Immune checkpoint expression in HNSCC patients before and after definitive chemoradiotherapy
Abstract
Background
Primary platinum-based chemoradiotherapy (CRT) remains the treatment of choice for nonresectable squamous cell carcinoma of the head and neck (HNSCC). Immune-checkpoint modulators are used as palliative therapy and studied in combination with definitive CRT. However, the immunological changes by CRT need yet to be understood.
Methods
A cohort consisting of 67 paired tissue biopsies (N = 134) of HNSCC patients before and after CRT was created. The expression of PD-1, PD-L1, and CD27 of tumor and immune cells by immunohistochemistry was evaluated.
Results
PD-L1 expression on immune cells of non-responders was significantly lower before CRT (P = .008). CD27 was expressed only on immune cells and not on cancer cells. A significant lower CD27-expression score was observed following CRT (P = .019).
Conclusions
Conventional CRT changes the expression of CD27 in the tumor microenvironment. Whether this is due to a loss of expression or a reduction of CD27+ cells must be evaluated in further analyses.
1 INTRODUCTION
Squamous cell carcinomas of the head and neck area (HNSCC) represent approximately 3%-4% of all new cancer cases and are responsible for about 2% of all tumor-associated deaths, according to data raised by the American Cancer Society in 2017.1 Primary chemoradiotherapy (CRT) is an established therapeutic option for advanced HNSCC. It is available for inoperable findings, patients not fit for general anesthesia or organ preserving strategy as an alternative to a surgical therapy. If possible, a therapy regimen consisting of radiation therapy in combination with platinum-based chemotherapy is chosen.2 However, not all patients respond to this type of therapy and thus, depending on the tumor location, in at least 20% of cases there are therapy failures with a residual tumor after completion of therapy.2, 3 Salvage surgery is a remaining curative option, but often associated with an increased rate of complications. Depending on the localization of the tumor, complications occur in 24%-59% of cases.4, 5 However, a large proportion of patients cannot be operated after CRT has been completed and can only be treated on a palliative basis.
Checkpoint inhibitors, which can modulate the immune system, have recently become available in the clinical setting. Recent studies on PD-1 inhibitors as first-line palliative therapy for HNSCC, which provided at least non-inferior results compared with the standard of care EXTREME regimen, finally led to the approval of nivolumab and later also of pembrolizumab in the United States and in Europe.6, 7 Yet, observed response rates of both checkpoint inhibitors have been below 20% and the search for reliable biomarkers continues. Pembrolizumab showed an increased response rate of about 23% for a score over 20 points based on the expression of PD-L1 on tumor and immune cells in relation to viable tumor cells (CPS).7
There are many ongoing studies on combinations of immunotherapeutic agents in order to increase response rates. A promising substance recently tested in combination with nivolumab for HNSCC is varlilumab.8 The investigational product is an agonistic anti-CD27 antibody. CD27 is expressed on most T cells, a subset of B cells and NK cells and acts as a costimulatory molecule in the activation of T cells and the expansion of memory B cells. Although it is constitutively expressed on T cells, CD27 is further upregulated in activated T cells.9 Further, high CD27 expression in the tumor microenvironment (TME) is associated with improved survival in other entities.10 However, the role and prognostic influence of CD27 in HNSCC is still unknown. A favorable factor for a possible response to immunotherapy with checkpoint inhibitors seems to be a so-called inflamed phenotype of the tumor. A high number of tumor-specific CD8+ T cells in the tumor microenvironment is of particular importance, as this leads to an increased expression of PD-L1 and other inhibitory molecules on the tumor cells.11 But so far there is hardly any data on how the composition of the tumor microenvironment and in particular the expression of checkpoint molecules on tumor cells and on tumor-associated leukocytes (TAL) are influenced by chemoradiotherapy. There have been only a few studies observing changes in the TME during CRT in patients with different tumor types, whereas most studies are limited on examination of pretreatment samples and the main focus was on changes of immune subsets in blood and tumor tissue. In these studies observations diverged based on the prevalence of cytotoxic T cells and regulatory T cells (Treg) and only very few data are available on checkpoint expression under CRT, indicating an increase of PD-L1 expression in response to cisplatin.12-16
Therefore, the primary aim of the study was to further investigate especially the modulation of PD-L1, PD-1, and CD27 expression on HNSCC cells and TAL by primary CRT, in vivo.
