Treatment of advanced squamous cell carcinoma of the external auditory canal: Critical analysis of persistent failures in diagnosis and surgery with a competing-risk model
Abstract
Background
A series of temporal bone squamous cell carcinomas (TBSCCs) was analyzed with the aim of (i) better understanding the causes for the persistent high failure rate in advanced SCCs and (ii) discussing a possible way out from this stalemate in treatment.
Methods
Forty-five TBSCCs consecutively treated surgically were reviewed.
Results
The 5-year cumulative incidence for postoperative local recurrence was 41.8%. At multivariable analysis, pT3-4 stages were associated with eightfold relative incidence of developing local recurrence during follow-up (sHR = 9.06, 95% confidence interval [CI] = 1.18–69.46, p = 0.034) and cause-specific death (sHR = 7.95, 95%CI = 1.01–62.27, p = 0.048).
Conclusions
The poor outcome in advanced TBSCC occurred because of local recurrence due to defective resection. The fundamental pitfall of surgery on advanced TBSCC appeared to be the insufficient knowledge of microscopic tumor growth in the different sites and subsites of the temporal bone. The serial histopathological study of the en bloc surgical specimen and autopsy temporal bones seems to represent a way to enhance our understanding of these tumors.
1 INTRODUCTION
Squamous cell carcinomas (SCCs) of the external auditory canal are classified as advanced tumors (T3-T4) when they grow beyond the canal walls.1-3 SCC erosion of the osseous canal, tumor growth in the temporal bone and close sites are categories of both TNM Pittsburgh1, 2 and AJCC3 classification systems. The Pittsburgh classification refers to full thickness erosion of the osseous canal and tumor growth into specific sites of the temporal bone and adjacent soft tissues.
The AJCC uses generic criteria of tumor size and cortical or gross bone erosion and skull base invasion. The prognosis of advanced SCCs is poor despite aggressive therapy combining surgery, radiotherapy, and chemotherapy. Growth beyond the external auditory canal walls as well as the dismal surgical outcome raised several problems which prompted this study. Our series of external auditory canal SCCs was critically analyzed with the aim of (i) better understanding the causes for the persistent high failure rate in advanced SCCs and (ii) discussing a possible way out from this stalemate in treatment.
2 MATERIALS AND METHODS
2.1 Patients
The medical charts of patients undergoing primary surgery for malignancies involving the temporal bone in the years between 1980 and 2015 were considered. The study was conducted in accordance with the principles of the Helsinki Declaration. All data were examined in agreement with Italian privacy and sensitive data laws and the in-house rules of the Otolaryngology Section at Padova University (Italy). Before undergoing surgery, all patients operated on between 2005 and 2015 preoperatively signed a consent form for disclosure of privacy in managing personal data for scientific purposes. In particular, they consented “to the use of their clinical data for scientific research purposes in the medical, biomedical, and epidemiological fields, also in order to be recalled in the future for follow-up needs.” Alive patients treated before 2005 were retrieved and signed a retrospective detailed informed consent. The medical charts of all patients from our group undergoing primary surgery for malignancies involving the temporal bone were considered. Non-SCC, tumors of the auricle or parotid extending to the EAC were excluded. This study retrospectively included 45 consecutive patients surgically treated for primary temporal bone SCC (TBSCC), according to the principles of en bloc resection.4
Preoperatively, the patients underwent micro-otoscopy with biopsy, temporal bone computerized tomography (CT) and/or contrast-enhanced magnetic resonance imaging (ceMRI), neck ultrasonography (with or without fine needle aspiration cytology), chest X-ray, and liver ultrasonography, as previously reported.5, 6 Positron emission tomography (PET) was used in selected cases. Tumors were classified according to the revised Pittsburgh classification system.2
The senior surgeon of our surgical team operated on all the cases. Parotidectomy and neck dissection were variably performed according to the following criteria. Superficial parotidectomy was performed as a prophylactic measure in all locally advanced cases (T3-T4) and in most T2 cases. Total parotidectomy was performed when intraparotid nodes were involved or direct tumor infiltration through the anterior wall of the external auditory canal was diagnosed. The cN0 cases were treated by elective selective neck dissection, the cN+ cases by type III modified radical neck dissection. Parotidectomy and/or neck dissection were not performed in a few elderly patients where adjuvant RT was planned or if already performed in cases of recurrences. Adjuvant RT was indicated in cases of advanced tumors, positive or close (<5 mm) margins, neck nodes metastases, extracapsular spread, and in all those cases where aggressive pathological features were evidenced at pathology, as reported in our previous paper.7
Patients were followed up clinically every 3 months in the first year, then every 6 months up until the fifth year, and then annually. ceMRI or contrast-enhanced CT (ceCT), if MRI was unavailable, was performed every 6 months in the first year and annually thereafter. Neck ultrasonography and chest X-rays were also performed at least annually.
