Causes of death among patients diagnosed with chronic lymphocytic leukemia: A population-based study in the Netherlands, 1996–2020
Graphical Abstract
Abstract
Chronic lymphocytic leukemia (CLL) manifests heterogeneously with varying outcomes. This population-based study examined causes of death (CODs), as registered by the physician who established the death, among 20,588 CLL patients diagnosed in the Netherlands between 1996 and 2020. Utilizing cause-specific flexible parametric survival models, we estimated cause-specific hazard ratios (HRs) and cumulative incidences of death due to CLL, solid malignancies, other hematological malignancies, infections, and other causes. Our findings reveal CLL as the predominant COD, contributing to around 40% of relative mortality, with a declining 5-year death probability from 16.8% in 1996–2002 to 7.6% in 2010–2020. Also, deaths attributed to solid malignancies, other hematological malignancies, and other COD diminished over time, as evidenced by respective HRs (95% confidence interval) of 0.68 (0.60%–0.77%), 0.45 (0.38%–0.53%), and 0.77 (0.66%–0.90%). In summary, our comprehensive, population-based analysis underscores a noticeable reduction in CLL-attributed deaths and other competing causes over the studied period. Nonetheless, CLL is registered as the most prevalent cause of mortality among contemporary diagnosed patients with CLL, emphasizing the continued relevance of CLL-centric clinical strategies and research.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) predominantly afflicts adults in the Western world, with an age-standardized incidence rate ranging from 3.8 to 5.0 per 100,000 person-years.1, 2 CLL exhibits a heterogeneous and complex landscape of patient trajectories, varying from indolent cases requiring no immediate treatment to rapidly progressing forms necessitating prompt intervention.3 Despite usually affecting an elderly demographic often plagued by comorbidities, the scarcity of data, mainly from the early 2000s, revealed that CLL and its complications, rather than non-CLL-related causes, have emerged as the primary leading causes of death (CODs) within this populace.4, 5 However, contemporary population-based studies are limited by outdated paradigms and small cohorts, failing to adequately reflect temporal trends in COD among CLL patients in the context of recent therapeutic advancements.4-7
Over recent decades, significant progress in CLL management—such as chemoimmunotherapy and novel therapy-based approaches like ibrutinib and venetoclax―has marked a transformative era, enhancing overall response rates, minimal residual disease negativity, and progression-free survival.8-16 Moreover, these advances heralded an era of improved relative survival rates and life expectancy for CLL patients at the population level.5, 17-20 Nonetheless, there is a growing recognition of long-term complications associated with both the disease and its treatment, such as increased infection rates and an increased propensity to develop second primary malignancies.21-24 Therefore, understanding the evolving patterns of COD in CLL patients is critical for optimizing long-term outcomes and refining treatment strategies. To address the gaps in the current literature, this nationwide population-based study aimed to examine trends in COD among CLL patients in the Netherlands between 1996 and 2020, considering the impact of advancements in treatment during this period.
METHODS
Data sources
The Netherlands Cancer Registry
Established in 1989, the Netherlands Cancer Registry (NCR) covers over 95% of all newly diagnosed malignancies in the Netherlands.25 It relies on comprehensive case notification via the Nationwide Network and Registry of Histopathology and Cytopathology and the National Registry of Hospital Discharges (i.e., inpatient and outpatient discharges). The latter notification source is vital to ascertain CLL cases in the NCR since it captures CLL cases solely diagnosed through immunophenotyping of the peripheral blood.
Basic information about each case on dates of birth and diagnosis, sex, disease topography and morphology, and receipt of anti-neoplastic therapy (i.e., yes or no) are routinely ascertained in the NCR by trained registrars through retrospective review of medical records within 1 year after diagnosis. Topography and morphology are coded following the International Classification of Diseases for Oncology (ICD-O) guidelines.26 Disease stage data, using the Rai classification system, and information on the exact therapeutic regimens became available in the NCR from 2014.27 Information on each patient's vital status (i.e., alive, dead, or emigrated) was retrieved by annually linking the NCR to the Nationwide Population Registries Network, which holds this information on all residents in the Netherlands. The vital status was available until December 31, 2023, when the data were extracted for this study.
Statistics Netherlands
Statistics Netherlands is a Dutch governmental institution established in 1899 that, on a statutory basis, gathers statistics on various domains in the Netherlands, including COD for all deceased domestically Dutch residents. These CODs, including primary and secondary causes, are registered by the treating physician. As of the calendar year 1996, the CODs are coded according to the International Statistical Classification of Diseases and Related Health (ICD-10, 10th revision) of the World Health Organization (WHO).28, 29 Our study utilized nonpublic microdata, with follow-up until December 31, 2023, accessible for statistical and scientific purposes under specific conditions. For further information on data availability, please contact [email protected].
