Sevuparin strongly reduces hepcidin expression in cells, mice, and healthy human volunteers
Graphical Abstract
Abstract
Hepcidin is an essential regulator of systemic iron availability mediating both iron uptake from the diet and its release from body stores. Abnormally high hepcidin levels resulting from inflammation in chronic diseases cause iron restriction with the onset of anemia. Restoring physiological levels of hepcidin could contribute to ameliorating anemia in these patients. Heparin derivatives are known to suppress hepcidin expression acting on the BMP/SMAD pathway. The novel heparin derivative sevuparin, modified to markedly reduce its anticoagulant activity, is proposed as a promising hepcidin antagonizing strategy. Sevuparin was tested for its anti-hepcidin properties in vitro in HepG2 cells, in vivo in mice, and in healthy volunteers. Sevuparin strongly suppressed basal, BMP6-, and IL6-dependent hepcidin expression in HepG2 cells in a dose- and time-dependent manner, modulating the essential BMP6/SMAD cascade. These effects were evident in C57BL/6J mice after intravenous injection of a single dose of sevuparin (20 mg/kg) with a 70% reduction of hepcidin mRNA. Remarkably, similar effects were observed in healthy volunteers following single subcutaneous doses at 3, 6, and 9 mg/kg with 40%–50% suppression at 3 and 6 mg/kg and 72% at 9 mg/kg. Moreover, sevuparin was able to reduce hepcidin upregulation in a mouse model of acute inflammation induced by LPS, also showing an amelioration of the inflammatory markers. Combined with its excellent safety profile, these data suggest a role for sevuparin in treating high-hepcidin disorders.
INTRODUCTION
Iron is an essential element for life, involved in many vital biological processes. Iron is, however, highly reactive and able to induce cell damage if present in excess and its homeostasis must be tightly controlled. At the systemic level, iron is regulated by hepcidin, a small peptide produced by the liver in response to body iron increase or inflammatory stimuli. Hepcidin binds and inhibits the only known iron exporter, ferroportin (FPN), either by inducing its internalization and degradation1 or by blocking the FPN central channel.2 The inhibition of FPN activity blocks dietary iron absorption and iron release from macrophages and hepatocytes, which reduces systemic iron availability. Hepcidin expression is transcriptionally regulated by iron status, inflammation, erythropoiesis, and hypoxia. The iron-dependent regulation involves the liver sinusoidal endothelial cells (LSEC) which monitor the circulating and tissue iron status and release bone morphogenetic protein (BMP) 2 and 6 in response.3-5 The BMPs bind specific receptors on hepatic cells called type I (ALK2 and 3) and II receptors (BMPR2 or ACTR2a) as well as the co-receptor hemojuvelin (HJV).6 BMP receptor binding leads to the phosphorylation of SMAD1/5/8, which associates with SMAD4, and subsequently translocates as a complex to the nucleus to trigger hepcidin expression.7 BMP6 is regulated by iron, through the antioxidant-responding NRF2 transcriptional factor, whereas BMP2 is present in the basal state and is less sensitive to iron.8 Inflammatory stimuli act via macrophages that release IL6 which binds its receptor to activate the JAK2/STAT3 pathway9 and requires a functional BMP/SMAD pathway to act synergistically on hepcidin expression.10 Iron deficiency, sustained erythropoiesis, and hypoxia suppress hepcidin expression, partially acting via erythroferrone (ERFE). ERFE is a hormone released by erythroid cells under the stimulation of erythropoietin (EPO) and suppresses hepcidin11 by sequestering BMP2/6 from BMPR, leading to higher iron intake to favor hemoglobin synthesis.12, 13 Another major negative regulator is matriptase-2 (MT2), a transmembrane serine protease, which cleaves HJV with the consequent inhibition of hepcidin.14 Recently, it has been reported that MT2 also cleaves BMPR ALK2, ALK3, ACTR2a, BMPR2, Hereditary Hemochromatosis Gene (HFE), and Transferrin receptor 2 (TfR-2)15 in the cell membrane, revealing a complex function.
Dysregulated hepcidin occurs in several iron-related disorders,16 ranging from iron overload such as in hemochromatosis to iron deficiency anemias. Iron deficiency is the most common nutritional disorder in the world, and has well-known sequelae including fatigue, disrupted metabolism, developmental disturbances, and cardiac morbidity to name but a few. Anemia is often associated with inflammation or chronic disease, such as inflammatory bowel disease, autoimmune disorders (e.g., celiac disease and rheumatoid arthritis), cancer, infection, and chronic kidney disease. This so-called “anemia of chronic disease” (ACD) or “anemia of inflammation” (AI) is more prevalent in patients with advanced disease and in those responding poorly to standard anemia therapy.17 In particular, the forms of anemia caused by high hepcidin levels (either from genetic mutations or chronic disease) are poorly responsive to iron supplementation due to poor iron absorption from the duodenum and its release from body iron stores provoked by high levels of hepcidin. To effectively treat these types of anemia, a key factor is restoring the physiological levels of hepcidin acting on the essential BMP6/SMAD pathway.