2.2 Statistical analysis
Continuous variables have been reported as median and range, categorical variables as numbers and percentages. The Fisher's exact test or the chi-square test were applied for comparison, as appropriate. Survival analysis considered local recurrence-free survival (expressed as the time between the end of primary treatment and TBSCC recurrence) and disease-specific survival (expressed as the time between the end of primary treatment and death due to TBSCC recurrence) as main outcome measures. For event-free patients, data were censored at date of last follow-up control. Given the long follow-up period and high mortality rate due to causes other than the TBSCC, a competing risk analysis was performed, estimating the probability of local disease recurrence or disease-specific death in the presence of a competing event, which was death from other causes. The cumulative incidence (CI) functions for the given outcomes were calculated at 5 years and stratified according to relevant patient and tumor variables. Gray's test was applied for CI comparison. Subsequently, the regression model for subdistribution hazard according to Fine and Gray was adopted to estimate the effect of the variables on the CI functions, resulting in the subdistribution hazard rates (sHR) determination. Multivariable analysis was conducted for covariates with an inclusion value of p < 0.05 at univariate analysis, after checking for multi-collinearity. All tests were two-sided. The best model was selected according to a stepwise selection based on the Akaike information criterion. p-values <0.05 determined statistical significance. EZR, a modified version of R Commander,8 was used for all analyses.
3 RESULTS
3.1 Demographics and surgical results
The 45 patients (25 females [55.6%] and 20 males [44.4%]) had a median age at diagnosis of 60 years (range 37–82). According to the revised Pittsburgh classification system, 8 cases were cT1 (17.8%), 10 cT2 (22.2%), 14 cT3 (31.1%), and 13 cT4 (28.9%); five patients resulted cN+ (11.1%). Thirty-one patients (68.9%) underwent en bloc lateral temporal bone resection (LTBR), and 14 (31.1%) en bloc subtotal temporal bone resection (STBR), of whom 2 were submitted to incomplete resection partly with a piecemeal technique for palliative intent, and were excluded from the analysis. Parotidectomy was performed in 43 cases (95.6%) and neck dissection in 38 (84.4%). At diagnosis, the facial nerve function was clinically impaired in 8 cases (17.8%); 21 patients (46.7%) underwent facial nerve sacrifice during surgery because of clinical involvement, or as part of an en bloc subtotal temporal bone resection on tumor-free margins. Pathological analysis revealed 7 pT1 cases (15.5%), 7 pT2 (15.5%), 8 pT3 (17.8%), and 23 pT4 (51.2%) at pT classification. Overall, 12 patients (26.7%) were reclassified after pathological analysis to higher revised Pittsburgh classification, namely 1 cT1 (8.3%), 3 cT2 (25%), and 8 cT3 cases (66.7%). Among those who underwent neck dissection, 29 patients were pN0 (76.3%), 5 pN1 (13.2%), 3 pN2a (7.9%), and 1 pN2b (2.6%). The pathological grading of most of the patients was G1 (25 cases, 55.6%); on the other hand, 15 cases were G2 (33.3%), and 5 G3 (11.1%). Postoperative radiotherapy was administered in 26 patients (57.8,7 but none received preoperative or postoperative chemotherapy.
3.2 Survival analysis
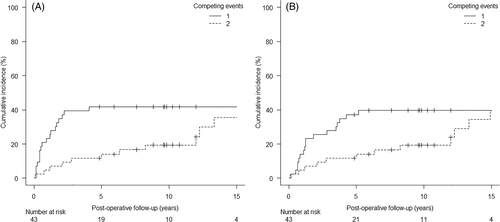
The analysis of survival was conducted on 43 patients, the two palliative cases being excluded. The median overall post treatment follow-up was 58 months (range 1–232), and 118 months for the censored cases (range 58–232). At last follow-up, 13 patients (30.2%) were alive without evidence of disease, 17 patients (39.6%) had died of the disease, and 13 (30.2%) had died of tumor-unrelated causes. Locoregional recurrence rate was 41.8% (18/43 cases): in particular, among these patients, 16 had developed a local recurrence (37.2%), while 2 (4.4%) only a nodal recurrence.