Study population
Our study encompassed all CLL patients diagnosed in the Netherlands between January 1, 1996, and December 31, 2020, as identified from the NCR using the ICD-O morphology code 9823. Exclusions were made for cases identified at autopsy (n = 71) and those with unknown COD (n = 652). Data on primary and secondary COD was retrieved by linking the NCR with data from Statistics Netherlands. CODs were categorized as follows: (i) CLL-related, (ii) solid malignancies, (iii) other hematological malignancies, (iv) infections, and (v) CODs other than in categories one to four, as detailed in Supporting Information S1: Table S1. Of note, organ-specific infections, including meningitis, intracranial abscess, endocarditis, myocarditis, pneumonia, lung abscess, skin infections/fasciitis, osteomyelitis, and pyelonephritis, were included in the infection category.5
According to the Central Committee on Research involving Human Subjects (CCMOs), this type of observational, noninterventional study does not require approval from an ethics committee in the Netherlands. The Privacy Review Board of the NCR approved the use of anonymous data for this study.
Statistical analysis
A flowchart of the patient selection and statistical analysis is presented in the Supporting Information S1: Figure S1. We employed cause-specific flexible parametric survival models, set within a competing risk framework, to derive cause-specific hazard ratios (CSHRs) and their corresponding 95% confidence intervals (CIs) as delineated in the references.30, 31 We applied restricted cubic splines to shape the baseline log cumulative hazard within the flexible parametric survival models. Following this, we devised a model to compute cumulative incidence functions (CIFs) and their 95% CIs up to 15 years after diagnosis. This model adopted the time elapsed since diagnosis as a time scale and factored in age groups (i.e., 18–59, 60–69, 70–79, and ≥80 years) and sex, aligning them in a two-way interaction with the delineated COD. Concurrently, the cause-specific relative contributions to the total mortality were estimated.
Next, we employed flexible parametric survival models within a competing risk framework, now utilizing the year of diagnosis as the time scale, to predict the cumulative 5-year probability of cause-specific death along with the associated 95% CIs. This model incorporated age and year of diagnosis as spline-modeled variables, adjusted for sex and including two-way interactions across all variables and time intervals. Further, a subgroup analysis was conducted on patients diagnosed between 2014 and 2020, integrating the Rai disease stage at diagnosis―categorized as low (i.e., Rai 0), intermediate (i.e., Rai I–II), and high (i.e., Rai III–IV)―as an augmented variable interacting bidirectionally with time.32
All flexible parametric survival models used 5 degrees of freedom (dfs) to model the baseline hazard for CLL-related death and 3 dfs for the remaining four COD categories. Time-varying effects were included using 2 dfs for CLL-related death and 1 df for the remaining four CODs. Further details about the flexible parametric models are provided in the Supporting Information S1: Methods. Study results were presented according to sex, age (i.e., 18–59, 60–69, 70–79, and ≥80 years), and the calendar period of diagnosis (1996–2002, 2003–2009, and 2010–2020) for a comprehensive view. Of note, the calendar periods were chosen as a proxy of the evolution of therapeutic modalities over time, representing the chemotherapy, introduction of chemoimmunotherapy, establishment of chemoimmunotherapy, and the novel-based agent eras, respectively. Data were graphically visualized using (stacked) cumulative incidence with 95% CIs and relative contributions to the total mortality plots. All statistical computations were executed using Stata Statistical Software version 17.0 (StataCorp, College Station, TX), utilizing the stpm2 and multistate packages.33, 34
RESULTS
Baseline characteristics
Our analytic cohort consisted of 20,588 patients diagnosed with CLL in the Netherlands between 1996 and 2020. The patients had a median age at diagnosis of 69 years, with an interquartile range (IQR) of 61–77 years. Most patients were males (61%), and 15% received antineoplastic therapy within 1 year after diagnosis. The baseline characteristics segmented by the calendar period of diagnosis are presented in Table 1. In the subgroup analysis of the 2014–2020 data (N = 6614), the distribution of Rai stage at diagnosis showed 52% at low risk, 33% at intermediate risk, and 15% at high risk (Supporting Information S1: Table S2).
