Risk factor analysis of dental implants in patients with irradiated head and neck cancer
Abstract
Background
We investigated dental implant outcomes in patients who had previously received radiotherapy (RT) for head and neck malignancies.
Methods
We reviewed 90 dental implants in 27 patients who received RT for head and neck cancer and received dental implants afterwards. The cumulative implant survival rate (CISR) was calculated. In addition, the implant quality was assessed using “Health Scale for Dental Implants.”
Results
The CISR at 3 years was 79.6%. The mean radiation dose at the implant site (Dmean) was identified as an independent prognostic factor for implant survival. No implant failed if Dmean was less than 38 Gy. Regarding implant quality, dental implants in grafted bone and Dmean were independent risk factors.
Conclusions
Dmean was identified as an independent prognostic factor for implant survival and quality. Dental implants can be safely considered when Dmean is lower than 38 Gy.
Abbreviations
-
- CISR
-
- cumulative implant survival rate
-
- Dmean
-
- the mean radiation dose at the dental implant site
-
- 3D-CRT
-
- three-dimensional conformal radiotherapy
-
- ICOI
-
- International Congress of Oral Implantologists
-
- IMRT
-
- intensity-modulated radiotherapy
-
- ORN
-
- osteoradionecrosis
-
- ROC
-
- receiver operating characteristic
-
- RT
-
- radiotherapy
-
- VIF
-
- variation inflation factor
1 INTRODUCTION
Artificial crowns, bridges, and dentures were previously used for missing teeth, but they are difficult to fix and provide low-quality masticatory function, frequently accompanied by pain, and poor esthetics.1 Thus, patients' preference for artificial crowns, bridges, and dentures is low.2, 3 Dental implants are a method of replacing missing teeth by implanting titanium screws directly into the mandibular or maxillary bone, preventing the aforementioned shortcomings (mastication, retention, and patient acceptance).4
Radiotherapy (RT) is commonly used as a primary treatment or in combination with surgery or chemotherapy in patients with head and neck malignancies.5, 6 However, several side effects have been reported in patients receiving RT to the head and neck, including xerostomia, mucositis, and irradiation caries.7, 8 In particular, radiation reduces the blood supply to the mucosa and underlying bone, causing tissue necrosis, which can lead to implant loss.9-12 Detailed research on RT has shown that the radiation dose at the dental implant site,13, 14 time lapse between RT and implantation,14, 15 implant location,16 and oral hygiene including smoking during RT17 are factors related to implant success. Therefore, in the past, dental implantation was carefully determined according to expert decision in patients who received RT to the head and neck.18
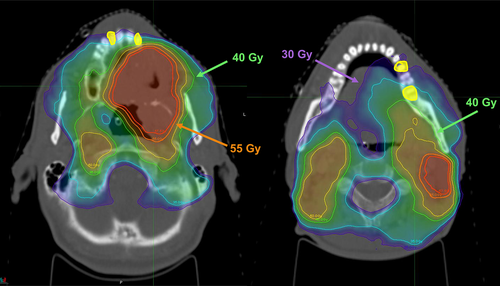
Over the past 30 years, the use of implants for dental rehabilitation has increased significantly due to advances in material science and surgical technology.19 Intensity-modulated RT (IMRT) has also been introduced as an advancement in technology. IMRT can precisely deliver a very high dose to the tumor while minimizing the amount of radiation received by normal tissue surrounding the tumor, thereby increasing the therapeutic ratio.20 Therefore, the dose to the maxilla or mandible can be minimized after head and neck RT, which allows dental implantation after RT21 (Figure 1); the number of patients receiving implants after head and neck RT is increasing. However, no comprehensive guidelines have been provided for specific radiation doses associated with implant failure in patients with head and neck cancer and the timing of dental extraction and implantation related to RT, and only one meta-analysis was published.22
In this study, we investigated implant outcomes and related risk factors in patients who received RT for head and neck malignancies.
2 METHODS
2.1 Patient selection
The medical records and radiology database at Yonsei Cancer Center and Yonsei University Dental Hospital were searched for patients who received RT for head and neck cancer between January 2008 and December 2018 (2901 patients) and those who received dental implants afterwards. Patients who did not have RT-related records or dental implant-related records or who did not complete RT were excluded (2874 patients). We evaluated 90 dental implants in 27 patients who were eligible for the study.