2 MATERIALS AND METHODS
2.1 Material
We retrospectively evaluated a total of 422 patients treated with primary CRT between 2005 and 2016. Inclusion criteria were a curative intent for CRT, the presence of a complete data set, absence of distant metastases, histology of HNSCC, and archival samples of pretherapeutic and posttherapeutic Formalin Fixed and Paraffin embedded (FFPE) biopsies available. All biopsies were taken routinely as part of response assessment about 3.7 months after end of CRT (range 1.3-9.5 months) and were representative for the primary tumor region. Nasopharyngeal and sinonasal tumors as well as skin cancer were excluded. After applying these criteria, 67 patients were left for analysis. The study has been approved by the local ethics committee of the Ulm University (#448/17).
2.2 Tissue specimen and IHC analysis
FFPE tissue specimen were obtained during routine endoscopy as part of the staging and varied from core biopsies to excisional biopsies and ranged from a few millimeters up to centimeters due to physician's preference. All biopsies were representative of the tumor core. Immunohistochemical staining (IHC) was performed on a Dako Autostainer Universal Staining System (Dako, Glostrup, Denmark) using manufacturers optimized protocols for each antibody. PD-1 was stained using mouse monoclonal antibodies NAT105 and JAD1 (Dianova, Hamburg, Germany) both at a dilution of 1:50 after antigen retrieval with low pH conditions. Two clones were used here because the production of NAT105 was discontinued. After validation for routine diagnostics, however, JAD1 was clearly equivalent in staining behavior, so that both clones illustrate PD-1 expression equally. Staining for PD-L1 was performed with the rabbit monoclonal antibody E1L3N (Cell Signaling, Danvers, Massachusetts) at a dilution of 1:200 after antigen retrieval with high pH conditions. CD27 expression was determined using mouse monoclonal antibody clone 137B4 (Novocastra, Leica Biosytems, Wetzlar, Germany) at a dilution of 1:500 after antigen retrieval with high pH conditions. Immunohistochemistry for p16 (clone 1D7D2, Thermo Scientific, Waltham, Massachusetts) was determined with a dilution of 1:400 and under high puffer conditions. Secondary detection was carried out with an anti-mouse/rabbit Dako REAL alkaline phosphatase detection system (Dako, Glostrup, Denmark) according to the manufacturer's recommendation. All antibodies were then visualized with Dako REAL Chromogen Red and hematoxylin (Dako, Glostrup, Denmark) was used for counterstaining. HPV analysis was done by PCR with HPV specific primer combinations and following sequencing of the PCR positive probes, according to established protocols.17, 18
For analysis whole slides were taken into account independent of tissue size. Here, only the representative tumor areas were considered for evaluation. First, proportions of tumor per tissue section (%) were determined before and after CRT. Second, immunohistochemical analysis was done using the modified H-Score and was documented by two observers (SEW and PMi).19, 20 If tumor cells showed a membranous expression for PD-L1 or PD-1, percentage and intensity (from 1 for low to 3 for high intensity) of stained tumor cells were determined (Figure 1A). We also determined percentage and staining intensity (1-3) of PD-L1, PD-1, and CD27 on TAL before and after chemoradiotherapy (Figure 1B). Immune cell content was analyzed and categorized into four groups (0%-5%, 6%-30%, 31%-50%, and above 51%), resulting in a score ranging from 2 to 7 points, described previously as immunoreactive score.21
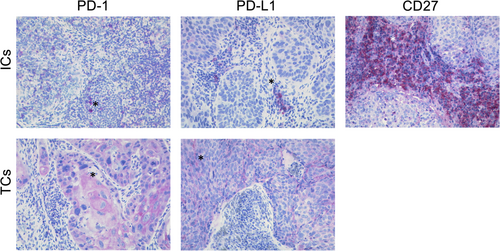
2.3 Literature review
We performed a literature search via PubMed using the terms “head and neck” AND “checkpoint” for the specific question of checkpoint expression in head and neck carcinoma after CRT. A total of 897 publications were screened and 253 of them analyzed. Relevant publications were selected by the authors (Table 3).
2.4 Statistics
Changes in expression by CRT were calculated using a paired t test. Differences between responders to CRT and nonresponders as well as differences between PD-1 antibodies were computed with an unpaired t-test for continuous variables and chi-square test for categorical variables. Possible influence of chemotherapeutic agent was calculated by Kruskal-Wallis test. Survival was analyzed by log-rank test. Overall survival was evaluated over 5 years, disease-free survival over 3 years. Univariate and multivariate analysis was performed using the Cox regression model. Parameters showing significance in at least one survival category in univariate analysis were chosen for multivariate analysis. Additionally, CD27 was included in multivariate analysis. A significance level of 0.05 was determined. All statistics were performed on SPSS v25 (IBM Corp., Armonk, New York).