| Calendar period | ||||
|---|---|---|---|---|
| 1996–2002 | 2003–2009 | 2010–2020 | Total | |
| Patient characteristics | No. (%) | No. (%) | No. (%) | No. (%) |
| Total no. of patients | 4035 | 5965 | 10,588 | 20,588 |
| Sex | ||||
| Male | 2418 (60) | 3625 (61) | 6594 (62) | 12,637 (61) |
| Female | 1617 (40) | 2340 (39) | 3994 (38) | 7951 (39) |
| Age (years) | ||||
| Median (IQR) | 69 (61–76) | 69 (60–77) | 69 (62–77) | 69 (61–77) |
| 18–59 | 909 (23) | 1383 (23) | 2038 (19) | 4330 (21) |
| 60–69 | 1128 (28) | 1765 (30) | 3286 (31) | 6179 (30) |
| 70–79 | 1345 (33) | 1819 (30) | 3405 (32) | 6569 (32) |
| ≥80 | 653 (16) | 998 (17) | 1859 (18) | 3510 (17) |
| Median follow-up (IQR) (years) | 11.5 (5.0–15.3) | 9.8 (4.3–14.6) | 7.0 (3.8–9.6) | 8.7 (3.8–13.1) |
| Antineoplastic therapya | ||||
| Yes | 993 (25) | 967 (16) | 1217 (11) | 3177 (15) |
| No/unknown | 3042 (75) | 4998 (84) | 9371 (89) | 17,411 (85) |
| Death | ||||
| Alive | 579 (14) | 2,038 (34) | 7105 (67) | 9722 (47) |
| ≤5 years | 1499 (37) | 1716 (29) | 2389 (23) | 5604 (27) |
| >5 and ≤10 years | 930 (23) | 1263 (21) | 1006 (9) | 3199 (16) |
| >10 years | 1027 (25) | 948 (16) | 88 (1) | 2063 (10) |
| Cause of deathb | ||||
| CLL-related | 1311 (38) | 1347 (34) | 1018 (29) | 3676 (34) |
| Solid malignancies | 581 (17) | 811 (21) | 780 (22) | 2172 (20) |
| Other hematological malignancies | 313 (9) | 314 (8) | 300 (9) | 927 (9) |
| Infections | 238 (7) | 309 (8) | 339 (10) | 886 (8) |
| Other cause | 1013 (27) | 1146 (29) | 1046 (30) | 3205 (30) |
| Cause of death (secondary)b | ||||
| CLL-related | 1948 (52) | 1851 (45) | 1072 (30) | 4871 (42) |
| Infections | 890 (24) | 771 (19) | 380 (11) | 2041 (18) |
- Abbreviations: CLL, chronic lymphocytic leukemia; ICD-10, International Statistical Classification of Diseases and Related Health, 10th revision; IQR, interquartile range; no., number.
- a Antineoplastic therapy was initiated as primary therapy within 1 year after the CLL diagnosis.
- b The causes of death are defined according to the ICD-10 codes as specified in Supporting Information S1: Table S1.
CODs
Over a median follow-up time of 8.7 years (IQR, 5.8–13.1 years), 53% of patients (N = 10,866) died, of whom 27%, 16%, and 10% within 5, 10, and ≥10 years after diagnosis, respectively (Table 1). Overall, death due to CLL (34%) was registered as the leading COD, followed by other causes (30%), solid malignancies (20%), infections (10%), and other hematological malignancies (9%; Table 1). Of note, when accounting for secondary COD, the total probability of death due to CLL and infections increased to 42% and 18%, respectively (Table 1).
The proportion of CLL-related deaths decreased over time due to a proportional increase in deaths due to solid malignancies, infections, and other causes (Table 1). In the most recent calendar period (2010–2020), mortality due to CLL or other causes was virtually on par (29% vs. 30%; Table 1). Cardiovascular system (46%) and respiratory system diseases (14%) were the most frequent contributors to the other COD category (Supporting Information S1: Table S3). Respiratory tract infections (35%) and COVID-19 infections (29%) were the leading infections contributing to mortality (Supporting Information S1: Table S3). Other specific CODs and their distribution are listed in Supporting Information S1: Table S3.
The probability of death for the overall cohort
Overall, at 15 years after diagnosis, the total probability of death (95% CI) was 35% (32%–39%), 57% (53%–60%), 83% (78%–87%), and 98% (92%–105%) for males aged 18–59, 60–69, 70–79, and ≥80 years, respectively. For females, the corresponding estimates were 26% (23%–29%), 46% (42%–50%), 73% (67%–77%), and 95% (89%–102%), respectively (Supporting Information S1: Figure S2).
The cumulative incidence of CLL-related death at 5, 10, and 15 years after diagnosis was 10.3% (95% CI, 9.9%–10.8%), 19.4% (95% CI, 18.8%–20.1%), and 25.5% (95% CI, 24.7%–26.3%), respectively. This cumulative incidence increased with advancing age and was markedly higher for males than females (Figure 1 and Supporting Information S1: Table S4).