We have previously shown that heparin is a strong inhibitor of hepcidin expression by interfering with the BMP/SMAD pathway.18-20 Since neither unfractionated nor low-molecular-weight heparins (LMWHs) are suitable therapeutics for chronic therapy on account of their anticoagulant activity, we also investigated non-anticoagulant heparin derivatives such as glycol-split or oversulfated heparins and found that some of these derivatives inhibit not only basal but also inflammation-induced hepcidin expression in vivo and that sulfation at sites 2O and 6O and a MW above 7 kDa are necessary for this functionality.21 This property appears to be specific to heparins because other glycosaminoglycans with a different disaccharide backbone and lacking highly sulfated regions, such as dermatan sulfate (DS) and chondroitin sulfate (CS), showed a marginal effect on hepcidin expression.18 Sevuparin is a novel heparin derivative in which the anti-thrombin binding domain has been modified resulting in abrogated anti-factor II and anti-factor X activities. Sevuparin is a polydisperse mixture of heparin chains with markedly attenuated anti-coagulant properties and an average molecular weight range of 6.5 to 9.5 kDa. The predominant disaccharide unit comprises N-sulfo-6O-sulfo-glucosamine and iduronic-2O sulfonic acid, lacking the 3-O sulfate group, thus impairing antithrombin binding.22 Sevuparin retains a limited effect on the activated partial thromboplastin time (APTT) activity, which is likely attributable to heparin co-factor II activity.23 The clinical significance of this effect on APTT is doubtful in the absence of effects on other coagulation parameters. Sevuparin is currently being developed as a treatment targeting systemic inflammation disorders such as sepsis but has also been tested in Phase II trials of acute vaso-occlusive crisis in sickle cell disease23 and falciparum malaria infection.22 At the time of this report, 209 human subjects have been exposed to sevuparin by the intravenous and subcutaneous routes without any specific safety or tolerability signals except for mild injection site pain following subcutaneous administration. In the present work, we report on the short-term, or acute, effect of sevuparin in inhibiting hepcidin in vitro in three hepatic cell lines, HepG2, Hep3B, and Huh7 cells, and its translation in vivo both to mice (in healthy conditions and in the presence of acute inflammation induced by Lipopolysaccharides (LPS) injection) and in healthy human volunteers.
MATERIALS AND METHODS
Cell culture and pharmacological treatments
The human hepatoma cell line HepG2 and Hep3b (American Type Culture Collection) were cultured in minimum essential medium (MEM; Gibco, Life Technologies) whereas the Huh7 cell line was cultured in Dulbecco's modified Eagle's medium (DMEM, Life Technologies). Both media were supplemented with 6 mM l-glutamine (Gibco, Life Technologies), 110 mg/L sodium pyruvate (Gibco, Life Technologies), 40 mg/L gentamicin (Gibco, Life Technologies), and 10% endotoxin-free fetal bovine serum (FBS, Gibco) at 37°C under an atmosphere of 5% CO2/95% air.
The pharmacological treatments are explained in detail in the Supporting Information.
Healthy mice
C57BL/J6 (Envigo RMS S.r.l.) mice were maintained on a 12-h light/12-h dark cycle and fed with water and standard rodent chow ad libitum. All animals were housed in vivaria approved by the Association for Assessment and Accreditation of Laboratory Animal Care in the University of Brescia (Italy) following standards and procedures approved by the Institution Animal Care and Use Committee for ethical use of animals in experiments. Animal experiments were approved by the Italian Ministry for Health under protocol number 616/2020 and the details of the experiments are reported in the Supporting Information.
Human subjects
Subject details are summarized in Supporting Information S1: Table 1. The study was conducted in accordance with Good Clinical Practice guidelines and the ethical principles outlined in the Declaration of Helsinki. The study (Clinical Trial Registration no: NCT03853421) was conducted at the Clinical Pharmacology of Miami (CPMI) facility in Miami, Florida, USA, in accordance with the FDA and International Conference on Harmonization (ICH) guidelines on current Good Clinical Practices (GCPs). Written informed consent was obtained from all participating human subjects. The protocol (PKSC01) was approved on January 4, 2019, by the IntegReview IRB, Austin, Texas. Written informed consent was obtained from all participating subjects.
Clinical study design
This was a randomized, double-blind, placebo-controlled, sequential cohort, single escalating dose study to explore the safety, tolerability, pharmacokinetics, and pharmacodynamics of sevuparin. The study comprised three sequential subcutaneous dose cohorts (3, 6, and 9 mg/kg sevuparin); details are reported in the Supporting Information.
Clinical analytical methods
Clinical laboratory evaluations (serum/plasma biochemistry and hematology) were performed according to clinical practice standards by Eccolab, while study-specific laboratory measures are further described in the Supporting Information.
Safety
Safety assessments included treatment-emergent adverse events (TEAEs), ECGs, vital signs, clinical laboratory evaluations (serum biochemistry, hematology, HIT), urinalysis, and physical examinations. All adverse events were coded using the Medical Dictionary of Regulatory Activities (MedDRA, Version 22.0) and listed. Treatment-emergent AEs were tabulated by categorical information of interest and summarized. Laboratory data were also summarized.
