 | 62 | M | N | λ light chain | IgGκ | 35 | 49 | 4.239b | 2.130 | −6.900 | CR |
| 19 | 65 | M | N | IgGλ | IgGλ + free κ |
42 | 232 | 2.825b | 3.275 | −5.977 | VGPR |
| 20 | 67 | M | N | IgAκ | IgGκ | 83 | 64 | 2.039b | 5.280 | 2.690 | CR |
| 21 | 52 | M | N | IgGλ | IgGλ + IgGκ | 107 | 91 | 0.679 | 29.75 | 9.550 | CR |
| 22 | 64 | M | N | IgGλ | κ light chain | 70 | 264 | 2.354b | 4.800 | −6.500 | PR |
| 23 | 54 | F | Y | IgAκ | IgGκ | 206 | 70 | 1.018 | 7.200 | 0.13 | CR |
- Abbreviations: APB, abnormal protein band; ASCT, autologous stem cell transplant; CR, complete response; dfLC, difference between involved and uninvolved free light chains; fLC, free light chain; ifLC, involved free light chains; Ig, immunoglobulin; PR, partial response; VGPR, very good partial response; M, male; F, female; Y, Yes; N, No.
- a Oligoclonality.
- b Abnormal fLC ratio.
Correlation of APBs with response rate
Patients who developed APBs exhibited a markedly superior response to LCAR-B38M therapy in contrast to those without APBs (p = 0.015; Figure 1). All patients with APBs achieved at least partial remission compared with an 83% ORR observed in patients without APBs. Among the APB cohort, 20 out of 23 patients (87%) attained complete remission, with 15 patients (65%) achieving MRD-negative CR. In contrast, in the non-APB group, the rates of CR and MRD-negative CR were 58% and 54%, respectively.

Correlation of APBs with prognosis
The median OS of patients who developed APBs was significantly prolonged compared to patients without APBs (86.1 vs. 22.1 months; p = 0.017; Figure 2A). In addition, patients who developed APBs post CAR-T showed a longer PFS than those without APBs (60.3 vs. 8.8 months; p = 0.0003; Figure 2B).
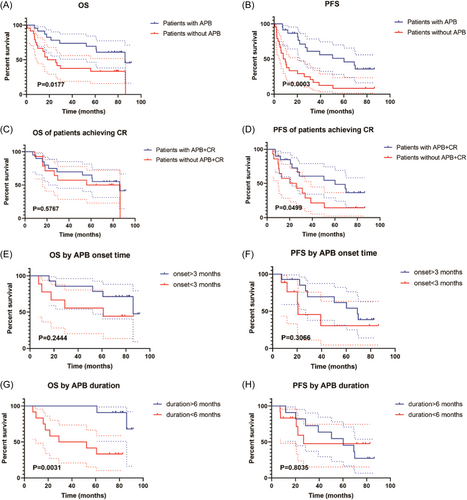
Given the correlation between APB development and improved treatment response, we conducted a comparative analysis of OS and PFS specifically among patients who achieved CR. Results showed that the median OS of patients who achieved CR with or without APBs was not significantly different (86.1 vs. 71.3 months; p = 0.5767; Figure 2C), while the median PFS of patients with APBs who achieved CR remained significantly longer than those without APBs (60.3 vs. 23.0 months; p = 0.0499; Figure 2D).
To further determine whether the presence of APBs was an independent prognostic factor, we conducted univariate and multivariate Cox regression analyses (Tables 3 and 4). Results showed that CAR-T efficacy and platelet count were independent prognostic factors for OS, and the appearance of APBs, CAR-T efficacy, platelet count, and β2-microglobulin were independent prognostic factors for PFS.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.000 (0.954–1.048) | 0.991 | ||
| EMD | 1.792 (0.748–4.293) | 0.19 | ||
| APB | 0.397 (0.180–0.874) | 0.022 | 0.579 (0.182–1.836) | 0.353 |
| CAR-T efficacy | 0.001 | 0.030 | ||
| CR | 1 (Reference) | 1 (Reference) | ||
| VGPR | 0.644 (0.085–4.860) | 0.67 | 3.955 (0.385–40.615) | 0.247 |
| PR | 2.727 (0.906–8.202) | 0.074 | 1.352 (0.284–6.436) | 0.705 |
| NR | 14.921 (4.150–53.653) | <0.0001 | 11.980 (2.300–62.406) | 0.003 |
| Platelet, ×109/L | 0.990 (0.983–0.997) | 0.003 | 0.987 (0.976–0.998) | 0.017 |
| β2M, mg/L | 1.099 (1.019–1.185) | 0.014 | 1.161 (0.831–1.623) | 0.382 |
| Serum creatinine, mmol/L | 1.005 (1.001–1.008) | 0.014 | 0.999 (0.983–1.016) | 0.931 |
| LDH, U/L | 1.001 (1.000–1.001) | 0.093 | ||
- Note: Bold values indicate significant difference.