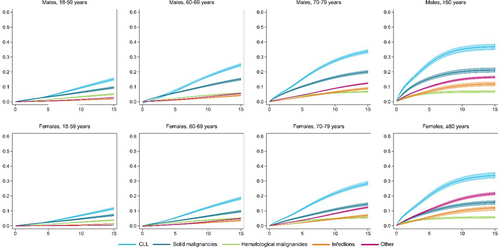
Death due to solid malignancies emerged as the second leading COD, with a cumulative incidence of 6.6% (95% CI, 6.3%–7.0%) at 5 years, 11.1% (95% CI, 10.7%–11.6%) at 10 years, and 14.5% (95% CI, 13.9%–15.2%) at 15 years after diagnosis, irrespective of sex and age (Figure 1 and Supporting Information S1: Table S4). However, in females aged ≥80 years, the 15-year cumulative incidence of death due to solid malignancies (16.6%; 95% CI, 15.1%–18.0%) was surpassed by death due to other CODs (22.8%; 95% CI, 20.8%–24.8%), making it the third leading COD in this subgroup of patients (Figure 1 and Supporting Information S1: Table S4). Death due to other hematological malignancies and infections were the least frequent CODs, irrespective of sex and age (Figure 1).
An analysis of relative mortality revealed CLL as a dominant contributor to death at diagnosis among males and females aged 60–69, 70–79, and ≥80 years, experiencing a slight decline in the first 3 years after diagnosis but stabilizing thereafter (Supporting Information S1: Figure S3). In patients aged 18–59 years, approximately 40% of the relative mortality was attributed to solid malignancies at diagnosis. Further, in this population, death due to CLL contributed to approximately 25% of the relative mortality at diagnosis and increased to approximately 40% at 15 years after diagnosis (Supporting Information S1: Table S5 and Figure S3).
The probability of death according to the calendar period of diagnosis
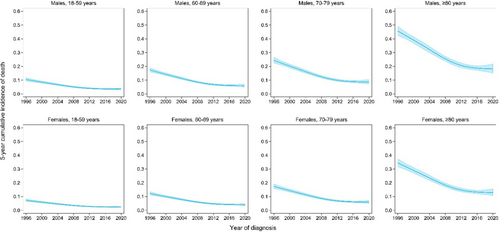
Next, we evaluated the 5-year cumulative incidence for the different CODs according to the calendar period of diagnosis, stratified by age and sex (Table 2). Overall, the 5-year cumulative incidence of CLL-related death decreased from 16.8% (95% CI, 15.5%–18.3%) in 1996–2002 to 11.2% (95% CI, 10.3%–12.2%) in 2003–2009 and 7.6% (95% CI, 6.7%–8.5%) in 2010–2020. This decrease was most pronounced in males aged ≥80 years, in which the 5-year cumulative incidence decreased from 40.4% (95% CI, 37.8%–43.1%) in 1996–2003 to 19.4% (95% CI, 17.4%–21.6%) in 2010–2020 (Figure 2 and Table 2). Likewise, the 5-year cumulative incidence of death due to solid malignancies, other hematological malignancies, infections, and other COD decreased over the calendar periods (Supporting Information S1: Figure S4–S7). Of note, an increase in the 5-year cumulative incidence due to infections was observed for patients diagnosed between 2010 and 2020, probably related to the SARS-CoV-2 pandemic (Supporting Information S1: Figure S6 and Table S6). Nevertheless, the overall total probability of death decreased with each year of diagnosis (Figure 3).