This study was approved by the Severance Hospital institutional review board (No. 4-2021-0718), and the requirement for the provision of informed consent was waived because of the retrospective nature of this study. All methods were carried out in accordance with relevant guidelines and regulations.
2.2 RT and dosimetric evaluation
All patients underwent RT using three-dimensional conformal RT (3D-CRT) or IMRT. During RT, the patient's head and neck were immobilized using a thermoplastic mask. The RT field was defined as the primary tumor bed or gross tumor lesion plus cervical lymph node area, if necessary. The Pinnacle (Philips Medical Systems, Cleveland, OH) was used for 3D-CRT plans, and TomoTherapy (Accuray, Sunnyvale, CA) or RayStation (RaySearch Laboratories, Stockholm, Sweden) was used for IMRT plans.
As the dosimetric variables can be cross-correlated, a single dosimetric covariate was preselected for univariate and multivariate analyses. Recent studies have shown that the mean mandibular dose seems an appropriate parameter rather than the maximum mandibular dose to predict the risk of osteoradionecrosis (ORN).23, 24 Therefore, in our study, we contoured the implant screw embedded in the bone and measured the mean dose of this contour, which is defined as Dmean (the mean radiation dose at the dental implant site).
2.3 Dental evaluation
In our institution, pre-RT dental care is provided by a dental oncologist before RT for head and neck cancer. Patients with poor dental condition receive pre-RT dental extraction at the discretion of the dental oncologist.
All patients in our study underwent dental implantation by a dental implantologist after RT. Whether to implant or not was decided after discussion between the radiation oncologist and dental implantologist. Dental implantation was not performed if wound healing was not achieved in the area to be implanted or if there was a periodontal infection. After dental implantation, the patients received a periodic clinical follow-up. Panoramic radiography or computed tomography (CT) was also performed.
The implant quality was assessed using “Health Scale for Dental Implants,” as presented at the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference.25 This scale incorporated clinical and radiographic evaluations to categorize implants into one of the following groups: success, satisfactory survival, compromised survival, or failure (Table S1, Supporting Information). To define implant success, the ICOI recommends that the evaluation be conducted at least 12 months after implantation. Therefore, in our study, the implant quality assessment via “Health Scale for Dental Implants” was conducted at a point in time 12 months after implantation.
2.4 Statistical analysis
The cumulative implant survival rate (CISR) was defined as the time frame between the date of dental implantation and the date of implant failure or the last follow-up. The CISR according to Dmean was analyzed using the Kaplan–Meier method and stratified log-rank test. Since there were multiple implants in one patient, there were clinical factors that were shared between implants; therefore, we used the Cox proportional-hazards regression model with robust variance to analyze the risk factors related to the CISR by considering these repeated measurements. The receiver operating characteristic (ROC) and area under the curve analyses were used to identify the optimal cutoff value of Dmean that best predicted implant survival. In addition, based on the Dmean of 50 Gy as suggested in the previous studies,13, 16, 26-28 we tried to analyze whether there was a significant difference in the CISR.
With respect to implant quality, we analyzed the risk factors associated with patients in a group with compromised survival or failure according to the “Health Scale for Dental Implants.” Considering the repeated measurements of multiple implants in one patient, univariate and multivariate analyses of the risk factors associated with patients in a group with compromised survival or failure were performed using a generalized estimating equation with a logit link function. The Youden index was used to identify the optimal cutoff value of Dmean that best predicts the implant quality group with compromised survival or failure.
Variables included in the risk factor analysis related to implant survival and quality were checked for multicollinearity using a variation inflation factor (VIF) >10. Statistical significance was set at p < 0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY), R package, version 3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria), and SAS (version 9.4, SAS Inc., Cary, NC).
3 RESULTS
3.1 Characteristics of the patients and dental implants
The median age was 63 years (range, 37–77 years). Of the total 27 patients, 14 were never smokers, 10 were former smokers who quit smoking before treatment, and 3 were active smokers who continued smoking after treatment. The oral cavity (12 patients, 44.5%) was the most common primary malignancy site, followed by the nasopharynx (4 patients, 14.8%). Most patients (23 patients, 85.2%) received IMRT. Twelve patients (44.4%) received concurrent chemoradiotherapy.