3 RESULTS
The median age at the time of diagnosis was 62 years with a median follow-up period of 26 months. In 82% of cases the initial diagnosis was made between 2010 and 2015. About 36 patients suffered of an oropharyngeal, 18 of a hypopharyngeal, 9 of a laryngeal, and 4 of an oral squamous cell carcinoma. 82.1% of the patients was seen with cT3 or higher and about 80% had clinically suspicious cervical lymph nodes at first diagnosis. All patients received primary CRT with curative intent. The average radiation dose applied was 67.66 Gy. Most patients received either cisplatin or carboplatin (73.1% and 14.9%, respectively). A small number of patients was treated concomitantly with mitomycin C or cetuximab (9% and 3%, respectively).
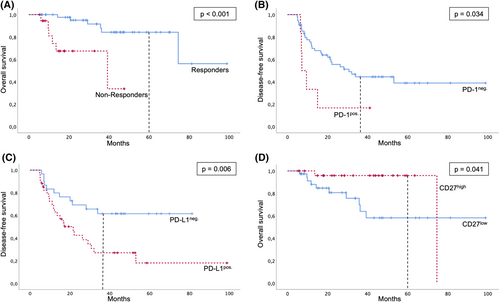
Response was evaluated by re-staging and an obligatory posttherapeutic biopsy. Pathological complete response was achieved in 71.6% of the patients, in 28.4% vital tumor cells were detected after therapy. These patients were classified in the study as non-responders. Influence of HPV on response was investigated for oropharyngeal cases. 27.8% of the cases were both, p16 and HPV DNA positive. All contained HPV16. HPV16 positive patients tended to respond better to CRT (P = .042) and had a significantly improved disease-free survival (P = .003). However, no influence on overall survival could be measured. As expected, overall survival was dramatically worse for non-responder, P < .001, 5-year survival 33.7% vs 56.2% (Figure 3A). A correlation of clinical data with the response to CRT did not reveal any significant associations (Table 1). Nonresponder and patients with recurrent/metastasis disease after initial complete response were treated mostly either with salvage surgery or with chemotherapy. Only one patient received anti-PD-1 treatment as part of a clinical trial (Table 2).
| Characteristic | Responder (n = 48; 71.6%) | Nonresponder (n = 19; 28.4%) | P-valuea |
|---|---|---|---|
| Sex | .949 | ||
| Men | 35 (72.9%) | 14 (73.7%) | |
| Women | 13 (27.1%) | 5 (26.3%) | |
| Age (average in years) | 60.9 | 62.05 | .621b |
| Smoking status | .095 | ||
| Current smoker | 17 (35.4%) | 2 (10.5%) | |
| Former smoker | 26 (54.2%) | 14 (73.7%) | |
| Never smoker | 5 (10.4%) | 2 (10.5%) | |
| Missing information | 1 (5.3%) | ||
| Alcohol consumption | .705 | ||
| Current heavy drinker | 2 (4.2%) | 1 (5.3%) | |
| Former heavy drinker | 12 (25%) | 3 (15.8%) | |
| Moderate daily drinker | 16 (33.3%) | 6 (31.6%) | |
| Occasional drinker | 11 (22.9%) | 3 (15.8%) | |
| Never drinker | 5 (10.4%) | 4 (21.1%) | |
| Missing information | 2 (4.2%) | 2 (10.5%) | |
| Tumor site | .149 | ||
| Oropharynx | 28 (58.3%) | 8 (42.1%) | |
| HPV16/p16 positive | 10 (35.7%) | 0 (0%) | .042 |
| HPV16/p16 negative | 17 (60.7%) | 8 (100%) | |
| Missing information | 1 (3.6%) | 0 (0%) | |
| Hypopharynx | 12 (25%) | 6 (31.5%) | |
| Larynx | 7 (16.4%) | 2 (10.5%) | |
| Oral cavity | 1 (2.1%) | 3 (15.8%) | |
| cT-classification | .328 | ||
| cT1-2 | 7 (14.6%) | 5 (26.3%) | |
| cT3-4 | 41 (85.4%) | 14 (73.7%) | |
| cN-classification | .120 | ||
| cN0 | 11 (22.9%) | 2 (10.5%) | |
| cN1 | 3 (6.3%) | 1 (5.3%) | |
| cN2-3 | 34 (70.9%) | 16 (69.4%) | |
| Grading | .320 | ||
| G1 | 1 (2.1%) | 2 (10.5%) | |
| G2 | 31 (64.6%) | 11 (57.9%) | |
| G3 | 16 (33.3%) | 6 (31.6%) | |
| Clinical stage | .266 | ||
| II | 1 (2.1%) | 0 (0%) | |
| III | 5 (10.4%) | 0 (0%) | |
| IVA | 36 (75%) | 14 (73.7%) | |
| IVB | 6 (12.5%) | 5 (26.3%) | |
| Chemotherapy | .063 | ||
| Cisplatin | 38 (79.2%) | 11 (57.9%) | |
| Carboplatin | 7 (14.6%) | 3 (15.8%) | |
| Mitomycin C | 3 (6.3%) | 3 (15.8%) | |
| Cetuximab | 0 (0%) | 2 (10.5%) | |
| Radiation dose (mean ±SD) | 68.36 ±3.91 | 65.9 ±9.37 | .279b |
- Note: Percentage is calculated within the response group.