| 5-year cumulative incidence (95% CI) | |||||
|---|---|---|---|---|---|
| CODsa | Sex | Age at diagnosis (years) | 1996–2003 | 2004–2009 | 2010–2020 |
| CLL | Male | 18–59 | 9.0% (8.0%–10.0%) | 5.7% (5.1%–6.4%) | 3.8% (3.3%–4.3%) |
| 60–69 | 14.9% (13.8%–16.2%) | 9.7% (8.9%–10.5%) | 6.4% (5.7%-7.2%) | ||
| 70–79 | 21.1% (19.6%–22.8%) | 14.0% (12.8%–15.1%) | 9.2% (8.24%–10.4%) | ||
| ≥80 | 40.4% (37.8%–43.1%) | 28.1% (26.1%–30.2%) | 19.4% (17.4%–21.6%) | ||
| Female | 18–59 | 6.2% (5.5%–7.0%) | 4.0% (3.5%–4.5%) | 2.6% (2.2%–3.0%) | |
| 60–69 | 10.5% (9.5%–11.5%) | 6.7% (6.2%–7.4%) | 4.4% (3.9%–5.0%) | ||
| 70–79 | 15.0% (13.7%–16.4%) | 9.7% (8.9%–10.7%) | 6.4% (5.7%–7.3%) | ||
| ≥80 | 30.2% (27.9%–32.6%) | 20.3% (18.8%–22.1%) | 13.8% (12.2%–15.5%) | ||
| Solid malignancies | Male | 18–59 | 4.8% (4.1%–5.7%) | 4.3% (3.7%–5.0%) | 3.8% (3.2%–4.6%) |
| 60–69 | 10.8% (9.6%–12.1%) | 9.7% (8.7%–10.8%) | 8.6% (7.6%–9.9%) | ||
| 70–79 | 14.0% (12.5%–15.7%) | 12.6% (11.3%–13.9%) | 11.2% (9.9%–12.8%) | ||
| ≥80 | 18.4% (16.4%–20.8%) | 16.7% (14.8%–18.6%) | 14.9% (13.0%–17.1%) | ||
| Female | 18–59 | 2.7% (2.3%–3.3%) | 2.4% (2.1%–2.9%) | 2.2% (1.8%–2.6%) | |
| 60–69 | 6.2% (5.4%–7.1%) | 5.6% (4.9%–6.3%) | 4.9% (4.2%–5.8%) | ||
| 70–79 | 8.1% (7.1%–9.2%) | 7.24% (6.3%–8.2%) | 6.5% (5.6%–7.5%) | ||
| ≥80 | 10.8% (9.4%–12.4%) | 9.7% (8.5%–11.0%) | 8.7% (7.5%–10.1%) | ||
| Other hematological cancers | Male | 18–59 | 2.9% (2.3%–3.5%) | 2.0% (1.6%–2.4%) | 1.5% (1.2%–1.9%) |
| 60–69 | 4.5% (3.8%–5.3%) | 3.1% (2.6%–3.7%) | 2.3% (1.9%–2.9%) | ||
| 70–79 | 6.0% (5.0%–7.1%) | 4.1% (3.5%–4.9%) | 3.1% (2.5%–3.9%) | ||
| ≥80 | 9.3% (7.8%–11.1%) | 6.5% (5.4%–7.7%) | 4.9% (3.9%–6.1%) | ||
| Female | 18–59 | 2.4% (2.0%–3.0%) | 1.7% (1.3%–2.1%) | 1.3% (1.0%–1.7%) | |
| 60–69 | 3.8% (3.2%–4.6%) | 2.6% (2.2%–3.2%) | 2.0% (1.6%–2.5%) | ||
| 70–79 | 5.1% (4.2%–6.1%) | 3.5% (2.9%–0.4%) | 2.7% (2.1%–3.3%) | ||
| ≥80 | 8.0% (6.6%–9.6%) | 5.6% (4.6%–6.7%) | 4.2% (3.3%–5.3%) | ||
| Infections | Male | 18–59 | 0.8% (0.6%–1.1%) | 0.7% (0.5%–1.0%) | 1.0% (0.7%–1.5%) |
| 60–69 | 1.9% (1.5%–2.4%) | 1.7% (1.4%–2.1%) | 2.5% (1.9%–3.2%) | ||
| 70–79 | 3.3% (2.7%–4.1%) | 3.0% (2.5%–3.6%) | 4.3% (3.4%–5.4%) | ||
| ≥80 | 8.7% (7.1%–10.5%) | 7.9% (6.6%–9.4%) | 10.9% (8.9%–13.5%) | ||
| Female | 18–59 | 0.6% (0.4%–7.9%) | 0.5% (0.4%–0.7%) | 0.7% (0.5%–1.1%) | |
| 60–69 | 1.4% (1.1%–1.7%) | 1.2% (1.0%–1.6%) | 1.8% (1.4%–2.3%) | ||
| 70–79 | 2.4% (1.9%–3.0%) | 2.2% (1.8%–2.7%) | 3.1% (2.4%–4.0%) | ||
| ≥80 | 6.3% (5.2%–7.7%) | 5.6% (4.8%–6.9%) | 8.1% (6.4%–10.1%) | ||
| Other | Male | 18–59 | 3.0% (2.5%–3.5%) | 2.28% (1.9%–2.7%) | 2.0% (1.7%–2.4%) |
| 60–69 | 8.5% (7.6%–9.4%) | 6.48% (5.9%–7.2%) | 5.7% (5.0%–6.5%) | ||
| 70–79 | 15.6% (14.2%–17.1%) | 12.0% (11.0%–13.1%) | 10.7% (9.49%–11.9%) | ||
| ≥80 | 36.5% (33.9%–39.3%) | 29.4% (27.3%–31.6%) | 26.4% (23.9%–29.2%) | ||
| Female | 18–59 | 2.1% (1.8%–2.5%) | 1.6% (1.4%–1.9%) | 1.4% (1.2%–1.7%) | |
| 60–69 | 6.0% (5.4%–6.8%) | 4.6% (4.1%–5.2%) | 4.1% (3.5%–4.7%) | ||
| 70–79 | 11.2% (10.2%–12.4%) | 8.7% (7.8%–9.6%) | 7.6% (6.7%–8.7%) | ||
| ≥80 | 27.8% (25.5%–30.2%) | 22.0% (20.2%–23.9%) | 19.6% (17.5%–21.9%) | ||
- Note: This table summarizes the causes of death according to age, sex, and calendar period. As a reader's guide, death due to CLL was the highest in male individuals, increased with age, and decreased over time.