- Abbreviations: APB, abnormal protein band; CAR, chimeric antigen receptor; CI, confidence interval; CR, complete response; EMD, extramedullary disease; HR, hazard ratio; LDH, lactate dehydrogenase; NR, no response; PR, partial response; VGPR, very good partial response; β2M, β2-microglobulin.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 0.994 (0.952–1.036) | 0.764 | ||
| EMD | 2.069 (0.928–4.617) | 0.076 | ||
| APB | 0.316 (0.155–0.645) | 0.002 | 0.124 (0.038–0.408) | 0.001 |
| CAR-T efficacy | 0.003 | 0.001 | ||
| CR | 1 (Reference) | 1 (Reference) | ||
| VGPR | 1.206 (0.284–5.126) | 0.8 | 3.427 (0.762–15.416) | 0.108 |
| PR | 5.324 (2.088–13.573) | <0.0001 | 5.132 (1.325–19.875) | 0.018 |
| NR | 4.436 (0.944–20.854) | 0.059 | 57.272 (5.650–580.550) | 0.001 |
| Platelet, ×109/L | 0.994 (0.989–1.000) | 0.037 | 0.992 (0.985–0.999) | 0.026 |
| β2M, mg/L | 1.080 (1.014–1.150) | 0.016 | 1.146 (1.058–1.241) | 0.001 |
| Serum creatinine, mmol/L | 1.003 (1.000–1.007) | 0.081 | ||
| LDH, U/L | 1.001 (1.000–1.001) | 0.067 | ||
- Note: Bold values indicate significant difference.
- Abbreviations: APB, abnormal protein band; CAR, chimeric antigen receptor; CI, confidence interval; CR, complete response; EMD, extramedullary disease; HR, hazard ratio; LDH, lactate dehydrogenase; NR, no response; PFS, progression-free survival; PR, partial response; VGPR, very good partial response; β2M, β2-microglobulin.
Then, we investigated the impact of onset time and duration of APBs on prognosis. Median OS (86.1 vs. 61.1 months; p = 0.2444; Figure 2E) and PFS (69.2 vs. 20.7 months; p = 0.3066; Figure 2F) showed no significant difference between patients who developed APBs >3 and <3 months post-CAR-T infusion. Notably, a longer duration of APBs (>6 vs. <6 months) was associated with significantly superior OS (not reached vs. 40.7 months; p = 0.0031; Figure 2G), while no significant difference was found in PFS between the two groups (60.3 vs. 26.9 months; p = 0.8085; Figure 2H).
Correlation of APBs with immune reconstitution
The occurrence of APBs has been recognized in previous studies as a marker of immune reconstitution.4, 21 Therefore, we investigated the correlation of APBs with peripheral immune cells as well as immunoglobin levels (Table 5). Our findings revealed that patients who developed APBs exhibited significantly higher counts of white blood cells, neutrophils, and lymphocytes compared to those who did not develop APBs at both 3 and 6 months post CAR-T treatment, with no significant difference in monocyte count between the two groups. To further explore the correlation of APBs with lymphocytes, we detected T-cell subtypes and NK cells from peripheral blood samples of patients after LCAR-B38M infusion. However, no significant difference was observed between the two groups in the percentage of CD4+ T cells, CD8+ T cells, T-helper cells, Treg cells, cytotoxic T cells, and NK cells of peripheral blood (Figure 3A–F).
