Quantifying NPM1 MRD in AML patients prior to allogeneic stem cell transplantation: Where to draw the line?
Today, assessment of measurable residual disease (MRD) increasingly allows a dynamic risk stratification and informs treatment decisions in acute myeloid leukemia (AML).1 For AML patients with NPM1 gene mutations quantitative PCR methods have proven to be reliable tools for MRD measurement.1 Detection of mutated NPM1 transcript levels is highly informative at various time points during the disease course,2-4 including before allogeneic hematopoietic stem cell transplantation (HSCT).3, 5-8 Published studies consistently indicate overall survival (OS) differences of approximately 40%–50% at 2 years after HSCT comparing NPM1 MRD-positive and NPM1 MRD-negative patients.3, 6-9 However, the MRD thresholds identified and adapted differ substantially between studies, ranging from the limit of detection of the respective test, often 0.001%,6, 7 to 0.1%3 or even 1%8 NPM1/ABL1 copies. Reasons for these differences are based on characteristics of the respective MRD assays, varying statistical approaches, or different priorities, for example, avoiding “overtreatment” versus identifying every patient at risk. This has resulted in distinct proportions of patients identified to be at high risk of adverse outcomes in each study.2, 6-8 Additionally, due to still insufficient interlaboratory harmonization, NPM1 MRD result comparability remains limited. Here we systematically analyzed different NPM1 MRD thresholds to identify NPM1-mutated AML patients at high risk of relapse following HSCT.
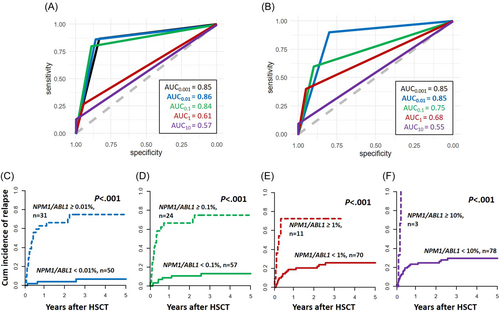
We retrospectively analyzed 81 NPM1-mutated AML patients who received an allogeneic HSCT at our institution. All had available material for MRD analysis obtained up to 28 days before HSCT in complete remission (CR) or CR with incomplete recovery (CRi) (bone marrow, n = 30, or peripheral blood, n = 51). Median age at HSCT was 61.4 (range: 33.1–76.4) years. Most patients received reduced-intensity or nonmyeloablative conditioning (81%), and 19% of patients were transplanted following myeloablative conditioning. Further patients' characteristics are given in Supporting Information S1: Table S1. Written informed consent was obtained in accordance with the Declaration of Helsinki. Median follow-up after HSCT was 4.4 years. NPM1 MRD was measured by digital droplet PCR using a competitive probe approach and normalized to ABL1 as previously described.7 For patients with a positive NPM1 MRD test result, distinct cut-offs were analyzed using receiver operator characteristics (ROC) curves to define the clinically most relevant threshold, starting from the limit of detection (0.001% NPM1/ABL1) in log steps up to 10% NPM1/ABL1. As potentially different detection levels in blood and bone marrow have been reported in some studies,2, 6, 10 ROC curves were analyzed separately for both tissues. However, the largest area under the curve was detected for a 0.01% NPM1/ABL1 cut-off to define MRD-positive or MRD-negative patients in both bone marrow and blood, respectively (Figure 1A,B and Supplementary Table S2), which is why for further analyses, both were analyzed together. Twenty-five patients (31%) relapsed after HSCT. Adapting the 0.01% NPM1/ABL1 cut-off, we observed the clearest separation of the cumulative incidence of relapse (CIR, p < 0.001, after 2 years 4% vs. 66%, Figure 1C, resulting in a 93.8% relative risk reduction for relapse after 2 years for MRD-negative [n = 50] compared to MRD-positive [n = 31] patients), as well as OS curves (p < 0.001, after 2 years 76% vs. 38%; Supporting Information S1: Figure S1A). Higher cut-offs resulted in steeply declining sensitivities and negative predictive values, while “relapse prediction” in MRD-positive patients did not improve much: Compared to a 0.01% NPM1/ABL1 cut-off, a stepwise higher proportion of MRD-negative patients experienced relapse for 0.1% and 1% NPM1/ABL1 cut-offs (at 2 years 11% vs. 67% and 