- Abbreviations: CI, confidence interval; CODs, causes of death; ICD-10, International Statistical Classification of Diseases and Related Health, 10th revision.
- a The causes of death are defined according to the ICD-10 codes as specified in Supporting Information S1: Table S1.
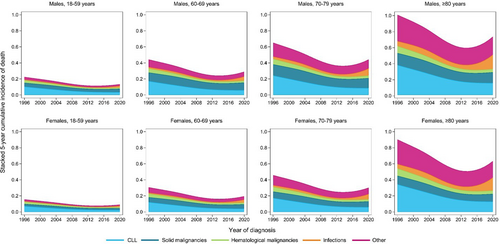
The probability of death according to the Rai disease stage
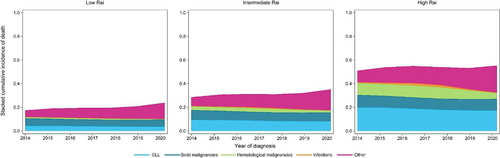
To evaluate COD in relation to the Rai disease stage, we further looked into a subcohort of patients diagnosed with CLL between 2014 and 2020. During a median follow-up of 5.3 years (IQR, 2.8–6.9), 25% of the patients died, of whom 15%, 7%, and 4% within 3, 5, and ≥5 years after diagnosis, respectively (Supporting Information S1: Table S2). The cumulative incidence of death for all CODs together remained relatively stable over the calendar years and increased with higher Rai stages (Figure 4 and Supporting Information S1: Figure S8). Also, this association holds for CLL-related deaths. More specifically, within 1 year after diagnosis, the relative probability of CLL-related deaths was 9%, 26%, and 52% for patients with low, intermediate, and high-risk Rai, respectively (Supporting Information S1: Figure S9). Moreover, the relative probability of CLL-related deaths increased slightly over time in patients with low-risk Rai, whereas it decreased over time in patients with high-risk Rai (Supporting Information S1: Figure S8).
CSHRs for CODs
Overall, the CSHRs for death due to any cause (i.e., for each of the five COD groups) were higher for males than females and increased with advancing age, albeit this association became less pronounced over time (Table 3). Also, this association essentially holds according to the calendar period of diagnosis. In other words, the CSHR is lower for more contemporary diagnosed patients than those diagnosed in earlier eras. However, the CSHR for death due to infections decreased during 2003–2009 (HR, 0.81; 95% CI, 0.67%–0.98%) but was comparable during 2010–2009 (HR, 0.87; 95% CI, 0.72%–1.05%) compared to 1996–2002, due to a higher proportion of infection-related mortality attributed to SARS-CoV-2.