| APB | No APB | p Value | |
|---|---|---|---|
| WBC, ×109/L | |||
| 3 mo | 5.80 ± 2.79 | 3.75 ± 1.12 | 0.020 |
| 6 mo | 6.54 ± 3.10 | 4.44 ± 1.84 | 0.032 |
| 12 mo | 5.40 ± 1.95 | 5.43 ± 2.40 | 0.962 |
| Neutrophil, ×109/L | |||
| 3 mo | 3.16 ± 2.05 | 1.99 ± 0.80 | 0.022 |
| 6 mo | 4.07 ± 2.55 | 2.61 ± 1.43 | 0.050 |
| 12 mo | 3.12 ± 1.30 | 3.05 ± 1.94 | 0.888 |
| Monocyte, ×109/L | |||
| 3 mo | 0.46 ± 0.25 | 0.33 ± 0.15 | 0.082 |
| 6 mo | 0.41 ± 0.17 | 0.35 ± 0.16 | 0.310 |
| 12 mo | 0.34 ± 0.13 | 0.76 ± 1.51 | 0.732 |
| Lymphocyte, ×109/L | |||
| 3 mo | 2.02 ± 1.37 | 1.33 ± 0.68 | 0.047 |
| 6 mo | 1.95 ± 0.72 | 1.45 ± 0.77 | 0.045 |
| 12 mo | 1.78 ± 0.79 | 1.50 ± 0.58 | 0.267 |
| Recovery rate of, number (%)a | |||
| IgG | 16 (73) | 5 (23) | 0.001 |
| IgA | 15 (68) | 5 (23) | 0.002 |
| IgM | 20 (91) | 10 (45) | 0.001 |
| Recovery time, median (range), d | |||
| IgG | 224.5 (38–867) | 302 (117–1405) | 0.350 |
| IgA | 586 (192–1006) | 954 (302–1405) | 0.087 |
| IgM | 239 (69–1315) | 239.5 (155–585) | 0.598 |
- Note: Bold values indicate significant difference.
- Abbreviations: APB, abnormal protein band; Ig, immunoglobulin; WBC, white blood cell.
- a N (APB) = 22; N (non-APB) = 22.

Additionally, the recovery of IgG, IgA, and IgM was significantly more pronounced in patients with APBs compared to those without APBs after CAR-T treatment (p = 0.001, 0.002, and 0.001, respectively). However, the time required for immunoglobulin recovery was not significantly different between the two groups (p = 0.350, 0.087, and 0.598, respectively).
DISCUSSION
Although the appearance of APBs in patients after treatment of HSCT or novel agents has been widely reported, there is currently a lack of data available regarding the occurrence and clinical significance of APBs in patients with MM receiving BCMA-targeted CAR-T cell therapy.
As previously reported, the frequency of APBs after HSCT or novel agent treatment varies from 6% to 73%.11, 12 In this study, we observed that 23 (48.9%) of 47 patients developed APBs after LCAR-B38M treatment, an occurrence rate comparable to or slightly higher than that seen after HSCT or novel agents, and surpassing that observed after conventional chemotherapy.8, 14, 20, 22 There are several possible reasons for the variable range of APB incidence, such as demographic and disease heterogeneity, the use of different definitions of APBs, different treatments, the sensitivity and frequency of serum immunofixation electrophoresis, and follow-up time. Since the appearance of APBs was closely associated with deeper remission, it is not surprising that patients treated with CAR-T cells showed a relatively high incidence of APBs. In addition, the most frequent heavy chain of APBs in the present study was IgG, with almost equal κ/λ distribution, which is consistent with previous studies.9, 11 Notably, 30.4% of patients developed the same isotype of APBs as the primary M-protein in our study, which is similar to the reported incidence of 27.6% by Schmitz et al.20 The high frequency of this phenomenon highlighted the clinical importance of distinguishing it from relapse or disease progression. Furthermore, despite initially being regarded as transient,10 increasing evidence has demonstrated that APBs could persist for years. Larrea et al.22 reported the persistence of APBs to be as long as 9.4 years. In the present study, we observed that the longest duration of APBs after CAR-T infusion was 21.1 months, suggesting the possibility of long-term persistence.