Statistics
Details are reported in Supporting Information.
RESULTS
Cellular assays
To verify the effect of sevuparin in suppressing basal and BMP6-stimulated hepcidin expression in vitro, HepG2 cells were treated with different concentrations of sevuparin (0.12–0.4–1.2–3.6–11–33 µg/mL) for 6 h in the presence or absence of BMP6 (25 ng/mL) (Figure 1). The effect of sevuparin was evaluated in comparison with the same concentrations of the glycol-split heparin derivative (RO, previously characterized as a potent hepcidin inhibitor11) and with other types of glycosaminoglycans without highly sulfated regions and already known to show little or no effect in suppressing hepcidin such as dermatan sulfate (DS) and chondroitin sulfate (CS) (Figure 1A,B) or 2O and 6O-desulfated heparin (less effective in suppressing hepcidin expression, as previously reported11, 14). The cells were harvested and analyzed for the level of hepcidin and ID1 mRNA (by qPCR) (Figures 1A–D and S2) and for the levels of pSMAD5/SMAD5 (by western blotting) (Figures 1E,F and S1A,B).
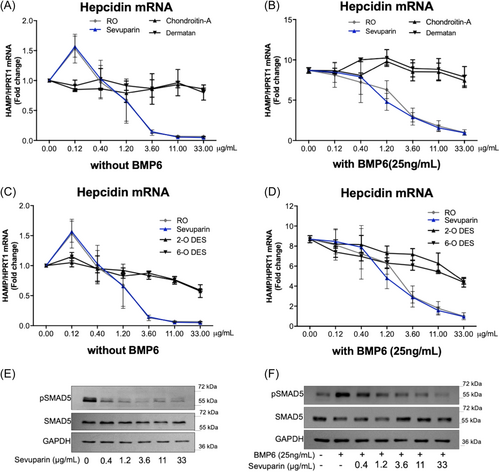
Sevuparin inhibited hepcidin (HAMP) mRNA transcription in a concentration-dependent manner, showing 50% inhibition of HAMP mRNA level at the 1.2 µg/mL concentration, and reaching a plateau of inhibition of about 95% at 11 µg/mL (Figure 1A,C). Sevuparin was as potent as RO-heparin since no significant difference between their effect was detected at any experimental point; the calculated IC50 values were 1.21 ± 1.03 and 1.05 ± 0.47 µg/mL for sevuparin and RO-heparin, respectively.
BMP6 was used to trigger hepcidin expression via BMP/SMAD signaling to test sevuparin-mediated suppression. BMP6 (25 ng/mL) induced a nine-fold increase of hepcidin mRNA compared to basal levels, (indicated as 0.00 in Figure 1F) and sevuparin blocked this upregulation in a concentration-dependent manner; specifically, the 1.2 µg/mL concentration reduced the BMP6-induced HAMP mRNA levels by approximately 50% compared to the BMP6-stimulated level, while a concentration of 33 µg/mL completely restored the baseline level (basal hepcidin level without BMP6, 1.0 value) (Figure 1B). The same results were obtained in treating two other human hepatocellular cell lines, Huh7 and Hep3B, with different doses of sevuparin in the absence or presence of BMP6 stimulation with a strong reduction of hepcidin mRNA (Figure S3).
In HepG2 cells, sevuparin was as potent as RO-heparin in inhibiting hepcidin expression also in the BMP6-stimulation setting (Figure 1B,D).
Conversely, CS and DS, with a different disaccharidic backbone and lacking highly sulfated regions compared to RO-heparin and sevuparin, showed only a marginal hepcidin reduction in HepG2 cells at the highest tested doses (5%–10% reduction at 33 µg/mL) in both the basal and BMP6-stimulated conditions.
Similarly, the 2O- and 6O-desulfated heparins showed a lower potency in inhibiting hepcidin as compared to sevuparin and RO-heparin, with an mRNA reduction of about 40% compared to the untreated cells (0.00), confirming the equal contribution of 2O- and 6O-sulfation (present in sevuparin and RO heparin) to the hepcidin inhibition both under basal and BMP6-stimulated conditions (Figure 1C,D).
As previously demonstrated,18 heparins can suppress hepcidin expression interfering with the BMP/SMAD pathway; the current study confirms that the same is true for sevuparin, which significantly reduced pSMAD5 both under basal and BMP6-stimulated conditions (Figures 1E,F and S1A,B).