Of the 90 dental implants, 50 (55.6%) underwent dental extraction before RT, and 40 (44.4%) underwent dental extraction after receiving RT. The median interval between the completion of RT and the dental extraction was 23.0 months (range 7.1–117.8 months). Seventeen implants (18.9%) were placed in the grafted bone. The median interval between the completion of RT and dental implantation was 28.2 months (range 1.7–121.5 months). The median value for Dmean was 35.7 Gy (range 0.0–64.8 Gy). The baseline characteristics of all patients and dental implants are listed in Table 1.
| Characteristic | Patients | |
|---|---|---|
| N | % | |
| Age (years, median [range]) | 63 (37–77) | |
| Sex | ||
| Male | 15 | 55.6 |
| Female | 12 | 44.4 |
| Smoking | ||
| Never | 14 | 51.9 |
| Former | 10 | 37 |
| Active | 3 | 11.1 |
| Diabetes mellitus | 1 | 3.7 |
| Primary site | ||
| Oral cavity | 12 | 44.5 |
| Nasopharynx | 4 | 14.8 |
| Paranasal sinus | 3 | 11.1 |
| Parotid gland | 3 | 11.1 |
| Oropharynx | 2 | 7.4 |
| Mandible | 2 | 7.4 |
| Thyroid | 1 | 3.7 |
| RT modality | ||
| 3D-CRT | 4 | 14.8 |
| IMRT | 23 | 85.2 |
| Concurrent chemotherapy | 12 | 44.4 |
| Characteristic | Dental implants | |
|---|---|---|
| N | % | |
| Location | ||
| Maxilla | 36 | 40.0 |
| Mandible | 54 | 60.0 |
| Dental extraction | ||
| Before RT | 50 | 55.6 |
| After RT | 40 | 44.4 |
| The end of RT to dental extraction (months, median [range]) | 23.0 (7.1–117.8) | |
| Dental implants in grafted bone | 17 | 18.9 |
| The end of RT to dental Implantation (months, median [range]) | 28.2 (1.7–121.5) | |
| Radiation dose at the dental implant site (Gy, median [range]) | ||
| Mean | 32.9 (0.0–63.4) | |
| Max | 35.7 (0.0–64.8) | |
- Abbreviations: 3D-CRT, three-dimensional conformal radiation therapy; Gy, gray; IMRT, intensity-modulated radiation therapy; RT, radiotherapy.
3.2 Implant survival
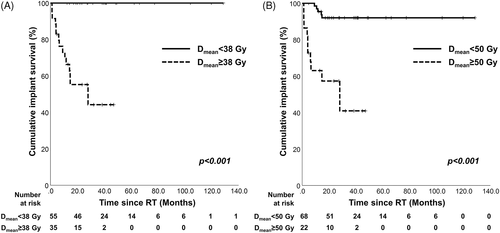
The median follow-up after implantation was 27.3 months (range, 1.2–128.6). Sixteen dental implants in four patients failed during follow-up. The 3-year CISR in all patients was 79.6%. The ROC analysis showed that Dmean could predict implant survival after RT (Figure S1). Based on the ROC analysis, the Dmean cut-off value for predicting implant survival was 38 Gy. Based on Dmean 38 Gy, no implants failed when Dmean < 38 Gy was irradiated (3-year CISR: 100.0% vs. 44.2%, p < 0.001; Figure 2A). In addition, based on Dmean 50 Gy, the group with Dmean less than 50 Gy had a significantly higher CISR than the group with more than 50 Gy (3-year CISR: 92.0% vs. 40.9%, p < 0.001; Figure 2B).
For analyzing the risk factors related to implant survival, univariate analysis using the Cox proportional hazards regression model with robust variance was performed. It revealed Dmean (hazard ratio 1.12, p = 0.001) as a significant factor (Table 2). The variables included in the model simultaneously did not have multicollinearity, using a VIF > 10. Since the number of events referred to as implant failure was smaller than the predictive factors, multiple regression models including these factors cannot be estimated; therefore, multivariate analysis was not performed.
| Univariate analysis | ||
|---|---|---|
| HR (95% CI) | p-value | |
| Age (years)a | 1.02 (0.95–1.10) | 0.553 |
| Smoking (no vs. former/active) | 0.31 (0.04–2.56) | 0.274 |
| Concurrent chemotherapy (no vs. yes) | 0.16 (0.02–1.34) | 0.091 |
| Dental extraction (before RT vs. after RT) | 1.65 (0.74–3.69) | 0.224 |
| Dental implants in grafted bone (no vs. yes) | 3.19 (0.55–18.40) | 0.194 |
| The end of RT to dental Implantation (months)a | 1.01 (0.99–1.03) | 0.229 |
| Dmean (Gy)a | 1.12 (1.05–1.19) | 0.001 |
- Note: Variables included in the model simultaneously do not have multicollinearity using variation inflation factor >10.