| Causes of deatha | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLL | Solid malignancies | Other hematological malignancies | Infections | Other | ||||||
| CSHR (95% CI) | p Value | CSHR (95% CI) | p Value | CSHR (95% CI) | p Value | CSHR (95% CI) | p Value | CSHR (95% CI) | p Value | |
| Sex | ||||||||||
| Male | (Ref.) | (Ref.) | (Ref.) | (Ref.) | (Ref.) | |||||
| Female | 0.69 (0.65%–0.74%) | <0.001 | 0.59 (0.54%–0.65%) | <0.001 | 0.82 (0.71%–0.94%) | 0.004 | 0.75 (0.65%–0.86%) | <0.001 | 0.89 (0.79%–0.99%) | 0.046 |
| Age (years) | ||||||||||
| 18–59 | (Ref.) | (Ref.) | (Ref.) | (Ref.) | ||||||
| 60–69 | 1.89 (1.68%–2.13%) | <0.001 | 2.33 (1.99%–2.73%) | <0.001 | 1.95 (1.57%–2.41%) | <0.001 | 2.41 (1.77%–3.28%) | <0.001 | 2.05 (1.58%–2.65%) | <0.001 |
| 70–79 | 3.76 (3.34%–4.25%) | <0.001 | 4.55 (3.92%–5.28%) | <0.001 | 3.07 (2.46%–3.83%) | <0.001 | 7.03 (5.17%–9.59%) | <0.001 | 7.47 (5.92%–9.42%) | <0.001 |
| ≥80 | 10.4 (9.17%–11.84%) | <0.001 | 7.33 (6.19%–8.68%) | <0.001 | 6.21 (4.89%–7.88%) | <0.001 | 25.6 (18.5%–35.5%) | <0.001 | 26.1 (20.5%–33.1%) | <0.001 |
| Period of diagnosis | ||||||||||
| 1996–2002 | (Ref.) | (Ref.) | (Ref.) | (Ref.) | (Ref.) | |||||
| 2003–2009 | 0.58 (0.54%–0.63%) | <0.001 | (0.77%–0.97%) | 0.016 | 0.61 (0.52%–0.72%) | <0.001 | 0.81 (0.67%–0.98%) | 0.026 | 0.77 (0.66%–0.90%) | 0.001 |
| 2010–2020 | 0.36 (0.33%–0.39%) | <0.001 | 0.68 (0.60%–0.77%) | <0.001 | 0.45 (0.38%–0.53%) | <0.001 | 0.87 (0.72%–1.05%) | 0.148 | 0.72 (0.61%–0.84%) | <0.001 |
- Note: This table summarizes the cause-specific hazard ratios for the different causes of death. As a reader's guide, the probability of death due to CLL was the highest in male individuals, increases with age, and decreases over time.
- Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; CSHR, cause-specific hazard ratios; ICD-10, International Statistical Classification of Diseases and Related Health, 10th revision; ref., reference.
- a The causes of death are defined according to the ICD-10 codes as specified in Supporting Information S1: Table S1.
As shown in Supporting Information S1: Table S7, subgroup analysis revealed that patients with a high Rai stage at diagnosis had a higher probability of death due to CLL (CSHR, 4.82; 95% CI, 3.82%–6.08%), solid malignancies (CSHR, 1.62; 95% CI, 1.22%–2.13%), other hematological malignancies (CSHR, 9.24; 95% CI, 6.07%–14.1%), infections (CSHR, 6.10; 95% CI, 2.43%–15.3%) and other CODs (CSHR, 1.86; 95% CI, 1.52%–2.28%).
DISCUSSION
This comprehensive, nationwide, population-based study meticulously elucidates the temporal trends of COD among 20,588 patients diagnosed with CLL in the Netherlands between 1996 and 2020. We revealed a significant decline in CLL-related deaths and other competing causes over time, reinforcing the positive impacts of advancements in CLL management due to chemoimmunotherapy and novel-based agents. Our findings align with a prior single-center study conducted at the Mayo Clinic involving 1274 patients diagnosed between 2000 and 2020 in the United States, as well as a population-based study in Sweden involving 13,009 patients diagnosed between 1982 and 2013.4, 5 However, we uniquely contribute by offering a nuanced, updated, and population-based insight into CLL patient COD up to 2020. Similar to the Swedish study, we reported a decreasing trend in CLL-related deaths―irrespective of sex and age―emphasizing the continuous necessity for innovative therapeutic approaches to further reduce CLL-associated mortality, which unfortunately remains predominant at around 40% 15 years after diagnosis in the contemporary era.5
As the longevity of CLL patients improves, long-term complications such as second primary malignancies and infections become more prominent.20 Studies indicate a higher risk of both solid and hematological malignancies in CLL patients due to environmental and lifestyle exposures, immune dysfunction, and treatment-related effects.22, 24 As for the latter, the advent of efficacious chemoimmunotherapies―particularly the combination of fludarabine, cyclophosphamide, and rituximab―may fuel the risk of SPM development, especially myelodysplastic syndromes, and acute myeloid leukemia.22, 24 Yet, it is crucial to highlight that SPM development in CLL did not necessarily culminate in excess mortality because we demonstrated that death from other hematological malignancies decreased while death from solid malignancies remained comparatively stable over time. This finding indicates an intricate interplay of therapeutic benefits and risks of these SPMs in a more contemporary era, particularly in those that can be curatively managed with modern approaches.
