High pretreatment disease burden as a risk factor for infectious complications following CD19 chimeric antigen receptor T-cell therapy for large B-cell lymphoma
Graphical Abstract
Abstract
Infection has emerged as the chief cause of non-relapse mortality (NRM) post CD19-targeting chimeric antigen receptor T-cell therapy (CAR-T) therapy. Even though up to 50% of patients may remain infection-free, many suffer multiple severe, life-threatening, or fatal infectious events. The primary aim of this study was to explore severe and life-threatening infections post licensed CAR-T therapy in large B-cell lymphoma, with a focus on the role of disease burden and disease sites in assessing individual risk. We sought to understand the cohort of patients who experience ≥2 infections and those at the highest risk of infectious NRM. Our analysis identifies a higher disease burden after bridging therapy as associated with infection events. Those developing ≥2 infections emerged as a uniquely high-risk cohort, particularly if the second (or beyond) infection occurred during an episode of immune effector cell-associated neurotoxicity syndrome (ICANS) or while on steroids and/or anakinra for ICANS. Herein, we also describe the first reported cases of “CAR-T cold sepsis,” a phenomenon characterized by the lack of an appreciable systemic inflammatory response at the time of detection of infection. We propose a risk-based strategy to encourage heightened clinician awareness of cold sepsis, with a view to reducing NRM.
INTRODUCTION
CD19-targeting chimeric antigen receptor T-cell therapy (CAR-T) has transformed the treatment of relapsed and/or refractory (r/r) large B-cell lymphoma (LBCL), leading to Food and Drug Administration and European Medicines Agency approval of axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel).1-3
Published reports indicate that infection, which occurs in 23%–42% of patients within the first month,4-6 is the chief cause of non-relapse mortality (NRM).7-10 The greatest morbidity and mortality from infection is observed in the first-month post-CAR-T, but ongoing infection-driven NRM is reported up to 12 months post infusion.7, 10 Early infections (<30 days post-CAR-T) are predominantly bacterial, and later infections are primarily viral,5, 11 while fungal infections are relatively less commonly observed.12
Published data suggest that risk factors for infection post-CAR-T include high-grade cytokine release syndrome (CRS),5, 6 corticosteroids,13, 14 number of prior lines of therapy,4 recent (preadmission) infection,4 prior hematopoietic stem cell transplantation, Immunoglobulin G (IgG) < 400 mg/dL,15 and bone marrow burden.16 More recently, Rejeski et al. reported that a high HEMATOTOX (HT) score was associated with infection, suggesting that impaired neutrophil, hemoglobin, and/or platelet counts pre-CAR-T combined with raised inflammatory blood markers (ferritin, C-reactive protein [CRP]) could potentially predict patients at higher risk of post-CAR-T cytopenia and infectious complications.8, 17
While the management of CAR-T immunotoxicity, such as CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) continues to evolve, with earlier immunomodulatory interventions leading to better control and lower morbidity, infections have therefore emerged as the main clinical barrier to the safe delivery of CAR-T in the clinic. Establishing which patients are at the highest risk of severe and life-threatening infection is key to improving safety, particularly in older patients and those with comorbidities.
Here we report the infectious complications arising in a cohort of 178 adults with r/r LBCL following axi-cel or tisa-cel. The objectives of the study were to identify pre-CAR-T factors associated with the highest risk of severe infection and infectious NRM, to raise clinician awareness, and to support the development of targeted monitoring and prevention strategies that could benefit patients at the highest risk.
METHODS
Patients
Data were extracted retrospectively from electronic medical records for consecutively treated patients at two CAR-T centers (University College London Hospital [UCLH] and King's College Hospital [KCH]) between January 2019 and March 2021 as part of a hospital service evaluation. CAR-T product selection (axi-cel; Kite Pharma, or tisa-cel; Novartis) was at the discretion of the treating center. Lymphodepletion (LD) with fludarabine and cyclophosphamide was administered as per the manufacturers' instructions. Uniform UK treatment eligibility criteria were applied, as previously described.18
Definitions and outcomes
The primary outcome was severe or life-threatening infection following CAR-T infusion. All-cause mortality was a secondary outcome. Infections during LD or on the day of CAR-T infusion were excluded from the analysis.
Infection was defined as positive microbiology, virology, histopathology, and/or radiological findings in conjunction with clinical symptoms. Invasive fungal infections were classified as proven, probable, and possible according to revised EORTC criteria.19 Culture-negative neutropenic fever was excluded due to the potential overlap with CRS.
Grading was mild (no treatment or oral antibiotics), severe (requiring intravenous therapy), or life-threatening (symptoms such as hemodynamic instability or requiring organ support) as previously established.6, 20 Fatal infection was defined as infection directly leading or contributing significantly to death, as determined by the responsible clinical team.
The date of onset of infection was recorded as the date of the diagnostic test. Early and late infections were defined as those occurring up to (≤90 days) and beyond 90 days of CAR-T infusion, respectively. Documented clearance of infection followed by subsequent recurrence were considered separate events. Blood cultures positive for more than one organism on the same day were classed as separate events. Routine monitoring for viral reactivation (cytomegalovirus [CMV], Epstein–Barr virus, adenovirus, and human herpesvirus 6 [HHV-6]) was not performed at either center.