- a Correlation of clinical parameters and response using chi-square test.
- b Correlation of age and radiation dose and response using unequal variance t-test.
| Responder (21/48 patients; 43.8%) | Nonresponder (19/19 patients) | |
|---|---|---|
| Salvage surgery | 5 (23.8%) | 6 (31.6%) |
| Re-irradiation | 1 (4.8%) | 1 (5.3%) |
| Chemotherapy (EXTREME) | 5 (23.8%) | 6 (31.6%) |
| Anti-PD1 | 1 (4.8%) | 0 (0%) |
| Best supportive care | 6 (28.6%) | 5 (26.3%) |
| Unknown | 3 (14.3%) | 1 (5.3%) |
- Note: The percentage is calculated within the response group.
| Author, year (Ref.) | Method | Material | Prognosis of PD-L1high | Prognosis of PD-1high | Prognosis of CD27high | Treatment |
|---|---|---|---|---|---|---|
| Strati et al.28 | RT-qPCR | Circulating tumor cells of 113 patients | Associated with worse progression-free and overall survival | n/a | n/a | Induction CT followed by CRT |
| Balermpas et al.2 | IHC | Surgical specimen of 161 patients | Associated with improved survival and lower recurrence rate | Associated with improved survival and lower recurrence rate | n/a | Surgery and adjuvant CRT |
| Fiedler et al.26 | IHC | Pretreatment biopsies of 82 patients | No significant effect | Associated with lower recurrence rate | n/a | Definitive RT or CRT |
| Fukushima et al.29 | IHC | Pretreatment biopsies of 92 patients | Associated with improved survival and lower recurrence rate | n/a | n/a | Definitive RT, CRT, or IRT |
| Schoenfeld et al.30 | IHC | Pretreatment biopsies of 81 patients | No significant effect | n/a | n/a | heterogenous |
| Moratin et al.31 | IHC | Surgical specimen of 175 patients | Associated with worse overall survival | n/a | n/a | Surgery and risk-adapted adjuvant treatment |
| Badr et al.32 | RNA-seq | TCGA data of 518 patients | Associated with worse survival | n/a | n/a | heterogenous |
| Schulz et al.33 | Flow cytometry, western blot | 6 HNSCC cell lines | Associated with radioresistant cell lines | n/a | n/a | Irradiation |
3.1 PD-1
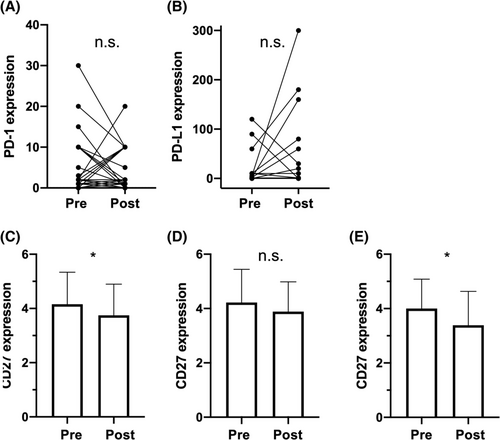
The overall expression of PD-1 was low with average H-Scores ranging from 1.87 pre-CRT on tumor cells to 3.78 pre-CRT on TAL. A possible batch bias due to the use of two different antibodies could be ruled out and the median expression scores are displayed in Figure S1. There were no significant changes in the expression of PD-1 on neither tumor nor TAL during therapy (Figure 2A shows results for immune cells). Further, no significant differences between responders and non-responders could be observed. The choice of chemotherapy had no influence on the change in expression, neither for tumor cells (P = .35) nor for TAL (P = .98). PD-1 expression was not predictive for response to CRT and did not differ depending on HPV status. Kaplan-Meier analyses revealed a significant advantage in disease-free survival of patients with no PD-1 expression on tumor cells before CRT compared with PD-1 expressing tumors, P = .039, 3-year survival 44.4% vs 16.7% (Figure 3B). However, when adjusting for HPV status, PD-L1 expression and CD27 expression, PD-1 expression did not remain an independent prognostic factor (Table S2).