Given that the absolute percentage of deaths from other causes was over 10%, a competing risk analysis was adopted.9 The 5-year CI for postoperative local recurrence was 41.8% (95% CI = 27.1–55.9), as depicted in Figure 1A. The 5-year CI stratified according to the considered variables is summarized in Table 1. Clinical and pathological T stages, clinical nodal stage, and tumor grading showed a significant association with a higher local recurrence incidence. Regression analysis results for local recurrence are shown in Table 2. At multivariable analysis, pT3-4 stage were associated with a ninefold relative incidence of developing local recurrence during follow-up (sHR = 9.06, 95% CI = 1.18–69.46, p = 0.034).
| Variable | Local disease recurrence | Disease-specific mortality | ||
|---|---|---|---|---|
| 5-year, % (95% CI) | Comparison of CI p-valuea | 5-year, % (95% CI) | Comparison of CI p-valuea | |
| Overall | 41.8 (27.1–55.9) | NA | 37.2 (23.1–51.3) | NA |
| Sex | ||||
| Male | 40.0 (19.3–60.0) | 0.900 | 30.0 (12.3–50.1) | 0.569 |
| Female | 43.5 (23.3–62.1) | 43.5 (23.3–62.1) | ||
| Age | ||||
| ≤60 years | 45.8 (25.6–64.0) | 0.628 | 37.5 (19.0–56.0) | 0.785 |
| >60 years | 36.8 (16.5–57.5) | 36.8 (16.5–57.5) | ||
| cT stage | ||||
| cT 1–2 | 11.2 (19.0–29.8) | 0.001 | 11.1 (1.9–29.8) | 0.0023 |
| cT 3–4 | 64.0 (42.2–79.4) | 56.0 (34.8–72.7) | ||
| pT stage | ||||
| pT 1–2 | 7.1 (0.5–27.5) | 0.0024 | 7.1 (5.0–27.5) | 0.0041 |
| pT 3–4 | 58.6 (38.8–74.0) | 51.7 (32.5–67.9) | ||
| N stage | ||||
| N0 (c/pN0) | 37.1 (21.6–52.7) | 0.152 | 31.4 (17.1–46.8) | 0.131 |
| pN+ | 62.5 (22.9–86.1) | 62.5 (22.9–86.1) | ||
| Grading | ||||
| G1 | 25.0 (10.2–43.1) | 0.02 | 16.6 (5.2–33.7) | 0.0078 |
| G2-3 | 63.2 (37.9–80.4) | 63.1 (37.9–80.4) | ||
- Note: Death from other causes is the competing event. p < 0.05 with statistical significance are evidenced in italics.
- Abbreviations: 95% CI, 95% confidence interval; CI, cumulative incidence.
- a Gray's test for comparison of cumulative incidence functions.
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| sHR (95% CI) | p-value | sHR (95% CI) | p-value | |
| Sex | ||||
| Male | Reference | 0.900 | ||
| Female | 1.06 (0.43, 2.61) | |||
| Age | ||||
| ≤60 years | Reference | 0.630 | ||
| >60 years | 0.79 (0.31, 2.01) | |||
| cT stage | ||||
| cT 1–2 | Reference | 0.0073 | ||
| cT 3–4 | 7.92 (1.74, 35.95) | |||
| pT stage | ||||
| pT 1–2 | Reference | 0.020 | Reference | 0.034 |
| pT 3–4 | 11.25 (1.46, 85.76) | 9.06 (1.18, 69.46) | ||
| N stage | ||||
| N0 (c/pN0) | Reference | 0.140 | ||
| pN+ | 2.11 (0.77, 5.76) | |||
| Grading | ||||
| G1 | Reference | 0.026 | Reference | 0.130 |
| G2-3 | 3.04 (1.14, 8.07) | 2.09 (0.80, 5.50) | ||
- Note: Death from other causes is the competing event; p < 0.05 is evidenced in italics.
- Abbreviations: 95% CI, 95% confidence interval; sHR, subdistribution hazard rate.
The 5-year CI for disease-specific mortality was 37.2% (95% CI = 23.1–51.3) (Figure 1B). Table 1 shows the 5-year CI stratified according to the relevant variables. As observed for recurrence-free survival, clinical and pathological T stages, clinical nodal stage and tumor grading had a significant impact on disease-specific mortality. Regression analysis results for disease-specific mortality are summarized in Table 3. At multivariate analysis, pT3-4 stage was associated with an almost eightfold relative incidence of cause-specific mortality (sHR = 7.95, 95% CI = 1.01–62.27, p = 0.048).