Severe neutropenia was defined as a neutrophil count of <0.5 × 109/L.21 Granulocyte-colony stimulating factor (GCSF) was not administered prior to Day +21 post infusion in the inpatient setting. The use of GCSF beyond the initial inpatient stay was not recorded for either center. The frequency of clinical follow-up post discharge was in line with European Society for Blood and Marrow Transplantation (EBMT) guidance.22
Bridging therapy (BT) was defined as any lymphoma therapy delivered between T-cell apheresis and LD. CRS and ICANS were graded according to American Society for Transplantation and Cellular Therapy (ASTCT) consensus guidelines23 and toxicity management strategies were at the discretion of the treating center. Steroid cumulative dosing for CAR-T toxicity was calculated as methylprednisolone equivalent (mg). Response to CAR-T therapy was determined locally and defined as per Lugano classification 2014.24
In the absence of metabolic tumor volume to quantify disease burden, a “high burden” after bridging therapy (BT) was defined a priori as lactate dehydrogenase (LDH) pre-LD > ULN (249 units/L) and (stage 3/4 disease and/or ≥3 extra-nodal sites). “Low burden” post-BT was defined as LDH < 250 units/L and (stage 1/2 disease and/or <3 extra-nodal sites).
Fitness for an autologous stem cell transplantation (ASCT) was determined by the treating clinician based on physical suitability (e.g., age, frailty, comorbidities), irrespective of disease status.
Infection prophylaxis and intravenous immunoglobulin (IVIG)
All patients received prophylaxis against herpes simplex/varicella zoster virus (HSV/VZV) and Pneumocystis jirovecii pneumonia (PJP) with oral acyclovir and either inhaled pentamidine, oral co-trimoxazole or azithromycin, respectively. The duration of HSV/PJP prophylaxis and antifungal practice differed between centers (Supporting Information S6: Table S1). Prophylactic antibiotics (such as fluroquinolones) were not administered at any stage in either center. IVIG was commenced post infusion with regional IVIG panel approval in those with an IgG level <4 g/L and recurrent confirmed bacterial infections.
Statistical analyses
All analyses were performed using Stata 17 (StataCorp). Patient baseline characteristics, toxicity profiles, and outcomes were compared between the two participating centers using chi-squared tests (or Fisher's exact test if any cell frequency ≤5) for categorical variables and the Kruskal–Wallis test for continuous variables. Patient time-at-risk was calculated in days from the date of CAR-T infusion and follow-up was censored at disease progression, death, or end of follow-up (to June 14, 2021). Survival analysis was performed using Cox regression. A final multivariable Cox regression model (including a priori potential confounders age, sex, center, and CAR-T drug) was fitted by carrying forward variables identified as associated with the outcome (α ≤ 0.1) in univariate analysis and performing stepwise backward and forward adjustments to identify and select those variables that were independently associated with the outcome, supported by consideration of causal pathways and assessment of collinearity. Exposures occurring as episodes of variable length during time-at-risk were included as time-varying effects. The proportional hazards assumption was assessed by inspection of plots of log(−log(survival)) and scaled Schoenfeld residuals against the log of survival time (with a test of non-zero slope for the residuals). Graphs and figures were created using Stata and GraphPad Prism v9.5.1 (GraphPad Software).
RESULTS
Patient characteristics
Across the cohort of 178 patients, the median age at the time of CAR-T approval was 58 years (range = 19–80 years) with a male predominance (61.8%, 110/178) (Supporting Information S6: Table S2). Approximately two-thirds (63.5%, 113/178) of patients had de novo diffuse large B-cell lymphoma (DLBCL), 28.1% (50/178) had transformed indolent disease, and 6.2% (11/178) had primary mediastinal B-cell lymphoma (PMBCL). One-fifth (20.2%, 36/178) had a prior ASCT, and approximately one-third (36.0%, 64/178) had received three or more lines of chemotherapy. Most patients (89.9%, 160/178) received BT (Supporting Information S6: Table S3), 56.9% (91/160) and 43.1% (69/160) achieved stable disease (SD)/progressive disease (PD) and complete remission (CR)/partial remission (PR), respectively. Disease characteristics pre/post-BT are summarized in Supporting Information S6: Table S2. Patients treated at the two participating centers had similar characteristics post-BT except for an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 2 in 13.3% (11/83) of KCH patients compared with only 1/95 UCLH patients (p = 0.004). Axi-cel was given to 139/178 (78.1%) of patients and tisa-cel to 21.9% (39/178) (Supporting Information S6: Table S4).
Where data were available (Supporting Information S6: Table S5), baseline median CRP (n = 142) and ferritin (n = 149) were 7.5 mg/L (interquartile range [IQR] = 2.7–34.4) and 595 µg/L (IQR = 264–1329), respectively; 6.9% (12/175) of patients started LD with neutrophils <1.2 × 109/L (median = 3.2, IQR = 2.0–4.5), potentially indicating marrow toxicity from prior therapies in a minority. Post infusion 23% (41/178) received GCSF at ≥Day 21 in the inpatient setting. GCSF use post discharge was not recorded for either center.
Incidence of total and severe/life-threatening infection after CAR-T infusion
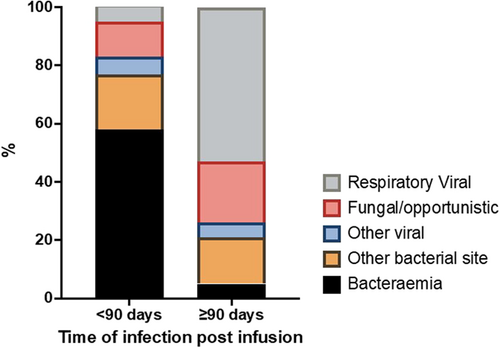
A total of 191 separate infectious episodes were recorded in 89/169 patients (52.7%) (9/178 were omitted as infection preceded or occurred on the day of cell infusion). 145/191 (75.9%) infectious events occurred within 90 days post-CAR-T infusion, and 46/191 (24.1%) events occurred later than 90 days (Supporting Information S6: Table S6). There was a clear predominance of early bacterial and later respiratory viral infections (Figures S1 and S2).