21% vs. 73%, respectively; Figure 1D,E). Only at the highest cut-off of 10% NPM1/ABL1 copy numbers, all MRD-positive patients relapsed, but at this cut-off “MRD-negative” patients had a CIR as high as 25% after 2 years (Figure 1F), with a negative predictive value of only 0.72 (Supporting Information S1: Table S3). Subsequently, we identified the 0.01% NPM1/ABL1 cut-off in remission before HSCT as the cut with the highest discriminative power for relapse after HSCT in our cohort. Patients in MRD-positive remission (at least 0.01% NPM1/ABL1) had a significantly higher white blood cell count (p = 0.04) and higher LDH levels (p = 0.02) at diagnosis. Even though the co-mutational profile at diagnosis did not differ between both groups (Supporting Information S1: Table S1 and Figure S2), patients remaining NPM1 MRD-positive at HSCT by trend more often had a pretreatment FLT3-ITD mutant-to-wild-type ratio > 0.5 (p = 0.08). Notably, the number of chemotherapy cycles prior to HSCT did not differ between MRD-positive and MRD-negative patients (p = 0.20), indicating that MRD-positive patients may not benefit from additional conventional chemotherapy cycles. Whether shifting treatment strategies to, for example, venetoclax plus hypomethylating agents before HSCT may lead to MRD-conversion and outcome improvements in these patients should be evaluated in clinical studies. NPM1 MRD-positive patients more often were transplanted in a second remission of their AML (p = 0.03) and patients transplanted in a second remission had higher NPM1/ABL1 copy numbers at HSCT compared to those transplanted in first remission (p = 0.02), a finding that may contribute to the previously described adverse outcomes of patients transplanted in second remission.11
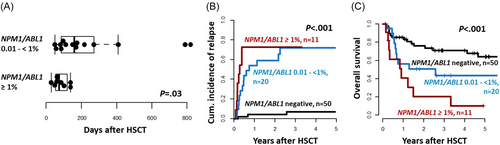
While AML patients with an MRD burden above 0.01% NPM1/ABL1 cut-off had significantly higher relapse rates, we observed a negative correlation between NPM1/ABL1 MRD levels before HSCT and time to relapse; the higher the NPM1 MRD burden at HSCT, the earlier the relapse occurred (r = −0.5, p = 0.03; Supporting Information S1: Figure S3). This correlation was strongest in patients with FLT3-ITD present at diagnosis (r = −0.82, p = 0.002). When introducing two cuts for patients with low (≥0.01% but <1%) or high (≥1%) NPM1/ABL1 MRD levels, there was a significantly shorter time to relapse in AML patients with high NPM1 MRD levels prior to HSCT compared to patients with low MRD burden (Figure 2A,B). In addition, compared to patients with low NPM1 MRD levels, patients with high NPM1 MRD levels also showed a trend for shorter OS (p = 0.10; Figure 2C), indicating that patients transplanted with higher NPM1 MRD may be harder to salvage after relapse following HSCT.
Recently, Dillon et al.6 presented data of 107 NPM1-mutated AML patients prior to HSCT, implicating that patients with low NPM1 MRD burden (less than 1% [blood] or 0.2% [bone marrow]) only had shorter survival when they harbored a FLT3-ITD (n = 22 vs. n = 8).6 However, in our cohort the negative impact of a low or high NPM1 MRD at HSCT was not modified by the presence or absence of an FLT3-ITD (Supporting Information S1: Figure S4), which might be a result of the restricted sample size. Of note, in our cohort, none of the patients without a diagnostic FLT3-ITD that were MRD-negative at HSCT suffered a relapse during the follow-up time.
Previous studies tried to identify the most informative NPM1 MRD thresholds at other time points than prior to HSCT. After chemotherapy consolidation, Krönke et al.2 and Shayegi et al.12 suggested 2% and 1% NPM1/ABL1 MRD, respectively, as the values most informative for relapse. This led to the implementation of a 1% cut in some studies evaluating preemptive treatment strategies in MRD-positive patients.13 However, after an allogeneic HSCT, also 10% NPM1/ABL1 was described as clinically relevant12—likely as a result of graft-versus-leukemia (GvL) effects providing continuous therapeutic effects.