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| sHR (95% CI) | p-value | sHR (95% CI) | p-value | |
| Sex | ||||
| Male | Reference | 0.560 | ||
| Female | 1.32 (0.51, 3.38) | |||
| Age | ||||
| ≤60 years | Reference | 0.780 | ||
| >60 years | 0.87 (0.34, 2.25) | |||
| cT stage | ||||
| cT 1–2 | Reference | 0.011 | ||
| cT 3–4 | 7.16 (1.56, 32.88) | |||
| pT stage | ||||
| pT 1–2 | Reference | 0.026 | Reference | 0.048 |
| pT 3–4 | 10.34 (1.33, 80.44) | 7.95 (1.01, 62.27) | ||
| N stage | ||||
| N0 (c/pN0) | Reference | 0.110 | ||
| pN+ | 2.27 (0.83, 6.22) | |||
| Grading | ||||
| G1 | Reference | 0.013 | Reference | 0.057 |
| G2-3 | 3.86 (1.33, 11.16) | 2.82 (0.97, 8.20) | ||
- Note: Death from other causes is the competing event; p < 0.05 is evidenced in italics.
- Abbreviations: 95% CI, 95% confidence interval; sHR, subdistribution hazard rate.
4 DISCUSSION
The alarming aspect of our experience in this specific field was the high local recurrence rate, which was by far the main cause of failure. The 5-year CI for postoperative local recurrence and for disease-specific mortality were 41.8% and 37.2%, respectively. The preoperative clinical classifications changed to higher postoperative pathological revised Pittsburgh classification for more than one fourth of patients (26.7%), of which 66.7% were initially considered as cT3 lesions. This occurred with a 40% rate in the 1980s, when imaging was provided only by CT, and thereafter dropped to 22% with combined CT-MRI. Our results were consistent with other studies, considering en bloc,10-19 piecemeal,20-23 and en bloc LTBR-piecemeal STTBR24-31 resections.
Starting from these premises, our management rationale has been critically analyzed. Other factors such as the complex anatomy of the temporal bone and the little room allowed for resection within safe margins were felt to be contributory to the persistent failure of surgery. The local recurrence/persistence, as new tumor from normal epithelium or from a residual tumor both left by the resection, was the starting point for our reappraisal of the surgery outcome. The hypothesis of a metachronous new tumor from residual epidermis remained unassessed but appeared to be weak because of the high rate of supposed surgical errors. Residual tumor was then considered the origin of recurrence.
The tumor extent was provided by imaging and steered the en bloc resection which was planned with radical intent within radiological safe margins. The presence of residual tumor implied however that both at the time of diagnosis and surgery the correct extent and margins were missed. This is also suggested by the overall change to a higher class from preoperative clinical to postoperative pathological categories. The subsites of the missed tumor could not be appreciated at postoperative imaging, which only showed a macroscopic mass in the surgical field and could not detail the potential subsites of microscopic diffusion. The persistent pitfall appeared to be a defective assessment of malignancy extent and pointed to the insufficient knowledge of the modalities of tumor growth in the temporal bone. This problem had been studied by Arriaga et al.1 with the clinical-radiographic-histopathological analysis of temporal bone surgical specimens. Serial sections of the full bone were performed in 13 cases. Six of them were staged as T4, and 12 critical areas were examined for CT-pathological correlations, which showed a correspondence between positive and negative findings in all but one case. As reported by the authors, substantial portions of the specimens were discarded without histological processing, namely portions of the otic capsule were included in 6 of the 13 specimens, the inner ear was included in the section in one case only. The petrous apex was not mentioned. The authors pointed out that microscopic tumor progression was definitely difficult to ascertain. The foresighted intent by Arriaga et al.1 to propose a clinical-histopathological classification was thus based on a setting where several data on microscopic tumor diffusion could have been missed, including tumor margins. In fact, DOD occurred in 25% of free margins cases and NED was found in 25% of margins positive cases, thus supporting the hypothesis that findings on margins could have been missed. Since the Arriaga et al.1 investigation, the lack of serial studies on full specimens has been a persistent pitfall that has prevented further progress in the evaluation of resection appropriateness at pathology.