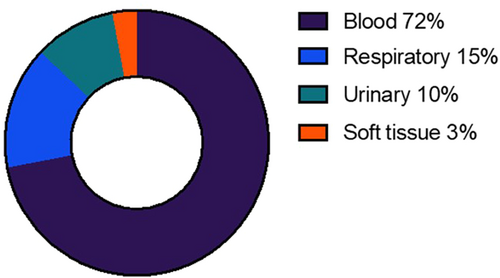
A total of 92 severe or life-threatening infections were reported in 53/178 patients (29.8%), of whom 20/53 (37.7%) experienced more than 1 severe/life-threatening event. The onset of the first infectious event was at a median of 14 days post-CAR-T infusion (IQR = 4–25 days, range = 1–504 days). Details of the causative organisms, subtype of infection, and bacterial site of recovery are illustrated in Supporting Information S6: Table S7, Figures 1 and 2, respectively, with a clear predominance of bloodstream infections. The most common organisms identified in blood were Staphylococcus species (n = 10), Escherichia coli (n = 7), Pseudomonas aeruginosa (n = 6), and Enterococcus spp. (n = 7); Escherichia coli (n = 2) or Klebsiella spp. (n = 2) in urine. 19/69 (27.5%) viral infections were deemed severe/life-threatening, the most common of which was SARS CoV-2 (n = 6). CMV reactivation was noted in the peripheral blood of seven patients, of whom two received systemic antiviral therapy due to concerns regarding high CMV titers. Evidence of end-organ disease was not confirmed in either case. Our group has previously published our experience of progressive multifocal leukoencephalopathy (JC polyomavirus) and HHV-6 encephalitis after CAR-T therapy.25-27 The illustrative timeline of infections is demonstrated in Figure 3. The remaining 125 patients were censored at death (n = 2), disease progression (n = 66), or end of active follow-up (n = 57).
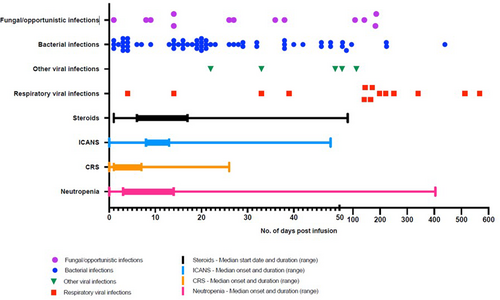
Of those who had ≥2 severe/life-threatening infections (n = 20), a median of 2 infections were reported per patient (range = 2–6) with a median onset of the second infection at 38 days post infusion (IQR = 19–48, range = 1–567). A total of 59 severe/life-threatening events occurred in those who had ≥2 severe/life-threatening infections, again with a predominance of early bloodstream and later viral infections. 6/29 (20.7%) who had one bloodstream infection isolated another organism in their blood on the same day (co-infection) and 4/29 (13.8%) had another episode of bacteremia a median of 14 days after the initial event (range = 6–18 days) with a different organism in 75% (n = 3/4). The infectious NRM rate in this group was 25% (5/20) (Table 1). Acknowledging the small numbers, we observed that 3/5 (60%) of deaths in this cohort occurred during the initial inpatient stay in the context of high-grade ICANS.
| No severe/life-threatening infection (n = 125) | 1 severe/life-threatening infection (n = 33) | 2+ severe/life-threatening infections (n = 20) | p value | |
|---|---|---|---|---|
| Age (years) | 58 (49–65) | 62 (53–66) | 60 (51.5–70) | 0.369 |
| Sex (male) | 79 (63.2%) | 18 (54.6%) | 13 (65.0%) | 0.629 |
| Subtype | ||||
| DLBCL | 82 (65.6%) | 23 (69.7%) | 8 (40.0%) | 0.175 |
| tFL/tMZL | 34 (27.2%) | 7 (21.2%) | 9 (45.0%) | |
| PMBCL | 6 (4.8%) | 2 (6.1%) | 3 (15.0%) | |
| Other/unknown | 3 (2.4%) | 1 (3.0%) | 0 (0.0%) | |
| CAR-T product | ||||
| Tisa-cel | 31 (24.8%) | 5 (15.2%) | 3 (15.0%) | 0.446 |
| Axi-cel | 94 (75.2%) | 28 (84.9%) | 17 (85.0%) | |
| Number of previous lines | ||||
| 1/2 | 83 (66.4%) | 21 (63.4%) | 10 (50.0%) | 0.365 |
| 3+ | 42 (33.6%) | 12 (36.4%) | 10 (50.0%) | |
| Pre-BT | ||||
| Stage pre-BT | ||||
| 1/2 | 29 (23.2%) | 2 (6.1%) | 3 (15.0%) | 0.049 |
| 3 | 17 (13.6%) | 9 (27.3%) | 1 (5.0%) | |
| 4 | 79 (63.2%) | 22 (66.7%) | 16 (80.0%) | |
| LDH (units/L) | 279 (219–387), n = 116 | 322 (223–639), n = 31 | 411 (252–560), n = 20 | 0.061 |
| Extra-nodal sites (≥3 vs. <3) | 5 (4.0%) | 6 (18.2%) | 3 (15.0%) | 0.008 |
| Post-BT | ||||
| Stage post-BT | ||||
| 0 | 12 (9.6%) | 4 (12.1%) | 3 (15.0%) | 0.230 |
| 1/2 | 23 (18.4%) | 2 (6.1%) | 2 (10.0%) | |
| 3 | 15 (12.0%) | 6 (18.2%) | 0 (0.0%) | |