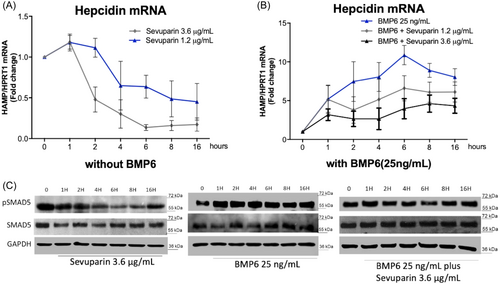
The inhibition of hepcidin in HepG2 cells by sevuparin at a concentration of 3.6 µg/mL was evident starting from 2 h posttreatment (with a reduction of about 50%), reaching a maximum effect at 6 h (90% reduction) and was still present at 16 h (Figure 2A). Hepcidin suppression was associated with a reduction of pSMAD5 (Figures 2C and S1C). Even in the presence of BMP6, the effect of sevuparin was rapid, with a significant suppression of hepcidin evident after 2 h of treatment which was maintained through to 16 h (Figure 2B). Already, the lower 1.2 µg/mL concentration (blue line in Figure 2A and light gray in Figure 2B) provided a time-dependent reduction of hepcidin mRNA with a similar trend compared to 3.6 µg/mL albeit with a lower magnitude.
To confirm the activity of sevuparin in suppressing pSMAD, we analyzed the expression of ID1, whose expression depends on the BMP/SMAD pathway. Sevuparin suppressed the basal and BMP6-stimulated ID1 expression in a concentration-dependent manner similar to RO-heparin (Figure S2A,B) and with a maximum effect between 2 and 6 hours both under basal and BMP6-stimulated conditions (Figure S2C,D), even if to a minor extent than for hepcidin. The same observations were made in Huh7 and Hep3B cells (Figure S3, C and D for Huh7 and G and H for Hep3B).
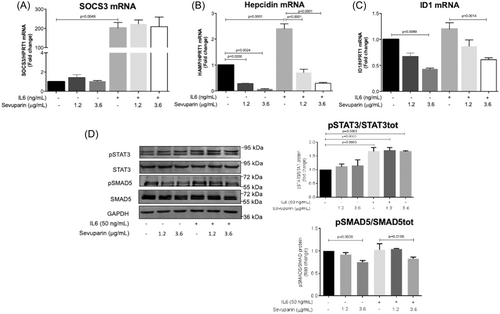
During inflammation, IL6 induces hepcidin expression synergistically to BMP/SMAD signaling by activation of the JAK/STAT3 pathway. To mimic acute inflammation in vitro, HepG2 cells were treated for 6 h with IL6 (50 ng/mL) at two concentrations of sevuparin (1.2 and 3.6 µg/mL) (Figure 3). IL6 was shown to activate SOCS3, the expression of which is triggered by the IL6/STAT3 pathway and is considered a marker of inflammation, confirming the intended effect of the IL6 treatment and the absence of sevuparin interference (Figure 3A). This was also supported by the IL6 induction of pSTAT3, which was not altered by the co-treatment with sevuparin (Figure 3D). On the other hand, IL6 increased the HAMP mRNA level by about 2.8-fold while sevuparin completely suppressed this stimulus, dampening IL6-induced hepcidin under basal conditions (black histogram in Figure 3B) and suppressing only pSMAD5 (Figure 3D). ID1 mRNA showed the same behavior as hepcidin (Figure 3C).
In vivo mouse studies
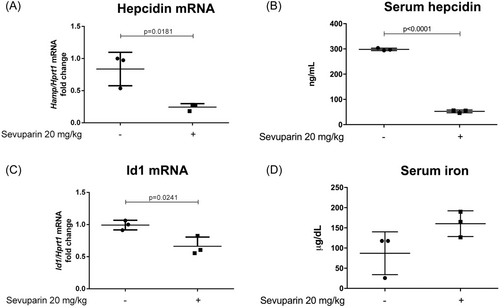
To assess the anti-hepcidin activity of sevuparin in vivo, healthy C57BL/6J mice (three mice per experimental group) were given single intravenous doses of saline or sevuparin (20 mg/kg); animals were then sacrificed 6 h post-dose. The treatment did not cause any adverse effects. The liver hepcidin and Id1 mRNA levels were evaluated by qPCR. Sevuparin treatment resulted in a 70% reduction of hepcidin mRNA (Figure 4A) and about a 40% reduction of Id1 expression (Figure 4C). The decrease in hepcidin mRNA (Figure 4A) after sevuparin treatment was supported by a similar reduction of serum hepcidin (Figure 4B) and a concomitant increase of serum iron (Figure 4D). These results suggest that sevuparin can suppress hepcidin not only in vitro but also in vivo.