One could speculate that the much lower NPM1 MRD levels observed as most discriminative in our cohort may result from the lower intensity conditioning regimens providing less disease control than myeloablative HSCT. However, in separate analyses for reduced-intensity/nonmyeloablative and myeloablative HSCT, despite limited patient numbers, the cut-off had prognostic power irrespective of the conditioning intensity (Supporting Information S1: Figure S5). Also in a recent study adapting NGS, adverse outcomes after HSCT were predicted by a very low MRD burden (0.01% variant allele fraction).9 Remarkably, outcomes of MRD-positive patients after reduced-intensity HSCT improved when the conditioning regimen was melphalan-based, showing potential for clinical intervention.9
Since mutated NPM1 has been described as immunogenic, NPM1-mutated patients may benefit from HSCT consolidation.14, 15 We evaluated the prognostic impact of a chronic graft-versus-host disease (GvHD) as a surrogate marker for GvL effects. In a landmark analysis for patients surviving longer than 100 days, CIR was reduced in NPM1 MRD-positive patients with chronic GvHD (25% after 2 years), compared to NPM1 MRD-positive patients who did not develop a chronic GvHD (92% after 2 years; Supporting Information S1: Figure S6). However, OS did not differ (p > 0.99) as MRD-positive patients with a chronic GvHD tended to have higher nonrelapse mortality (NRM; p = 0.06). Thus, patients transplanted with positive NPM1 MRD may benefit from immunologic interventions such as donor lymphocyte infusions to augment GvL but should be selected very carefully to avoid increasing NRM.
Our study has some limitations, including the small sample size and use of blood and bone marrow for MRD assessment. Additional studies—or meta-analyses of existing ones—will be needed to confirm our findings. In conclusion, in our cohort, NPM1/ABL1 MRD levels prior to allogeneic HSCT were most informative when applying a 0.01% cut-off. Higher NPM1/ABL1 thresholds resulted in lower sensitivity and negative predictive values without a meaningful gain of specificity. These results were independent of the analyzed tissue (blood vs. bone marrow), conditioning intensity, or patients' age (Supporting Information S1: Figure S7). Higher levels of NPM1 MRD burden at HSCT are associated with a shorter time to relapse after HSCT and may be harder to salvage following relapse after HSCT.
ACKNOWLEDGMENTS
The authors thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo, and Dagmar Cron for their help in determining cytogenetic, morphologic, and immunological analyses, and Christine Günther, Scarlett Schwabe, Ines Kovacs, and Kathrin Wildenberger for their help in sample processing. The current and past members of Sebastian Schwind's lab would like to express their deepest gratitude for his guidance, mentorship, patience, and friendship over the past 10 years. Although he left us much too early, he left a lasting impact on us as physicians and researchers.
AUTHOR CONTRIBUTIONS
Madlen Jentzsch and Sebastian Schwind contributed to the design and analysis of this study and the writing of the manuscript. Madlen Jentzsch, Lara Bischof, Marius Bill, Juliane Grimm, Jule Ussmann, Donata Backhaus, Tung Pham Thanh, and Dominic Brauer carried out the laboratory-based research. Madlen Jentzsch and Sebastian Schwind performed statistical analyses. Maximilian Merz, Marco Herling, Klaus H. Metzeler, Vladan Vucinic, Georg-Nikolaus Franke, Uwe Platzbecker, and Sebastian Schwind provided administrative support. Madlen Jentzsch, Lara Bischof, Marius Bill, Juliane Grimm, Jule Ussmann, Donata Backhaus, Dominic Brauer, Tung Pham Thanh, Maximilian Merz, Georg-Nikolaus Franke, Klaus H. Metzeler, Vladan Vucinic, Marco Herling, Uwe Platzbecker agreed on the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This study was supported by the Deutsche Gesellschaft für Innere Medizin (Clinician Scientist Program, M. J.), the Verein Zusammen gegen den Krebs e.V. (S. S.), the Deutsche Jose Carreras Stiftung (04R/2016 [S. S.] and PS15/05 [J. G.]), an intramural scholarship of the University of Leipzig (L. B.), and the Studienstiftung des Deutschen Volkes (T. P. T.).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.