CLL impairs the immune system, thereby increasing infection susceptibility, which can be further aggravated by treatments such as CD20 monoclonal antibodies and small molecule inhibitors.35-39 Despite our cohort exhibiting a relatively low 5-year incidence of death due to infections compared to the Danish study, it aligned with estimates from Sweden.5, 18, 40 Notably, infection-related deaths increased from 2010 to 2020, primarily due to a surge in COVID-19 cases. Indeed, studies indicated a higher COVID-19 mortality rate among lymphoproliferative disease patients, particularly those with CLL, often elderly and grappling with additional health issues.41 Although immunosuppressed CLL patients exhibit a poor response to COVID-19 vaccines, the anticipation of enhanced herd immunity and booster shots aims to mitigate infection-related deaths.42
Recently, the single-center Mayo Clinic unveiled compelling insights that patients with a high International Prognostic Index for CLL (CLL-IPI) score face a roughly threefold higher risk of dying from CLL progression or associated complications—which was defined as infections or SPMs—compared to those with an intermediate or low CLL-IPI index.6 This mirrors findings in follicular lymphoma (FL), in which patients with a higher disease stage exhibited a greater risk of FL-related death.43, 44 Building on these insights, our study extends the understanding of these dynamics by elucidating that the distribution of CODs, the overall probability of mortality, and their relative contributions vary based on the Rai stage at CLL diagnosis. This provides crucial perspectives on how disease severity influences mortality and underscores the significance of prognostic staging systems in guiding patients and their physicians by conveying the likelihood of different CODs.
Our study stands robust with several commendable strengths that merit discussion. Primarily, our study utilizes a well-established, comprehensive cancer registry. With its nationwide, population-based information, the NCR provides a solid foundation for discerning the temporal trends in COD among patients with CLL. The extensive nature of this registry, paired with the reliable COD data sourced from Statistics Netherlands―showing high reliability of over 90% for leading COD groups―amplifies the credibility of our findings, propelling them as a significant asset for future research endeavors.29 Also, rigorous statistical techniques have allowed for a precise estimation of CSHRs, CIF, and relative contributions to mortality, enriching our understanding of CLL's overall impact on different CODs.
While our study is foundational, it does harbor limitations that warrant attention. A significant nuance lies in the granularity of data, where details such as IGHV mutational status, TP53 aberrations, and information on the exact therapeutic regimens are sparse, somewhat limiting the depth of our insights. Potential underreporting of infection-related deaths also warrants cautious interpretation, especially given Denmark's higher reported incidence of such deaths.18, 40 In addressing differences regarding the COD data's reliability as sourced from Statistics Netherlands and other population-based sources, it is imperative to underscore the intrinsic value of these population-based national registry data. While there might be a tendency to view single-center, academic studies—often enriched with retrospectively reviewed, finely detailed COD data—with a degree of heightened credibility, it is essential to recognize the robustness and broader representation encapsulated in national registry data.
In summary, our nationwide, population-based study illuminates a multidimensional perspective on the temporal mortality trends among patients with CLL, epitomizing the triumphant strides and persistent challenges in CLL management. Nevertheless, the probability of all-cause death decreased over time, irrespective of sex and age. The insights gleaned from our study may guide future therapeutic innovations and strategic patient management to reduce CLL-related mortality among contemporarily diagnosed CLL patients. The current study serves as a benchmark to assess and monitor how the spectrum of CODs may alter in the future with a broader application of targeted therapies and other therapeutic advances.
ACKNOWLEDGMENTS
The authors would like to thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. The nationwide population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation (IKNL).
AUTHOR CONTRIBUTIONS
Avinash G. Dinmohamed, Lina van der Straten, and Manette A.W. Dinnessen designed the study; Lina van der Straten analyzed the data; Otto Visser was responsible for the data collection; Lina van der Straten wrote the manuscript with contributions from all authors, who also interpreted the data and read, commented, and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Arnon P. Kater has received personal fees from AbbVie, LAVA, Genmab, Janssen, AstraZeneca, Roche/Genentech, and Bristol Myers Squibb; and research funding from AbbVie, Janssen, AstraZeneca, Roche/Genentech, and Bristol Myers Squibb. Mark-David Levin has received personal fees from AbbVie, Janssen, and Roche; and research funding from AbbVie, Janssen, AstraZeneca, and Roche/Genentech. Anton W. Langerak has received research funding via an unrestricted grant from Roche-Genentech and speaker fees from Janssen. The remaining authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available via the Netherlands Comprehensive Cancer Organisation and Statistics Netherlands. These data are not publicly available, and restrictions apply to the availability of the data used for the current study. However, the data are available upon reasonable request and with permission of the Netherlands Comprehensive Cancer Organisation and Statistics Netherlands.