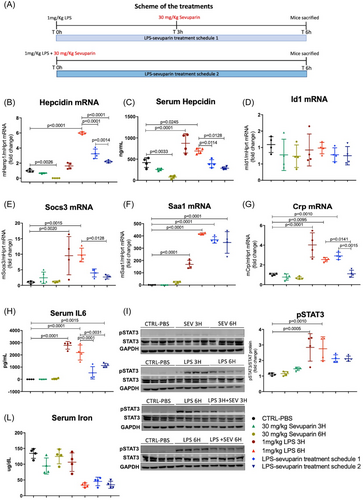
To assess the effect of sevuparin in suppressing hepcidin in acute inflammation, mice were treated intraperitoneally with LPS (1 mg/kg), a dose that is known to cause an upregulation of hepcidin through IL6 induction, with a maximum effect at 6 h after injection. Subcutaneous treatment with sevuparin (30 mg/kg) was administered in combination with LPS (to prevent hepcidin induction, LPS–sevuparin treatment schedule 2, Figure 5A) and after 3 h from LPS (to suppress the inflammation-dependent hepcidin upregulation, already present after 3 h of LPS of about two-fold, LPS–sevuparin treatment schedule1, Figure 5A) (Figure 5B brown circle). After 6 h of LPS, mice were sacrificed for analysis. Sevuparin caused a reduction of basal mRNA hepcidin levels of about 30% in 3 h (Figure 5B green triangle) and 95% in 6 h (Figure 5B green circle), whereas LPS only increased hepcidin expression about six-fold at 6 h (Figure 5B red triangle). Sevuparin in combination with LPS significantly reduced LPS-dependent hepcidin upregulation from a six-fold change (Figure 5B red triangle, only LPS) to a two-fold change (Figure 5B dark blue inverted triangle, LPS plus sevuparin) compared to the control group (Figure 5B black circle, phosphate-buffered saline only). This inhibition was also significant when sevuparin was injected at 3 h (Figure 5B light blue vs. red triangles) with a 50% reduction compared to the LPS group for 6 h (Figure 5B). The hepcidin mRNA levels in the experimental groups were confirmed by serum hepcidin which showed a similar trend (Figure 5C). As expected, Id1 was not affected by LPS while sevuparin reduced its expression even if to a minor extent and with some variability within the groups (Figure 5D). The mRNA level in the liver of three markers of inflammation (Socs3, Saa1, and Crp1) were upregulated after LPS injection, as expected, but was reduced after sevuparin treatment (Figure 5E–G) in line with a significant reduction of serum IL-6 and a slight decrease of pSTAT3 after sevuparin treatment compared to the LPS-treated group (Figure 5H and I, respectively). Serum iron content, strongly reduced after 6 h of LPS, showed a tendency to increase following treatment with sevuparin, although a single dose of sevuparin may not be sufficient to induce relevant increases in serum iron (Figure 5L).
Human volunteers
The study enrolled 24 subjects at one investigational site. All subjects completed the study with the exception of one subject in the 6 mg/kg dose group who discontinued early and lost to follow-up. There were no protocol deviations considered to have affected conclusions regarding the pharmacokinetics, safety, or tolerability of the study medication. Most subjects were female (14 of 24 subjects; 58.3%), White (20 of 24 subjects; 83.3%), and Hispanic/Latino (23 of 24 subjects; 95.8%). The mean age of the study subjects was 48.5 years (range of 19–65 years). The mean BMI of the study subjects was 26.1 kg/m2 (range of 19.1–29.0 kg/m2). Similar demographic profiles were observed between treatment groups (see Supporting Information S1: Table 1 for baseline demographics and subject disposition).
Pharmacokinetic results
Following subcutaneous administration, sevuparin was absorbed with a median time of maximum plasma concentration (tmax) of 2.0–3.0 h. The maximal sevuparin concentration (Cmax), area under the plasma concentration–time curve to the last detectable concentration (AUC0–tlast), and area under the plasma concentration–time curve to infinity (AUC0–∞) increased in a dose-related manner; over the dose range, Cmax increased in a dose-proportional manner (slope 1.07 [90% confidence interval [CI]: 0.874, 1.263]). AUC0–tlast (slope 1.57 [90% CI: 1.319, 1.824]), and AUC0–∞ (slope 1.27 [90% CI: 1.037, 1.512]) appeared to increase in a slightly greater than dose-proportional manner. Sevuparin was eliminated with mean terminal half-life (t½) values ranging from 6.5 to 10.5 h. The oral clearance of sevuparin (CL/F) was greater at the lower dose (6.22 L/h) compared to the higher doses (4.15 and 4.67 L/h), which may in part be attributable to plasma concentrations being measurable to 8 h in only four of the six subjects. The volume of distribution (Vd/F) ranged from 43.8 to 61.1 L and did not appear to show dose dependency. Systemic exposure parameters showed moderate (20%–30%) between-subject variability, Supporting Information S1: Table 2. There were no gender- or race-related differences in systemic exposure parameters. Compartmental pharmacokinetic parameters are presented in Supporting Information S1: Table 3 and were used in subsequent pharmacokinetic/pharmacodynamic (PK/PD) modeling.