| 4 | 75 (60.0%) | 21 (63.6%) | 15 (75.0%) | |
| Extra-nodal sites post-BT (≥3 vs. <3) | 15 (12.0%) | 5 (15.2%) | 3 (15.0%) | 0.726 |
| Response to BT (no BT = SD/PD) | ||||
| SD/PD | 75 (60.0%) | 19 (57.6%) | 14 (75.0%) | 0.398 |
| CR/PR | 50 (40.0%) | 14 (42.4%) | 5 (25.0%) | |
| Post-BT disease burden (N = 161)a | ||||
| Low | 63 (57.3%) | 13 (40.6%) | 8 (42.1%) | 0.163 |
| High | 47 (42.7%) | 19 (59.4%) | 11 (57.9%) | |
| Full blood count pre-LD | ||||
| Hemoglobin ×109/L | 110 (98–121), n = 122 | 106 (95–118) | 101 (90–117) | 0.258 |
| Neutrophils ×109/L | 3.1 (2.0–4.0), n = 122 | 2.8 (1.9–4.2) | 4.8 (2.9–11.9) | 0.023 |
| Lymphocytes ×109/L | 0.62 (0.34–0.93) | 0.82 (0.55–1.51) | 0.26 (0.22–0.46) | 0.001 |
| Platelets ×109/L | 185 (133–248) | 208 (124–259) | 190 (152–266) | 0.836 |
| Marker of disease or inflammation pre-LD | ||||
| LDH pre-LD (units/L) | 255 (192–347), n = 110 | 272 (216–432), n = 32 | 325 (238–604), n = 19 | 0.033 |
| CRP pre-LD or infusion (mg/L) | 6.8 (2.6–30.0), n = 96 | 4.7 (2.2–18.0), n = 30 | 42.7 (5.8–138.8), n = 16 | 0.033 |
| Ferritin pre-LD or infusion (µg/L) | 530 (193–1087), n = 101 | 600 (309–1507), n = 32 | 1368 (844–2822), n = 16 | 0.002 |
| Max CRS grade | ||||
| 0 | 27 (21.6%) | 10 (30.3%) | 2 (10.0%) | 0.383 |
| 1 | 42 (33.6%) | 9 (27.3%) | 4 (20.0%) | |
| 2 | 49 (39.2%) | 12 (36.4%) | 12 (60.0%) | |
| 3/4 | 7 (5.6%) | 2 (6.1%) | 2 (10.0%) | |
| Max ICANS grade | ||||
| 0 | 88 (70.4%) | 24 (72.7%) | 8 (40.0%) | 0.030 |
| 1/2 | 22 (17.6%) | 4 (12.1%) | 4 (20.0%) | |
| 3/4 | 15 (12.0%) | 5 (15.2%) | 8 (40.0%) | |
| Severe neutropenia | 106 (84.8%) | 23 (69.7%) | 17 (85.0%) | 0.124 |
| Steroids (any) | 42 (33.6%) | 16 (48.5%) | 15 (75.0%) | 0.001 |
| Steroid dose (if any) | 627 (160–2016), n = 42 | 760 (333–1197), n = 16 | 2245 (987–5970), n = 15 | 0.004 |
| Infectious NRM | 0 (0.0%) | 2 (6.1%) | 5 (25.0%) | <0.001 |
- Abbreviations: BT, bridging therapy; CR/PR, complete/partial remission; CRP, C-reactive protein; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; ICANS, immune effector cell-associated neurotoxicity syndrome; LD, lymphodepletion; LDH, lactate dehydrogenase; PMBCL, primary mediastinal B-cell lymphoma; SD/PD, stable/progressive disease; tFL/tMZL, transformed follicular/marginal zone lymphoma.
- a High burden post-BT = LDH > ULN (249 units/L) AND (stage 3/4 and/or ≥3 extra-nodal sites); Low burden post-BT = LDH < 250 units/L AND (stage 1/2 disease and/or <3 extra-nodal sites).
Risk factors for severe or life-threatening infection after CAR-T infusion
In univariate analyses, pre-BT factors associated with an increased risk of severe or life-threatening infection after infusion included stage 3/4 (vs. 1/2) disease, ≥3 extra-nodal sites, and LDH. Significant post-BT factors included ferritin, LDH, ECOG PS, and “high burden” pre-LD (Supporting Information S6: Table S8; and Figure S3). Toxicity management strategies, such as the use of tocilizumab, anakinra, and steroids, and ≥grade 3 ICANS were also associated with increased risk in univariate analyses. In multivariable models, an independent signal was observed between a high burden of disease post-BT and an increased risk of severe/life-threatening infection (Supporting Information S6: Table S9A). The impact of EN sites on infection risk was weakened after bridging therapy and our “high burden” variable reflected mainly disease stage 3–4 and LDH pre-LD (Table 9B). Patients with high burden were almost twice as likely as low burden to experience severe/life-threatening infection (hazard ratio [HR] = 1.72; 95% confidence interval [CI] = 0.97–3.04; p = 0.064). Neither the use of BT nor the response to BT had a clear impact on infection risk. However, those achieving CR/PR versus SD/PD with BT were less likely to suffer ≥2 infection events (25% vs. 75%) (Table 1).