It has been widely recognized that APBs are more likely to appear in patients with a high degree of response to antimyeloma treatment.5, 11, 23 In our cohort, all patients with APBs achieved a partial response or better, compared to 83% of patients without APBs. Among patients with APBs, 87% achieved CR, with 65% achieving MRD-negative CR, while patients without APBs had lower rates of CR (58%) and MRD-negative CR (54%). Furthermore, the appearance of APBs has been identified as a benign sign of improved prognosis. We also observed that patients with APBs had significantly longer OS and PFS compared to those without (OS, 86.1 vs. 22.1 months; PFS, 60.3 vs. 8.8 months). In addition, we found that patients with APBs lasting longer than 6 months had a prolonged OS, rather than PFS, which is consistent with a previous study.24 Intriguingly, patients who achieved CR and developed APBs had significantly longer PFS, rather than OS, than those who achieved CR but did not develop APBs. Consistently, the appearance of APBs was an independent protective factor for PFS, rather than OS. Fujisawa et al.14 and Tovar et al.24 also reported that patients with APBs showed better PFS and OS than those without APBs, but ABPs were not associated with prolonged PFS or OS in patients who achieved CR. However, the prognostic significance of APBs is still controversial. Some research failed to show improved prognosis in patients with APBs,6, 7, 11, 15 while other studies demonstrated APBs as an independent prognostic factor for both PFS and OS.12, 13, 20, 23
There is no evidence linking the appearance of APBs with the emergence of a new malignant myeloma clone, or disease recurrence.5, 16 In our study, all patients with APBs showed no signs of relapse or progression during the period of APB persistence, and APB levels were below the detection threshold, with no detectable M spike, which is consistent with previous research.4, 20 However, the origin of APBs and the mechanisms underlying their prognostic role remain elusive. One widely recognized explanation for the presence of APBs is B-cell immune reconstitution. Notably, we observed that patients with APBs demonstrated enhanced recovery rates of IgG, IgA, and IgM compared to those without APBs, aligning with findings from Mitus et al.10 that reported higher immunoglobin levels in patients with APBs. In addition, we found that patients with APBs were more likely to have a higher serum-free kappa light chain to free lambda light chain ratio, even in patients developing λ light chain type of APBs. This discordance between APB patterns and the overproduction of κ light chain has also been reported by other studies.14, 22, 25, 26 The recovery of immunoglobulin and overproduction of κ light chain most possibly reflects the recapitulation of normal B-cell ontogeny.
Furthermore, the presence of APBs could also be associated with T cell–B cell cooperation. Schmitz et al observed that T-cell depletion caused a lower occurrence of post-HSCT APBs.20 Aina et al. reported27 that the most frequent overrepresented T-cell receptors (TCRs) were Vβ 8 and 13.1 in patients with APBs, while TCR Vβ 13.2 was predominant in patients without APBs. In this study, we found that patients with APBs showed higher counts of white blood cells, neutrophils, and lymphocytes compared to those without APBs at 3 and 6 months post CAR-T treatment. Subsequently, we explored the proportion of T-cell subtypes and NK cells in peripheral blood; however, no significant difference was observed between the two groups in the percentages of CD4 T cells, CD8 T cells, T-helper cells, Treg cells, cytotoxic T cells, and NK cells. Ye et al.21 investigated the immune signature of the bone marrow in patients with MM post-HSCT. They observed a correlation between APBs and lower CD8 T-cell percentages along with a trend toward B-cell recovery, while no significant difference was noted in NK cells. Our findings, along with previous research, consistently imply a potential connection between APBs and lymphocytes.
This study had several limitations. First, its retrospective nature introduced inherent bias, and infrequent serum immunofixation electrophoresis and short follow-up time of a few patients potentially led to an underestimation of APB occurrence. Second, our investigation solely focused on peripheral samples, omitting the analysis of immune signatures within bone marrow samples. Third, while protein quantitation results for APBs are often below the detection threshold, we did not quantify APBs of all patients in our study.
In conclusion, the presence of APBs in patients with MM following CAR-T treatment was correlated with achieving a deeper remission and a more favorable prognosis. Patients with APBs demonstrated more frequent recovery of immunoglobulins and lymphocytes, indicating a strong humoral response and subsequent immune reconstitution.
ACKNOWLEDGMENTS
We especially thank all the patients and their families who participated in the study. Editorial support was provided by Eloquent Scientific Solutions.
AUTHOR CONTRIBUTIONS
Rui Liu: Methodology, formal analysis, data curation, original draft. Gongzhizi Gao: Methodology, formal analysis, data curation, validation, investigation. Hongli Chen: Methodology, formal analysis, writing—review and editing. Ruijun Dong: Data curation, validation, investigation. Wanggang Zhang: Investigation, writing—review and editing. Wanhong Zhao: Investigation, writing—review and editing. Jie Liu: Investigation, writing—review and editing. Jianli Wang: Investigation, writing—review and editing. Bo Lei: Investigation, writing—review and editing. Baiyan Wang: Investigation, writing—review and editing. Jiali Liu: Methodology, investigation. Xuezhu Xu: Validation, investigation. Zujie Lin: Validation, investigation. Ruoyu Yang: Validation, investigation. Yiwen Wang: Validation, investigation. Aili He: Conceptualization, supervision, writing—review and editing, project administration. Fangxia Wang: Conceptualization, supervision, writing—review and editing. Ju Bai: Conceptualization, supervision, writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional independent ethics committee of the Second Affiliated Hospital of Xi'an Jiaotong University (No. 2016002). All patients gave written informed consent. All authors read and approved the final manuscript and consent to publication.
FUNDING
This work was supported by the Science and Technology Program of Shaanxi Province (Grant number: 2018ZDXM-SF-039, Aili He).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