- Abbreviations: CI, confidence interval; Dmean, mean radiation dose at the dental implant site; Gy, gray; HR, hazard ratio; RT, radiotherapy.
- a Age, the end of RT to dental implantation, and Dmean were treated as a continuous variable.
3.3 Implant quality
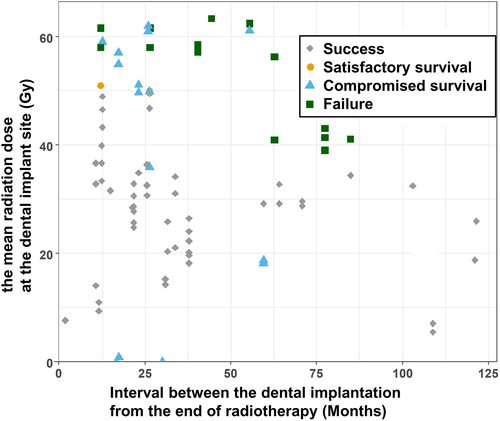
When evaluating the quality of implants using the “Health Scale for Dental Implants,” 49 implants showed success, 3 showed satisfactory survival, 20 showed compromised survival, and 18 were in the failure group, 16 of which showed absolute failures. A scatter plot according to Dmean and the interval till dental implantation from the end of RT are presented in Figure 3. When each implant case was classified based on a Dmean of 50 Gy and implantation 2 years after the end of RT, which were suggested as significant results in previous studies,29 7.4% of implants with Dmean of less than 50 Gy were the failure group, and all of them were implanted more than 2 years after the end of RT. When the Dmean was higher than 50 Gy, 28.6% of implants in the group implanted within 2 years after the end of RT and 73.3% of implants in the group implanted more than 2 years after the end of RT belonged to the failure group.
ORN occurred at four dental implant sites, all of which were in the failure group. The median value for Dmean at the site of ORN was 60 Gy.
The Youden index was used to find the optimal cut-off value of Dmean, and as a result, the compromised survival or failure group was well predicted when it was 38 Gy or more. Table S2 shows the results of evaluating the diagnostic performance based on a cut-off value of 38 Gy.
For risk factors related to implant quality, univariate and multivariate analyses were performed using a generalized estimating equation with logit link function. As a result, dental implantation on grafted bone (odds ratio 32.93, p = 0.012) and Dmean > 38 Gy at the dental implant site (odds ratio 15.10, p = 0.018) were significant factors (Table 3). The variables included in the model simultaneously did not have multicollinearity, using a VIF >10.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years)a | 1.04 (0.98–1.11) | 0.192 | 1.06 (0.95–1.17) | 0.316 |
| Smoking (no vs. former/active) | 0.32 (0.08–1.27) | 0.105 | 0.17 (0.02–1.88) | 0.150 |
| Concurrent chemotherapy (no vs. yes) | 1.17 (0.29–4.67) | 0.824 | 2.14 (0.21–21.69) | 0.521 |
| Dental extraction (before RT vs. after RT) | 0.80 (0.38–1.69) | 0.560 | 1.08 (0.35–3.37) | 0.890 |
| Dental implants in grafted bone (no vs. yes) | 18.08 (4.29–76.26) | <0.001 | 32.93 (2.16–502.86) | 0.012 |
| The end of RT to dental Implantation (months)a | 0.99 (0.98–1.01) | 0.401 | 1.01 (0.98–1.04) | 0.536 |
| Dmean (<38 vs. ≥38 Gy) | 10.98 (1.39–86.81) | 0.023 | 15.10 (1.58–144.03) | 0.018 |
- Note: International Congress of Oral Implantologists, Pisa, Italy, Consensus Conference, 2007. Variables included in the model simultaneously do not have multicollinearity using variation inflation factor >10.