CAR-T immunotoxicity (CRS/ICANS) was evaluated and an association between CRS and infection was not observed. An overall effect of ICANS was evident (p = 0.056), but patients with ≥grade 3 ICANS had almost threefold higher odds of severe or life-threatening infection in multivariate analyses (HR = 3.67; 95% CI = 1.42–9.51; p = 0.007) compared with patients without ICANS. The use of tocilizumab, steroids, and anakinra was collinear with ICANS and did not retain significance in the multivariable model. In our data set, neither baseline neutrophil count pre-LD, neutrophils below the lower limit of normal (<1.2 × 109/L) pre-LD nor severe neutropenia (<0.5 × 109/L) were associated with an increased risk of infection (Supporting Information S6: Table S8). Using time-varying analysis, considering the effect of an episode of severe neutropenia (of variable duration) during the patient's time at risk, that is, after CAR-T infusion and before the onset of infection or censoring event, no overall time-varying effect of severe neutropenia on the risk of severe/life-threatening infection in our cohort was found. Patients without ICANS had severe neutropenia (<0.5 × 109/L) for a median of 6 (IQR = 0–11) days compared with 9 (6–23.5) days for grades 1/2 and 7.5 (5–21) days for ≥grade 3 (p = 0.012). Among patients who developed ICANS (any grade), we found no effect of severe neutropenia on infection risk (HR = 0.88; 95% CI = 0.29–2.67; p = 0.82) and no effects if stratified by grade of ICANS.
Further exploration of severe or life-threatening infection by early (n = 48) versus late (n = 5) onset or by subtype of infection (bacterial n = 61, viral n = 19, fungal n = 13) was limited by small numbers.
Higher disease burden and infection risk
Patient demographics for those with a low and high burden of disease pre-LD were well matched in terms of age, number of prior lines of treatment, and CAR-T product choice (Supporting Information S6: Table S10). Those with a higher burden had higher baseline markers of inflammation (CRP and ferritin) and a lower baseline hemoglobin level. This cohort was also noted to have relatively higher baseline neutrophil counts and comparable lymphocyte counts pre-LD, suggesting that a prior chemotherapy-related immunological deficit was not contributing to infection risk. The risk of a severe/life-threatening event was higher for those with high versus low disease burden (39% vs. 25%), as was the risk of ≥2 events (14% vs. 10%); 100% (4/4) of early infectious NRM (<90 days) occurred in those with high-burden disease and 75% (3/4) who had ≥2 severe/life-threatening events (Table 1, and Supporting Information S6: Table S10). There were no clear differences in CAR-T immunotoxicity (CRS and ICANS), neutropenia, and steroid use between low and high-burden patients.
Looking specifically at those who developed ≥2 severe/life-threatening infectious events (n = 20), these patients were a particularly high-risk group with an infectious NRM rate of 25% (5/20) (Table 1). Pre-BT, patients in this high-risk cohort were more likely to have a higher stage of disease and higher LDH. Prior to LD, these patients also had a higher LDH and higher markers of inflammation (CRP and ferritin). Although these patients had comparable neutrophil counts, a lower lymphocyte count was noted, potentially suggestive of poor recovery post-BT. Post-infusion, this cohort was more likely to develop high-grade ICANS and require steroids and higher doses thereof. The second isolate was detected during an episode of ICANS or ongoing steroid use for ICANS in 37% of cases (7/20) with an associated mortality of 43% (3/7).
Extra-nodal sites and risk of infection
We observed a pattern of infection that appeared to correlate with commensals at the EN site or infection of the EN site itself. A total of 122 patients (68.5%) had lymphomatous involvement of ≥1 EN site at the time of CAR-T approval, of whom 39 (32%) acquired a severe/life-threatening infection. Of those with extra-nodal gastrointestinal (GI) tract involvement pre and/or post-BT (gastric, mesenteric, and small/large intestinal wall) and a severe/life-threatening event (n = 14), a GI commensal organism was isolated in blood in eight patients (57%) with ≥2 GI commensals detected in four patients (29%). Likewise, of those with extra-nodal lung parenchymal involvement pre and/or post-BT and a severe/life-threatening event (n = 7), 86% (6/7) developed a severe/life-threatening event involving the lung tissue during follow-up with 29% (2/7) suffering ≥2 lung-related events (Figures S4A and S4B).
Although GI organisms can commonly complicate the neutropenic posttreatment phase and lung tissue is a high-risk site for infection, it is possible that abnormal anatomy at EN sites, combined with T-cell-driven tumor lysis and necrosis may facilitate the breach of immunological defenses and entry of commensal organisms at these sites.
Infectious NRM and the risk of “cold sepsis”
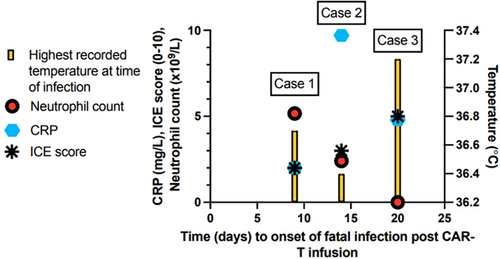
There were 66 deaths among the 178 patients, with a 12-month overall survival of 68% from the date of CAR-T infusion (Supporting Information S6: Table S11). Of 66 deaths, the vast majority were due to progressive disease (PD), but a total of seven were directly due to infection (Supporting Information S6: Table S12) and occurred at a median of 46 days post infusion (range = 15–257). Overall, 3.9% of 178 infused patients died post-CAR-T from infectious complications before progression or end of follow-up. In 2/7 cases, death was due to SARS CoV-2 complications in two tisa-cel recipients. In 5/7 cases, death from infection occurred in axi-cel recipients with active (n = 4) or resolved (n = 1) high-grade ICANS treated with corticosteroids (median total methylprednisolone dose of 1867 mg by time of death, range = 213–7133 mg). Of those with high-burden disease pre-LD who subsequently developed high-grade ICANS (n = 15, 19.5%), mortality from infection was 27% (n = 4). This cohort accounted for 100% of early NRM deaths (<90 days). All four patients had active high-grade ICANS and were still receiving steroids at the time of death, 3/4 were receiving anakinra and 3/4 did not mount a fever during the fatal infectious event. Two patients (29%) had no neutrophil recovery at the time of death. Figure 4 illustrates the clinical trajectory of our three cases of cold sepsis, demonstrating the issue of normothermia with no CRP response in this high-risk group. No early infectious deaths occurred in low-burden patients.