Clinical hepcidin data
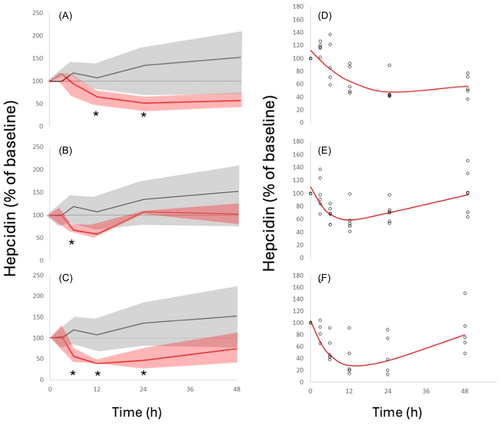
All baseline hepcidin values were below the upper limit of the reference range (1–12 nM).24 A clear and statistically significant reduction in hepcidin levels was seen at all three dose levels following sevuparin administration (Figure 6A–C); levels then slowly returned to baseline. Hepcidin suppression was maximal at time points beyond 6 h, while sevuparin levels reached maximum values at 2–3 h resulting in a clockwise hysteresis loop in the sevuparin versus hepcidin concentration response curve, suggesting an indirect hepcidin response. To allow a quantitative description of the concentration response, hepcidin data were fitted to an indirect response model; results of the fitting are presented in Figure 6D–F and Table 1. Hepcidin suppression was 40%–50% at the 3 and 6 mg/kg dose levels and reached 72% at the 9 mg/kg dose.
| 3 mg/kg | 6 mg/kg | 9 mg/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | %CV | Estimate | SE | %CV | Estimate | SE | %CV |
| Kin (1/h) | 6.67 | 2.79 | 45.2 | 22.27 | 17.80 | 70.6 | 18.50 | 6.10 | 33.3 |
| Kout (1/h) | 0.06 | 0.02 | 36.6 | 0.19 | 0.14 | 66.1 | 0.18 | 0.04 | 22.1 |
| IC50 (µg/mL) | 0.36 | 0.19 | 54.9 | 3.12 | 2.07 | 67.8 | 1.51 | 0.55 | 40.5 |
| Imax (%) | 51.8 | 20.7 | 40.0 | 41.2 | 20.6 | 49.9 | 72.0 | 31.9 | 44.3 |
Safety
No serious or severe adverse events were reported, and no subject discontinued the study due to an adverse event. In general, no differences were observed between the sevuparin and placebo treatment groups. Overall, a total of seven of 24 subjects (29.2%) reported TEAEs during the study. All TEAEs were nonserious, mild in intensity, and resolved by the completion of the trial, Table 2.
| Safety population | ||||
|---|---|---|---|---|
| 3 mg/kg | 6 mg/kg | 9 mg/kg | ||
| Sevuparin (N = 6) | Sevuparin (N = 6) | Sevuparin (N = 6) | Placebo (N = 6) | |
| System organ classpreferred term | n (%) | n (%) | n (%) | n (%) |
| Any adverse event | ||||
| Overall | 1 (16.7) | 1 (16.7) | 2 (33.3) | 3 (50.0) |
| Cardiac disorders | ||||
| Overall | 0 | 0 | 0 | 1 (16.7) |
| Sinus tachycardia | 0 | 0 | 0 | 1 (16.7) |
| Gastrointestinal disorders | ||||
| Overall | 0 | 0 | 0 | 1 (16.7) |
| Abdominal pain | 0 | 0 | 0 | 1 (16.7) |
| Investigations | ||||
| Overall | 0 | 0 | 0 | 1 (16.7) |
| Electrocardiogram PR prolongation | 0 | 0 | 0 | 1 (16.7) |
| Nervous system disorders | ||||
| Overall | 0 | 1 (16.7) | 1 (16.7) | 0 |
| Headache | 0 | 1 (16.7) | 1 (16.7) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Overall | 0 | 0 | 1 (16.7) | 0 |
| Dyspnea | 0 | 0 | 1 (16.7) | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Overall | 1 (16.7) | 0 | 0 | 0 |
| Erythema | 1 (16.7) | 0 | 0 | 0 |
| Skin burning sensation | 1 (16.7) | 0 | 0 | 0 |
Similar changes from baseline were observed in the mean, median, or min/max values in all laboratory safety parameters. Similar changes from baseline were observed across treatment groups; none were considered clinically significant. Sporadic individual abnormal values were observed in some clinical laboratory parameters, but none were considered clinically significant. One subject receiving 9 mg/kg sevuparin had elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) tests on Day 7; all were <1.5 × the upper limit of normal (ULN) and returned to normal on Day 15. In addition, two subjects given 3 mg/kg had elevations of AST on Day 7; both were <1 × ULN and returned to normal on Day 15. As expected from previous clinical experience with sevuparin, dose-related changes from baseline were observed in mean/median APTT values, peaking at the 3-h timepoint, and returning to normal by 24 h. Slight dose-related increases in mean/median values within normal ranges were also observed in prothrombin international normalized ratio (INR) and prothrombin times. No dose-related effects were observed in anti-Factor IIa or anti-Factor Xa results. No heparin-induced thrombocytopenia events or suspected events occurred during this study as demonstrated by 4 T scores25 being consistently low for all treatment groups at all time points, which is also in line with previous studies.23
Overall, no clinically important observations were noted in the change from pre-dose in the mean, median, or min/max vital sign values. Similar changes were observed across study time points. One subject receiving a placebo reported an adverse event of mild sinus tachycardia on Day 1. No notable differences were observed between treatment groups in the mean/median or min/max electrocardiogram (ECG) parameters; two ECG findings (mild sinus tachycardia and mild PR prolongation, both in the placebo group) were reported as adverse events.