3.2 PD-L1
Average expression scores of PD-L1 ranged from 2.0 on TAL after therapy to 46.89 on remaining tumor cells after CRT. Mean expression on TAL before CRT was 2 points (95% CI [0.55, 3.47]) lower for nonresponders, t (57.1) = 2.76, P = .008. There were no significant changes by CRT and no significant differences for PD-L1 expression on tumor cells. However, it could be observed that score values measured on remaining tumor cells after CRT showed a greater scattering (Figure 2B). HPV had no influence on PD-L1 expression. The choice of chemotherapy had no influence on the change in expression, neither for tumor cells (P = .28) nor for TAL (P = .08).
For survival analyses samples with scores ≥1 were considered positive. PD-L1 expression was not predictive for response to CRT. PD-L1 positive tumor cells before CRT were significantly associated with tumor recurrence (P = .013) and disease-free survival was worse for patients with PD-L1 positive tumor cells, P = .006, 3-year survival 61.5% vs 27.0% (Figure 3C). This remained independent in multivariate analysis adjusted for HPV status, PD-1 and CD27 expression (P = .007; Table S2).
3.3 CD27
As expected, expression of CD27 was only present on immune cells (TAL). The average expression score was significantly lower after CRT, t (62) = 2.40, P = .019, d = 0.30 (Figure 2C). When comparing oropharyngeal cases, HPV status was significantly associated with a higher expression score before therapy (P = .021). There were no significant differences comparing nonresponders with responders before and after CRT. However, the significant lower expression score after CRT described was only significant in nonresponders, t (17) = 2.27, P = .037, d = 0.53 (Figure 2D+E). The choice of chemotherapy had no influence on the change in expression (P = .81).
For further analyses levels were dichotomized with a cut-off at 5 points as 4 was the median score in both groups and samples with values above 4 were considered CD27high. CD27 expression was not predictive for response to CRT, but patients with CD27high had a significant longer overall survival, P = .041, 5-year survival 95.8% vs 58.2% (Figure 3D). This was not revealed an independent prognostic factor in univariate or multivariate analysis (Tables S1 and S2). Expression of CD27 was not associated with disease recurrence and had no measurable influence on disease-free survival.
4 DISCUSSION
In this study, we present data on the expression change of the immune-checkpoint molecules PD-1/PD-L1 and CD27 in tumor biopsies before and after definitive chemoradiotherapy. To the best of our knowledge, this has not been investigated in samples of head and neck squamous cell carcinoma before. We could observe no significant changes in the PD-1/PD-L1 axis when comparing pretreatment and posttreatment samples. The expression score of CD27 however decreased significantly through CRT and this decrease remained significant in nonresponders when stratifying the cohort for complete response to CRT. As CD27 is known to be expressed by T cells,22 it could only be stained on immune cells. Our observation of an impaired response to antitumor treatment of patients with decreased CD27 expression during therapy is supported by studies which could prove antitumor immunity mediated by CD27/CD70 signaling.23 Although our study is purely descriptional and comprises no mechanistic insights, our findings should be further investigated in order to establish a reliable basis for the application of an anti-CD27 agonist antibody. However, it has to be clarified first by single cell analysis what leads to the lower expression scores of CD27 as this can be due to a loss of expression on the cells or decreased numbers of CD27+ cells.
Safety and clinical activity of varlilumab, a related substance has already been shown in early clinical trials.8, 24 The resulting question is, whether the observed expression loss can be stopped or compensated by an agonistic CD27 stimulation. An earlier study has shown that the CD27 ligand, CD70, on human peripheral dendritic cells is upregulated by irradiation, in vitro.25 This might be an explanation for the downregulation of CD27 receptors. Further analyses are needed to investigate if the upregulation of CD70 also occurs as a result of irradiation of HNSCC and if it corresponds to CD27 downregulation. A rationale for a combination of irradiation and varlilumab would be to stop the downregulation by enhancing the T cell co-stimulation via CD27.