Consideration of antibacterial prophylaxis in a selected subgroup
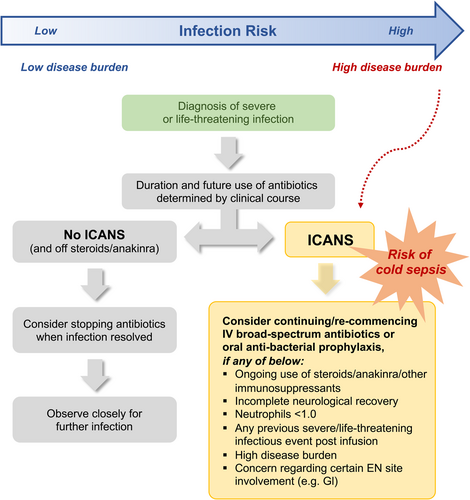
None of our patients received a prophylactic antibacterial agent. In our data set, 22/28 (79%) Gram-negative organisms isolated from 20 patients with a severe/life-threatening infection during the CAR-T inpatient stay were deemed sensitive to a fluoroquinolone (ciprofloxacin), including in two cases of early infectious NRM. Hence, this agent may have conferred benefits in select cases (data not shown). In our data set, those with higher disease burden were at higher risk of severe/life-threatening infection. However, disease burden alone may not warrant anti-bacterial prophylaxis. The subset of high-burden patients who develop high-grade ICANS, requiring ongoing steroids/interleukin blockade with/without neutrophil and/or neurological recovery, should be considered for antibacterial prophylaxis. This subset of patients is at high risk of sepsis, including cold sepsis and early infectious NRM. Lymphomatous involvement of specific EN sites (e.g., GI tract) may confer an additional risk of infection with commensals. We propose a risk-based strategy to guide prophylactic antibiotic use and encourage heightened clinician awareness of cold sepsis, with a view to reducing early NRM (Figure 5).
Use of IVIG after CAR-T therapy
Of 172 patients alive beyond 30 days, 17/172 (9.9%) received IVIG, commenced at a median of 3 months postinfusion (range = 1–21), and continued until death or end of follow-up in 88.2% (n = 15/17).
Median age was 55 years (range = 19–70) and the majority (82.4%; 14/17) had received axi-cel. In 13/17 (76%) patients, IVIG was commenced for recurrent sinopulmonary infections (Supporting Information S6: Table S13) and 46% (6/13) of these patients had sinopulmonary EN involvement on a PET scan prior to LD. Of those with sinopulmonary involvement at the point of submission for CAR-T (n = 22), 23% (n = 5) ultimately required IVIG post-CAR-T.
Only one episode of infectious NRM occurred in this cohort (SARS CoV2). Infection was a cause or significant contributor to death in the context of progressive disease in two other cases.
Data on T-cell reconstitution post-CAR-T therapy was available in only a subset of patients but demonstrated incomplete T-cell recovery in a significant proportion at 12 months (36%). Small numbers limited further analysis of these patients (Supporting Information S6: Table S14).
Infection in the context of progressive disease post-CAR-T therapy
With a median follow-up of 291 days (IQR = 175–484 days, range = 4–886 days), median progression-free survival from infusion for the entire cohort (n = 178) and those alive beyond 30 days was 251 days (IQR = 97–432 days, range = 4–886 days) and 261 days (IQR = 133–460, range = 33–886), respectively. Of the 83 patients who progressed after CAR-T, 65% (n = 54) commenced further therapy. A total of 17 patients (20.5%) developed a severe or life-threatening infectious event after CAR-T relapse. In 9 of 17 patients (53%), infection contributed significantly or directly led to death (Supporting Information S6: Table S15).
DISCUSSION
A myriad of host and treatment-related factors contribute to infection risk post CAR-T infusion.7, 12, 28-30 These factors are likely to have a cumulative effect. The incidence and distribution of all grade (52.7%) and severe/life-threatening (29.8%) infections in our cohort is similar to other published series in this patient group.8, 14 Acknowledging an exclusively LBCL population, we did not find a signal for variables previously reported by others, such as high-grade CRS5, 6 or a number of prior lines of therapy.4 Akin to other groups, we have demonstrated that impaired ECOG PS, steroids, markers of inflammation such as ferritin, and high-grade ICANS are associated with infection.8, 14, 31 However, our multivariate analysis identifies disease burden as independently associated with severe or life-threatening events in our cohort. Rejeski et al. retrospectively stratified infection risk within 90 days by the HT score, potentially identifying a subset that may benefit from antibacterial prophylaxis.8 Our study builds on this work in a large cohort of LBCL patients receiving standard-of-care CAR-T products, with a focus on the role of lymphoma disease burden and site of disease in helping to identify those with the highest mortality risk from infection.