- Abbreviations: CI, confidence interval; Dmean, mean radiation dose at the dental implant site; Gy, gray; OR, odds ratio; RT, radiotherapy.
- a Age and the end of RT to dental implantation were treated as a continuous variable.
4 DISCUSSION
The main finding of this study was that implant survival and quality were significantly associated with the mean radiation dose at the implant site. Previous studies that suggested this association suggested a cut-off value of 50 Gy, and there was a significant difference based on 50 Gy in our study as well. In addition, based on the cut-off value of 38 Gy suggested in our study, no implant showed failure when a dose lower than 38 Gy was irradiated. The mean radiation dose at the implant site was the only risk factor for implant survival, and the mean radiation dose at the implant site and dental implantation on grafted bone were significant risk factors for implant quality.
The relationship between dental implantation and radiation dose is an important issue that has been continuously reported since the early 2000s. In a meta-analysis, a tendency was observed for lower implant survival rates in patients receiving higher radiation doses.22 High doses are more likely to cause vessel damage, connective tissue fibrosis, and muscle and epithelial damage, which ultimately lead to ORN.30 In our study, all implants at the site of ORN were either compromised survival or failure groups. As implant failure and ORN are closely related, studies have previously been conducted to obtain cut-off values of radiation doses that can predict implant failure or ORN. According to one study, dental implant failures were more commonly observed at doses greater than 65 Gy.31 In addition, it was reported that the risk of ORN was highest when mandibular tooth extraction was performed in a radiation field with a dose of 60 Gy or more.32 In addition, studies have shown that 50–55 Gy, a dose lower than 60 Gy, is selected as the cut-off value, and values higher than this reduce implant survival.14, 15, 33 Most studies have used 50 Gy as a cut-off value, which is lower than the aforementioned doses, and 50 Gy is still used as a cut-off value in many studies even recently.13, 16, 26-28 Nowadays, by using IMRT, it is possible to reduce the amount of radiation to surrounding normal tissues, with the exception of the treatment site as much as possible. Therefore, studies on cut-off values lower than 50 Gy need to be conducted, but there are few studies on implant failure when the radiation dose to the implant site is 40 Gy or more. A recently published study argued that ORN could be considered only when the mean radiation dose was greater than 40 Gy.34 In our study, the median value for Dmean at the site of ORN was 61.0 Gy, so radiation dose could be considered as a risk factor for ORN. In addition, there was a significant difference in implant survival even when the previously suggested 50 Gy was used as the cut-off value, and there was no implant failure when irradiated below the newly proposed cut-off value of 38 Gy.
In general, various dosimetric factors are associated with ORN. Since these dosimetric factors are related to each other, the choice of the factor is still a matter of debate. The M. D. Anderson Head and Neck Cancer Symptom Working Group showed that the maximum dose to the mandible did not correlate with the risk of ORN.24 Aarup-Kristensen et al. also believed that the mean dose could reflect the general damaging mechanism based on the fact that the mandible is a parallel organ, and their study found that the mean dose could be an appropriate parameter for predicting ORN.23 In our study, the mean dose of the implant screw was analyzed as a representative dosimetric parameter. The mean dose was found to be an effective predictor of implant failure.
With respect to the timing of dental implantation, to avoid bone necrosis, implantation should be performed prior to RT if possible.35 However, if implantation is possible only after RT, it is not yet known how long to wait for implantation after RT. Several studies have shown that implant survival is not affected by the timing of implantation 6 months after RT.14, 15 In a meta-analysis, there was no statistically significant difference in implant survival when implantation was performed 12 months before and after RT.22 However, other studies have recommended that an interval of at least 12 months is necessary to obtain sufficient time for muscle recovery and bone remodeling.36 Additionally, as mentioned earlier, some studies suggest that implants should be inserted at intervals of more than 2 years.29 In our study, 84 implants (93.3%) were inserted more than a year after RT, making it difficult to apply the previous two criteria (6 months and 12 months); thus, we divided the implants into before and after based on the insertion after 2 years of RT. Among our study patients whose Dmean was less than 50 Gy, all patients in the failure group received implants more than 2 years after the end of RT. In addition, among the patients who received 50 Gy or more, the number of patients who belonged to the failure group was higher among those who received an implant after 2 years or more after the end of RT than among those who received an implant within 2 years (73.3% vs. 28.6%). To summarize our results, the greater the gap of more than 2 years between RT and implant insertion, the more the number of implants that failed. The reason for this result is that the worse the condition of the site scheduled for implant insertion (delayed wound healing, infection, severe inflammation, etc.), the more likely the implant insertion would have been postponed after RT.