DISCUSSION
Under normal conditions, hepcidin levels are maintained and controlled by an essential regulating signaling mechanism in the liver involving BMPs and the SMAD transcription factors.3, 26 Some heparinoid glycosaminoglycans have been demonstrated to be potent suppressors of hepcidin by interfering with the essential signaling and suppression at the transcriptional level.18-20 There is an array of chronic disorders in which suppression of systemic hepcidin levels could be clinically beneficial to counteract concomitant ACD.27 However, the use of available heparins to achieve a clinically meaningful suppression of hepcidin in humans is limited by their anticoagulation characteristics and the associated bleeding risk. Interestingly, there is one brief communication in which the authors showed a strong inhibition of hepcidin in critically ill patients nursed in the Intensive Care Unit treated with an unfractionated heparin as thromboprophylaxis or anticoagulant treatment.28 Therapeutics (small molecules, antibodies, etc.) that safely address the essential regulation of hepcidin are rare, while there are several approved drugs that antagonize direct or indirect mechanisms leading to increased hepcidin levels during inflammation, for example, the anti-IL6 antibody ziltivekimab,29 JAK/STAT inhibitors,30 lactoferrin,31 or even corticosteroids.32 The hepcidin upregulation due to inflammation requires an active SMAD pathway9, 33, 34 suggesting that its suppression has the potential to have a more profound effect on systemic hepcidin expression compared to the inhibition of the IL-6/STAT3 pathway. Clinically, this could mean that an inhibitor of BMP/SMAD could address both the high hepcidin expression seen in disorders in which the BMP/SMAD pathway drives its expression as well as in anemia of inflammation where IL6/STAT3 activation is the cause of high hepcidin.35
In this report, we observed that sevuparin, like some previously reported heparin derivatives,18-20 potently inhibited hepcidin expression in HepG2 cells both under baseline conditions and in the presence of BMP6 in a dose-dependent manner. It was further noted that hepcidin inhibition was followed by down-regulated SMAD5 phosphorylation as well as decreased expression of the ID1 gene confirming a robust inhibition of this pathway by sevuparin. In addition, when IL-6 was added to HepG2 cells, there was an induction of hepcidin mRNA that was abrogated by concomitant sevuparin treatment. Interestingly, sevuparin did not affect the phosphorylation status of STAT3, nor did it inhibit the expression of STAT-driven genes such as SOCS3, although it caused a significant inhibition of the SMAD pathway, required for the IL-6-dependent upregulation of hepcidin. The mechanistic elucidation of the effects of sevuparin on the systemic levels of hepcidin reinforces its potential as a therapeutic agent in disorders that involve hepcidin suppression as part of the treatment. In particular, the present data show that sevuparin antagonizes both mechanisms considered to be strong contributors to hepcidin dysregulation in disease (SMAD- and inflammation-driven hepcidin expression) acting on the essential SMAD signaling in the HepG2 cell line. In many disorders associated with high hepcidin levels, its upregulation is due to multiple factors; in CKD, these high levels are attributable to a combination of chronic inflammation, metabolic and cardiovascular dysregulation, and impaired elimination. The successful targeting of hepcidin expression by putative therapeutics may therefore require high potency based on the ability to broadly address hepcidin levels in order to achieve clinically meaningful reductions.
Sevuparin is presently under clinical development for systemic inflammation disorders such as endotoxemia and sepsis as well as for severe malaria and has also been tested in a Phase 2 study in patients with sickle cell vaso-occlusive crisis.23 The purpose of the clinical study reported as part of this work was to evaluate the pharmacokinetics and safety of sevuparin administered by subcutaneous injection. In this study, three different doses of subcutaneous sevuparin, 3, 6, and 9 mg/kg, were evaluated versus placebo. In line with the observations in earlier clinical studies22, 23 administering sevuparin by intravenous bolus injection, sevuparin was safe and well tolerated also when administered by the subcutaneous route. There were instances of the well-known heparin class effect on transient liver enzyme elevations, but no observations indicative of clinical HIT reactions as assessed by the 4T-score, which is consistent with earlier clinical trials.23 As previously, dose-dependent elevations in APTT were seen while no clinically significant differences in other coagulation-related measures (PT, INR, anti-factor IIa, and anti-factor Xa activity) were observed as compared to placebo.
In vivo, hepcidin regulation and signaling are parts of a physiological multifactorial context that involves inputs such as erythropoiesis, inflammation, infection, iron exposure, blood transfusions as well as kidney and liver function. This underlines the importance of understanding the in vivo relevance of any significant findings with hepcidin in vitro. In the present study, healthy mice treated with a single dose of sevuparin showed a 70% reduction in liver hepcidin gene expression with a concomitant reduction in Id1 mRNA indicating sevuparin-induced inhibition of the SMAD pathway that could also be observed in vivo. Remarkably, similar levels of suppression were observed in circulating hepcidin in healthy volunteers after a single dose of sevuparin with a maximal suppression between 12 and 24 h after the dose. This is a significant delay from the maximal sevuparin systemic levels (tmax of 2–3 h), strongly suggesting modulation of transcriptional and translational processes. While no clear dose dependency in terms of maximal effect was observed, the two higher doses appeared to provide a longer duration of effect, which is also supported by the increase in the Kin in the PK/PD model at the two higher doses (Table 1). From a pharmacodynamic point of view, therefore, maintaining hepcidin suppression would appear to be feasible using a once-daily dosing regimen with sevuparin.