Active malignancy is known to predispose to infection via a number of mechanisms. Disease alone, even in untreated patients, is thought to blunt innate and adaptive immune responses.32, 33 Cancer-associated cachexia with associated inflammation and malnutrition is a catabolic state that can compromise host defenses, such as tissue healing, mucosal integrity, secretory IgA function, complement pathways, and cellular immunity.34-36 Tumor infiltration and obstruction may disrupt the integrity of anatomical barriers, such as the skin and mucosal surfaces. Pulmonary tissue immunology may be particularly vulnerable to local tumor invasion leading to impaired muco-ciliary clearance and postobstructive atelectasis. Likewise with gut, oropharyngeal, and skin infiltration by cancerous tissue, local or systemic infection with tissue-specific resident flora is a recognized phenomenon.37-39 Acknowledging small numbers, our data raise the possibility that EN involvement, particularly of the GI and sinopulmonary tract, may increase susceptibility to infection, often with resident commensal organisms. Awareness of this phenomenon may allow for a higher index of suspicion for potential offending pathogens and tailored antibiotic use. For instance, a longer duration of antibiotics may be considered for certain patients, particularly those with EN GI tract involvement in the context of neutropenia.
The potential correlation of EN site with site-specific infection is also supported by our data on IVIG use post infusion. Although both congenital40, 41 and treatment-related42-44 hypogammaglobulinemia are known to manifest with sinopulmonary complications, our data suggest that patients with EN sinopulmonary involvement prior to CAR-T infusion are at higher risk of this clinical phenotype. Acknowledging the absolute numbers for immunoglobulin replacement were small (n = 17, 9.9%), 46% of those requiring IVIG for sinopulmonary infection post CAR-T (6/13) had radiological evidence of sinopulmonary EN disease prior to LD. This supports our theory that anatomical disruption at high-risk sites in susceptible individuals, such as the sinopulmonary tract, may contribute to infection risk. This encourages vigilance in select patients and supports early instigation of IVIG replacement to prevent later infections and further sinopulmonary damage in this cohort.
Higher disease burden and markers of inflammation are known to correlate with higher grades of CAR-T immunotoxicity in similar real-world cohorts45, 46 and other disease indications.47 Therefore, it could easily be inferred that the infection risk in high-burden patients may be attributable to the immunotoxicity itself or the employment of steroids and interleukin blockade to mitigate high-grade toxicity. However, in our data set, the risk of a severe/life-threatening event was substantially higher for those with high versus low disease burden (39% vs. 25%) and there were no clear statistically significant differences in CRS or ICANS grade or steroid/tocilizumab use between those with low and high burden, especially when considering those who developed any severe or life-threatening infectious event. The use of tocilizumab, anakinra, and steroids was significant for infection on univariate analysis only. On multivariate analysis, their use was collinear with ICANS and did not retain significance. Our data suggests that immunotoxicity and agents to control immunotoxicity were not the main drivers of severe/life-threatening infection, particularly when considering those who experienced a single event. However, those developing ≥2 severe or life-threatening infection events emerged as a uniquely high-risk cohort, with significantly higher rates of high-grade ICANS, steroid use, and infectious NRM (25%). The occurrence of a second (or beyond) severe/life-threatening event during an episode of ICANS or while on steroids ± anakinra for ICANS led to 43% mortality.
Therefore, we propose that disease burden and inflammation increase baseline infection risk. Additionally, we propose that the emergence of ICANS with the associated immunosuppressive treatments in high-burden patients selects out those at the highest risk of repeated infections and infectious NRM. All early infectious deaths (<90 days) (n = 4) occurred in those with high burden who subsequently developed high-grade ICANS. The incidence of infectious NRM in our data set is comparable with other groups.7, 8, 48 We also report the first cases of “cold sepsis” after CAR-T therapy, namely the lack of an appreciable systemic inflammatory response, such as fever and raised CRP, at the time of detection of infection. The phenomenon of “cold sepsis” has been described in other patient groups and is historically associated with a higher mortality.49-53 While the pathophysiology is debated,54, 55 the risk of a delayed diagnosis of sepsis is undoubtedly higher when the conventional clinical and laboratory flags for infection, namely fever and raised CRP, are absent. In the context of CAR-T therapy, the use of steroids and/or interleukin blockade may suppress a hyperthermic or inflammatory response in patients, many of whom may have incomplete neutrophil and/or neurological recovery. We highlight three cases of cold sepsis, occurring with or without neutrophil recovery in the context of ongoing steroid and/or anakinra use for high-grade ICANS. The absence of clinical or biochemical signs of infection supports the need for heightened clinical awareness, enhanced surveillance, and education of ward-based staff. It is possible that a risk-based mitigation strategy for cold sepsis incorporating biomarkers may permit risk stratification, earlier detection, and even pre-emptive treatment with early antibiotics.56-58 Such a tool would require robust, validated biomarkers for infection and prospective validation in a clinical study. Pending the above, we propose a clinical risk-based strategy for cold sepsis and antibiotic use for patients receiving CAR-T therapy (Figure 5).