Regarding dental extraction, there is a common consensus that it should be avoided after RT to reduce ORN. However, if dental extraction must be performed after RT for unavoidable reasons, atraumatic extraction should be performed to minimize mucoperiosteal disruption and bone injury.37, 38 Therefore, at our institution, pre-RT dental extraction is often performed on teeth that are likely to be removed after RT in patients receiving RT for head and neck cancer. However, pre-RT dental extraction has also been reported as an explainable factor for the development of ORN,23, 39-41 and a previous study reported that the incidence of ORN in the area after pre-RT dental extraction was 75.9%.42 Therefore, the authors of the aforementioned study argue that pre-RT dental extraction does not prevent the occurrence of ORN and that pre-RT dental extraction guidelines should consider more conservative measures. In our study, there was no significant difference in implant failure regardless of whether dental extraction was performed before or after RT. Therefore, conservative management after RT is more important for implant failure than the timing of dental extraction.
Maxillofacial reconstruction with vascularized bone, which restores facial contour and provides structural support and a foundation for dental rehabilitation, is widely used in head and neck cancer surgery. Some studies suggested unchanged implant survival in grafted bone versus native bone in patients with irradiated head and neck cancer,43, 44 while others have demonstrated lower implant survival rates in grafted bone.45 In addition, implants placed in grafted bone before RT may demonstrate superior survival compared to implants placed after.46 Our study also reported that the risk of implant failure was high not only when radiation dose was high, but also when implants were placed in grafted bone. Although it was difficult to analyze in our study, which analyzed only patients who received dental implantation after RT, in patients who underwent reconstruction with vascularized bone, considering that receiving dental implantation before RT may reduce implant failure.
This study has some limitations owing to its retrospective nature. First, since we collected patients with detailed implant-related records, the majority of our study subjects received dental implantation at our institution. Therefore, patients who received dental implantation at other institutions may have been excluded from the analysis. Second, since all patients who received dental implantation after head and neck RT were analyzed, there is a limitation in that the tumor and treatment characteristics are heterogeneous. Lastly, the condition of the gingiva or bone at the implant site is also an important factor that can affect implant failure, but it was excluded from the analysis because the related records were not written in detail. Despite these limitations, this study has the strength that the procedure, examination, and clinical follow-up were consistent because most of the analyzed patients received implants at our institution. In addition, since there are clinical factors shared between implants when there are multiple implants in one patient, we tried to reduce statistical bias by performing a statistical analysis called the Cox proportional-hazards regression model with robust variance and generalized estimating equation with logit link function. Third, we measured the mean radiation dose at the dental implant site by contouring each implant screw for a more accurate measurement in dosimetric evaluation. Fourth, since most of the patients in our study were treated with IMRT, our newly proposed cut-off value can be practically applied in the IMRT era. Finally, although additional prospective study is needed, this study suggested a cut-off value of 38 Gy that can be applied in clinical practice.
The results of this study showed that dental implantation after RT is feasible in patients with head and neck cancer. The mean radiation dose at the implant site and dental implantation on the grafted bone were significantly associated with implant quality. Dental implants can be safely considered in patients with a mean radiation dose lower than 38 Gy. In the case of >50 Gy, the implant failure rate was high even if the implant was placed more than 2 years after RT. If any of the above risk factors are present among patients receiving head and neck RT, careful monitoring should be considered for the proper management of dental implantation.
AUTHOR CONTRIBUTIONS
Chang Geol Lee and Joongyo Lee conceived and designed the study. Chang Geol Lee and Joongyo Lee performed the study. Jason Joon Bock Lee and Joongyo Lee analyzed the data. In-Ho Cha, Joongyo Lee, Kyung Ran Park, and Chang Geol Lee contributed materials and analysis tools. Chang Geol Lee, Joongyo Lee, and Jason Joon Bock Lee prepared all figures and tables and composed the main manuscript. All authors reviewed the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ETHICS STATEMENT
This study was approved by the Severance Hospital institutional review board (No. 4-2021-0718), and the requirement for the provision of informed consent was waived because of the retrospective nature of this study. The procedures followed in this retrospective study were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.