The current debate on TBSCC prognostic factors local failure and outcome of advanced tumors1, 2, 5, 6, 12, 13, 16, 18, 22, 26, 27, 30-45 continued with the original bias of defective data provided by imaging and histopathology. The understanding of local failure due to an undetected tumor emerged as the main unsolved problem. Although already recognized by Arriaga et al.,1 histopathological evaluations and images of the full temporal bone were not reported in clinical studies, but only in the pathology atlas of the ear46 until recent papers.47-50 Ungar et al.50 described the carcinoma growth from mastoid and tympanum to the apex along the peri-labyrinthine cells, as well as the areas of resistance to growth, and offered a fundamental contribution to surgery. On the other hand, the correlation between tumor extent at histopathology and radiological evidence could not be investigated, due to the lack of appropriate imaging, as pointed out by the authors.
The current staging systems for TBSCC,2 as well as the recently proposed systems18, 43, 51-53 are based on various tumor features, of which its “extent” plays the main role. Tumor extent includes more than quantitative data, as malignancy growth is also affected by involved subsites and varying temporal bone architecture. Thus, the meaning of “extent” as an index of severity relates to (i) extent, (ii) subsites involved, (iii) bone architecture. Current staging2, 3 AJCC and Pittsburgh follows the association tumor-treatment-outcome, but each of these categories has a precarious basis in advanced cases (T3-T4). “Tumor” relates to the pitfall of dubious extent; “treatment” implies variable surgical techniques and heterogeneous procedures, in which crucial steps for radicality are not standardized and differ from one surgeon to another; “outcome” lacks information on undetected microscopic diffusion and subsites where recurrence occurs. Inconsistency of both extent and surgery add up to hinder the foundation of current classifications. It seems to us that an ideal classification should be based on safe categories not liable to errors. The recent Padova scoring system51, 53, 54 on advanced tumor proposed a classification on easy assessable categories (Table 4). It preliminarily showed a promising prognostic predictivity54 deserving further prospective trials, though including some of the weaknesses of the Pittsburgh and AJCC staging systems.
| Variables | Variable class | Score |
|---|---|---|
| Revised Pittsburgh staging system: T category | 1 | 1 |
| 2 | 2 | |
| 3 | 3 | |
| 4 | 4 | |
| Dural involvement if T4 category | Non-involved | +0 |
| Involved | +1 | |
| Tumor spread if T4 category | Anterior | +0 |
| Non-anterior | +1 | |
| Histological grade (G) | 1 | +0 |
| 2 | +1 | |
| 3 | +2 |
- Note: Non-anterior tumor spread to subsites other than peri-auricular soft tissues or parotid space (medially, inferiorly, posteriorly into the temporal bone and skull base). A total score <5 identified tumors with a better prognosis, while scores of ≥5 identified cases with a worse prognosis (see Zanoletti et al.51).
From our viewpoint, the basic step of histopathological study with TBSCC serial sections of the autopsy temporal bone or undivided en bloc resection specimens is expected to provide the picture of tumor extent by adding the potential microscopic growth to the macroscopic extent given by imaging. In particular, it could (i) assess the tumor extent and obtain reliable data on margins status; (ii) provide information on the modalities of tumor growth in the various sites of the bone; (iii) set the relation between preoperative imaging and histopathological evidence; (iv) verify the rationale of appropriateness of a surgical resection approach; and (v) explore the sites of missed removal and the nature of persistence/recurrence.
The main strengths of this study lie in the homogeneity of the series of patients considered: (i) all tumors originated in the temporal bone (SCCs in the periauricular area and adjoining sites were excluded); (ii) all patients underwent primary temporal bone en bloc surgery; (iii) their surgical treatment was performed consecutively by the same team; (iv) the histological diagnosis was SCC in all cases. On the other hand, the main weaknesses of the study are related to the retrospective setting and the relatively limited number of cases considered.
5 CONCLUSION
The poor outcome in advanced TBSCC occurred because of local recurrence due to defective resection. Defective resection reasonably depended on the erroneous diagnosis of tumor extent as supplied by clinical, radiological and intraoperative data. It appeared that this pitfall hindered the tumor–treatment–outcome canon on which the oncological directions depend. The result of surgery, as explored by imaging, did not provide adequate details on the site/subsite of recurrence from which the erroneous step could be deducted. The fundamental pitfall of surgery on advanced TBSCC appeared to be the insufficient knowledge of microscopic tumor growth in the different sites and subsites of the temporal bone. A serial histopathological study of the en bloc surgical specimen and autoptic temporal bones definitely seems to represent a way to enhance our understanding of these tumors.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Padova within the CRUI-CARE Agreement.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.