The role of antibacterial prophylaxis post-CAR-T infusion is unclear. Rejeski et al. retrospectively stratified infection risk within 90 days by the HT score, potentially identifying a subset that may benefit from antibacterial prophylaxis.8 IDSA guidance supports fluoroquinolones for those at high risk of febrile or prolonged neutropenia.59 Its routine use to cover the neutropenic period post-LD and an infusion is not recommended by EBMT/European Haematology Association but can be considered at a local level in the context of prolonged neutropenia.22 Updated best practice recommendations suggest prophylaxis in select cases considering immune effector cell-associated hematotoxicity grading.60 Based on our experience with this patient cohort, we propose that patients with “high burden” post-BT who subsequently develop high-grade ICANS, requiring ongoing steroids/interleukin blockade with/without neutrophil recovery, particularly those with ≥1 prior severe/life-threatening event, should be considered for prophylactic antibiotics to mitigate the risk of cold sepsis and early NRM. Consideration can also be given to those with EN involvement at specific sites (e.g., GI tract). However, the use of ciprofloxacin prophylaxis has to be balanced against the risk (both for the individual patient and the wider healthcare setting) of promoting the emergence of quinolone-resistant Gram-negative bacterial infection. Additional risks to be considered are breakthrough infections with intrinsically less susceptible organisms such as enterococci, as well as antibiotic-related adverse events including Clostridium difficile infection.59, 61, 62
There is no consensus on the early or prophylactic use of myeloid growth factors to facilitate count recovery post CAR-T therapy and prospective clinical studies are required to establish the safety and optimal use of these agents. Historically, caution has been advised given the theoretical risk of exacerbating CRS and ICANS.63, 64 However, some have demonstrated the safety of this approach with a reduced incidence of febrile neutropenia in the absence of increased immunotoxicity risk.65 A retrospective study by Miller et al. (n = 197) demonstrated that prophylactic GCSF led to faster neutrophil recovery and comparable treatment outcomes without increasing the risk of high-grade ICANS. Higher rates of ≥grade 2 CRS were reported in this study.66
SARS CoV-2 was the most common severe or life-threatening viral infection in our cohort with two late fatalities. The reported incidence of severe disease and mortality in CAR-T patients has fallen, likely due to a combination of less virulent variants, vaccination programs, and pre-emptive SARS-CoV-2-directed pharmacotherapies.67-69 Vaccine-induced T-cell responses may provide some protection in those with ongoing B-cell aplasia and impaired antibody responses.70 However, despite advances, morbidity and mortality risk in this vulnerable cohort persists. Annual vaccination of CAR-T recipients and close contacts against Influenza (inactivated) is also recommended to reduce the risk of severe disease and hospitalization.64 Reactivation of latent viruses such as CMV has been reported, with the incidence ranging from 21% to 44%.71, 72 CMV reactivation was noted in seven of our patients. However, the probability of progression to end-organ disease in this patient group remains unknown. In the absence of systematic viral polymerase chain reaction monitoring in most centers, Marquez-Algaba et al. propose a risk-based surveillance strategy for CMV reactivation.72
This retrospective real-world study has limitations. In the absence of the ready availability of metabolic tumor volume, low and high disease burdens after bridging therapy were categorized using LDH, stage, and number of extra-nodal sites. The authors accept that this is not a universally accepted definition and larger data sets will be required to validate our findings. It is also possible that some severe/life-threatening infections, particularly post relapse, may not have been communicated to our teams. Skin commensals were included as potential pathogens if the clinical team treated them as such; some of these isolates may represent contaminants. Our data set did not include information on recent infections (including during BT), neutrophil recovery patterns, or HT scores (due to missing data points). Such variables are recognized risk factors for infection post-CAR-T infusion4, 8 and the authors acknowledge this as a limitation of our work.
With rapidly expanding CAR-T use, a clear understanding of the core factors driving CAR-T toxicity and the projected burden on hospital resources has become increasingly important. Outpatient or ambulatory care delivery is an attractive option for the substantial proportion of patients who are at lower risk of high-grade complications.73 Proposed CRS, ICANS, and cytopenia risk scores have the potential to identify higher-risk LBCL candidates in the real world17, 74, 75 with continued improvement in postinfusion care and burden of morbidity. However, infection, the chief cause of NRM, has emerged as the biggest clinical challenge, leading to prolonged hospital admissions, intensive care unit involvement, and significant morbidity. The risk of infection post infusion is highly variable, with up to 50% of patients remaining infection-free8, 28, 31 while others may suffer multiple severe, life-threatening, or fatal infectious events. Knowledge of predisposing factors could allow a more nuanced patient-specific CAR-T management pathway, with targeted interventions in acute and longer-term settings.
In summary, disease burden is a key risk factor for infectious complications post CAR-T therapy. The site of the disease may have implications for offending pathogens and targeted antibiotic use. The requirement for IVIG in our data set was low but concentrated in those with a history of sinopulmonary EN involvement. Better bridging strategies aimed at achieving disease control pre-infusion should be explored, with the potential added benefit of reducing the risk of high-grade toxicity. Cold sepsis represents a significant mortality risk to patients receiving steroids and/or interleukin blockade for immunotoxicity post CAR-T and additional measures are required to maintain safety in this cohort. Prospective validation of pre-emptive strategies, such as the use of antimicrobial prophylaxis, early GCSF, and the development of novel infection biomarker-based screening approaches should be encouraged in the real world to support their use.
AUTHOR CONTRIBUTIONS
Maeve A. O'Reilly, Lorna Neill, Claire Roddie, and Simon M. Collin led the concept and design of this document and prepared the manuscript. Simon M. Collin also analyzed the data. Deborah Springell, Jeremy Mensah, Kathleen P. L. Cheok, and Katarzyna Jalowiec contributed to data collection. All authors contributed to the preparation and approval of the final submitted version.
CONFLICT OF INTEREST STATEMENT
M. O. R.: Honoraria Kite Gilead, Novartis, and Janssen, Advisory boards Kite Gilead and Autolus, conference support Kite Gilead and Novartis. S. M. C.: Employee of AstraZeneca Ltd. N. S.: Speaker fees Kite Gilead, Pfizer and Shionogi, research grant Kite Gilead. K. P. L. C.: Honoraria Kite Gilead. A. K.: Kite Gilead conference support, honoraria, advisory board, Novartis: honoraria, research funding, Advisory board: Roche, Abbvie, BMS. C. R.: Advisory boards and speakers fees Novartis, Kite Gilead, BMS, Amgen, Autolus. R. S.: Kite Gilead speakers bureau, honoraria, conference travel, Novartis speakers bureau, honoraria, conference travel. The other authors declare no conflict of interest.
FUNDING
This research received no funding.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.