Even if it is not fully understood how the tissue-specific in vivo interplay between liver endothelial cells and liver parenchyma brings about the regulation of hepcidin via BMP signaling following the well-known physiological stimuli (i.e., iron, inflammation), it is notable that the hepcidin inhibition in this study translates from the hepcidin mRNA levels in three different human hepatic cell lines (HepG2, Hep3b, and Huh-7) in vitro to the in vivo suppression of baseline mRNA hepcidin levels in mouse liver tissue and hepcidin protein levels in both mouse and human serum/plasma at a similar order of magnitude and comparable exposures of sevuparin. The normal reference range for human hepcidin in this study was 1–12 nM and the mean baseline in this healthy population was 3.65 ± 3.21 nM. Hepcidin levels in patients with high hepcidin anemia disorders such as CKD can reach 25–30 nM36 and even higher depending on the condition and method of assessment.37, 38
In the mouse model of acute inflammation due to LPS challenge, characterized by systemic inflammation and a two-fold peak level increase of hepcidin expression compared to normal mice, sevuparin showed the capacity to significantly reduce the high hepcidin expression levels. Interestingly, sevuparin also caused a reduction of biomarkers of systemic inflammation in this model, such as circulating IL6, and a trend toward suppression of STAT3 phosphorylation. It is noteworthy that sevuparin was effective in reducing hepcidin both when given concomitantly with LPS (LPS–sevuparin treatment schedule 2, Figure 5) and when LPS-induced inflammation was already established (LPS–sevuparin treatment schedule 1, Figure 5).
However, our study has limitations as it does not consider the potential differences in SMAD signaling and hepcidin regulation between the healthy and the chronic disease states in which high hepcidin levels may be a part of the pathophysiology, as the present work focuses on the short-term aspects of hepcidin regulation. Furthermore, it does not assess sevuparin and its effects in combination with any standard treatment used today in relevant disorders, such as the use of erythropoiesis-stimulating agents (ESAs) in CKD, which is believed to be an important aspect for future treatments in these anemias. To further increase the understanding of the potential for sevuparin as a treatment of anemia in chronic inflammation, exploration of its effects in a relevant preclinical chronic disease model and combination treatments could therefore be useful.
In conclusion, the heparinoid sevuparin, with markedly attenuated anticoagulation properties compared to LMWHs, demonstrates hepcidin antagonizing properties consistent with a broad-acting Class I antagonist27 with a potential to abrogate both essential and inflammation-triggered hepcidin expression.
ACKNOWLEDGMENTS
We are grateful to the DMMT (University of Brescia) where Michela Asperti, Magdalena Gryzik, Andrea Denardo, and Maura Poli performed the research, for the use of the Animal facility, qualified from the Italian Health Ministry (D. L.vo 116/92), and to the Facility for Genetics and Genomics analysis. We also acknowledge Maria Klockare, Modus Therapeutics AB, Stockholm, Sweden, ProSoft Clinical, PA, USA, and the research site CPMI Miami, FL, USA, for their support in conducting the clinical research presented in this work. Open access publishing facilitated by Universita degli Studi di Brescia, as part of the Wiley - CRUI-CARE agreement.
AUTHOR CONTRIBUTIONS
Michela Asperti planned the preclinical experiments, performed the preclinical research, analyzed data, and authored the paper. Magdalena Gryzik and Andrea Denardo performed the research in cells and mice. Kristina E. M. Persson performed the clinical hepcidin analyses and contributed to writing the paper. John Öhd and Göran Westerberg designed, planned, and oversaw the conduct of the clinical research as well as contributed to the planning of the preclinical experiments and contributed to writing the paper. Maura Poli oversaw and planned the preclinical experiments, analyzed data, and authored the paper.
CONFLICT OF INTEREST STATEMENT
Michela Asperti, Magdalena Gryzik, Andrea Denardo, Kristina E. M. Persson, and Maura Poli state no conflict of interest. G.W. Modus Therapeutics AB: Consultancy. John Öhd Modus Therapeutics AB and Modus Therapeutics Holding AB: Current Employment, Current equity holder (Modus Therapeutics Holding AB is a publicly traded company).
FUNDING
This work was supported by Modus Therapeutics AB (research funding to M.P.) and by Fondazione Cariplo (Project code: 2021-1558, research funding to M.A.).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data required to produce the results of the present study are provided within the manuscript or via supplemental files (Supporting Information figures or Supporting Information Table or Supporting Information). The original data are available from the corresponding author upon reasonable request ([email protected]).