Additionally, we investigated whether the expression has any predictive or prognostic relevance. Prediction of response to CRT could not be revealed. We could see a slight advantage in disease-free survival for patients without PD-1 expression on tumor cells, which was not independent in multivariate analysis. However, as PD-1 is usually expressed by lymphocytes and not tumor cells and expression on TAL was not prognostic, these results are of limited relevance. A literature review identified two groups having investigated a possible influence of PD-1 on survival, with the observation of a lower recurrence rate and improved survival of patients with high PD-1 expression on lymphocytes.26 The discrepancy to our results could be explained by the observation of Balermpas et al. They saw no influence on survival when analyzing all tumor compartments at once, but only for intratumoural lymphocytes and lymphocytes at invasive margings.27
PD-L1 expression on tumor cells was associated with tumor recurrence leading to impaired disease-free survival. As this observation is contrary to previous studies to a certain extent, we performed a literature review on this subject. Our review revealed no obvious consensus about the prognostic role of PD-L1 expression (Table 2).26-33 Among these studies, Schulz et al were the only group to look at changes of PD-L1 expression by radiation. They compared known radio-resistant (rr) with radio-sensitive (rs) cell lines and could measure high PD-L1 expression in rr cells before radiation. This is in line with our observation of decreased disease-free survival. Moreover, they saw a time-dependent increase of PD-L1 in rs cells and decrease in rr cells after irradiation.33 It has to be assumed that cancerous tissue consists of both, rr and rs cells and this might be the explanation for the fact that we could measure no significant changes of PD-L1 by CRT.
In general, prognostic analyses were performed on heterogenous cohorts consisting of different percentages of primary tumor location. Treatment differed widely and ranged from induction chemotherapy followed by CRT to surgery with risk-adapted adjuvant therapy.28, 31 Two recent meta-analyses came to the conclusion that there is no clear predictive value of PD-L1 expression for any anti-cancer therapy. They saw the major limitation in a high heterogeneity of the studies regarding cancer sites and stages, anti-cancer therapies and PD-L1 assays.34, 35 Furthermore, different assays were used and even immunohistochemistry was not fully comparable, because different antibodies were applied in the studies. This aspect has been addressed by several authors in the past few years. Results of an analysis in lung cancer indicate an interchangeability of the PD-L1 antibody clones SP142, E1L3N, 9A11, SP263, 22c3, and 28-8.36 Another study, however, compared SP263 with E1L3N, which has been used in our study, and found SP263 to be more sensitive than E1L3N.37 A large metanalysis came to the conclusion that is more important to use a well-tested laboratory-specific antibody than just to exchange an FDA-approved antibody with another approved antibody, which might not have been designed for the respective purpose.38
Although the study is of high value, because of the analyzation of paired samples, there are some limitations. The sample size is small and consists of heterogenous tumor localizations. Immunohistology is a subjective method, which can lead to measurement errors and distortion of results, especially during evaluation. In order to minimize the subjectivity of the evaluation, the sections were assessed in a 4-eye principle. And finally, the samples were obtained in a period from 2005 to 2016. Age, fixation time as well as the storage location of tissue blocks can influence the staining quality. This can result in different intensities of the same expression.
Our results indicate that conventional CRT changes TAL on a molecular basis. This was especially evident for co-stimulatory CD27, which showed a lower expression score on TAL of nonresponders. These results have to be understood as preliminary and should serve as a basis for further investigation on how curative standard therapies influence the tumor microenvironment and should be looked into in prospective approaches in different tumor compartments, preferably at single cell level.
ACKNOWLEDGMENTS
Open access funding enabled and organized by Projekt DEAL.
AUTHOR CONTRIBUTIONS
Stephanie Ellen Weissinger, Peter Minkenberg, and Ulrike Kostezka performed experiments; Stephanie Ellen Weissinger, Peter Minkenberg, Simon Laban and Johannes Doescher prepared figures; Patrick Johannes Schuler, Simon Laban and Johannes Doescher provided patient samples; Johannes Doescher and Stephanie Ellen Weissinger designed research and wrote the article; Adrian von Witzleben, Patrick Johannes Schuler, and Thomas Karl Hoffmann edited the article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




