Chromophoric macrocycles from the oxidation of bis(aminofurazanylic) ethers of 1,2-diols
Corresponding Author
Aleksei B. Sheremetev
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, RussiaSearch for more papers by this authorElena V. Shatunova
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorBoris B. Averkiev
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Street B-334, 119991 Moscow, Russia
Search for more papers by this authorDmitrii E. Dmitriev
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorViktor A. Petukhov
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorMikhail Yu. Antipin
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Street B-334, 119991 Moscow, Russia
Search for more papers by this authorCorresponding Author
Aleksei B. Sheremetev
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, RussiaSearch for more papers by this authorElena V. Shatunova
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorBoris B. Averkiev
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Street B-334, 119991 Moscow, Russia
Search for more papers by this authorDmitrii E. Dmitriev
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorViktor A. Petukhov
N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47, Leninsky Prosp., 119991 Moscow, Russia
Search for more papers by this authorMikhail Yu. Antipin
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Street B-334, 119991 Moscow, Russia
Search for more papers by this authorAbstract
A series of chromophoric azofurazan-containing macrocycles 6a–c and 7a–d were synthesized from bis(aminofurazanylic) ethers of 1,2-diols 4a–d by dibromoisocyanurate oxidation. The macrocycle closure is a result of NN bond formation. An ion-binding ability of these compounds was tested. The macrocycles were characterized by NMR, MS, IR, and UV spectroscopy. The X-ray crystal structures of the macrocycles 6a, 7c, and linear counterpart 12 are reported. © 2004 Wiley Periodicals, Inc. Heteroatom Chem 15:131–145, 2004; Published online in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/hc.10226
REFERENCES
- 1 Shiga, M.; Takagi, M.; Ueno, K. Chem Lett 1980, 1021–1022.
- 2 Shinkai, S.; Nakaji, T.; Nishida, Y.; Ogawa, T.; Manabe, O. J Am Chem Soc 1980, 102, 5860–5862.
- 3 Shinkai, S.; Honda, Y.; Kusano, Y.; Manabe, O. J Chem Soc, Chem Commun 1982, 848–850.
- 4 Shinkai, S.; Honda, Y.; Minami, T.; Ueda, K.; Manabe, O.; Tashiro, M. Bull Chem Soc Jpn 1983, 56, 1700–1704.
- 5 Shinkai, S.; Honda, Y.; Ueda, K.; Manabe, O. Israel J Chem 1984, 24, 302.
- 6(a) Wei, W.; Tomohiro, T.; Kodaka, M.; Okuno, H. J Chem Soc, Perkin Trans 1, 1999, 3397–3398; (b) Wei, W.; Tomohiro, T.; Kodaka, M.; Okuno, H. J Org Chem 2000, 65, 8979–8987.
- 7 Dziomko, V. M.; Ostrovskaya, V. M.; Zhukova, T. E. Khim Geterotsikl Soedin 1979, 8, 1039–1040 (in Russian).
- 8 Sultanov, A. V.; Savvin, S. B. USSR Patent 1532560, 1989.
- 9 Ibrahim, Y. A.; Barsoum, B. N.; Elwahy, A. N. M.; Kuella, S. Supramol Chem 1998, 9, 5–12.
- 10 Skwierawska, A.; Inerowicz, H. D.; Biernat, J. F. Tetrahed Lett 1998, 39, 3057–3060.
- 11 Abbas, A. A. Tetrahedron, 1998, 54, 12421–12428 and references therein.
- 12 Shinkai, S.; Minami, T.; Kusano, Y.; Manabe, O. J Am Chem Soc 1983, 105, 1851–1856.
- 13(a) Luboch, E.; Biernat, J. F.; Simonov, Yu. A.; Dvorkin, A. A. Tetrahedron 1998, 54, 4977–4990; (b) Pipoosananakaton, B.; Sukwattanasinitt, M.; Jaiboon, N.; Chaichit, N.; Tuntulani, T. Tetrahed Lett 2000, 9095–9100.
- 14 Dziomko, V. M.; Ostrovskaya, V. M.; Zhukova, T. E.; Ryabokobylko, Yu. S. Zh Obshch Khim 1981, 51, 2324–2331 (in Russian).
- 15 Sitnikova, R. V.; Krylova, A. N.; Zelichenok, S. L.; Dziomko, V. M.; Ostrovskaya, V. M.; Zhukova, T. E.; Tolmacheva, E. I. Zh Anal Khim 1982, 37, 611–613 (in Russian).
- 16 Kitazawa, S.; Kimura, K.; Shono, T. Bull Chem Soc Jpn 1983, 56, 3253–3257.
- 17 Lin, I. C.; Pirio, M. US Patent 4,742,010, 1988; Chem Abstr 1989, 110, 88600.
- 18 Ostrovskaya, V. M.; Dyakonova, I. A.; Khim Geterotsikl Soedin 1987, 7, 867–882 (in Russian).
- 19 Newkome, G. R.; Sauer, J. D.; Roper, J. M.; Hager, D. C. Chem Rev 1977, 77, 513–597.
- 20 Sheremetev, A. B.; Kulagina, V. O.; Ivanova, E. A. J Org Chem 1996, 61, 1510–1511.
- 21 Sheremetev, A. B.; Kulagina, V. O.; Batog, L. V.; Lebedev, O. V.; Yudin, I. L.; Pivina, T. S.; Andrianov, V. G.; Starchenkov, I. B. In Proceedings of Twenty-Second International Pyrotechnics Seminar, July 15–19, 1996, Fort Collins, CO, pp. 377–388.
- 22 Batog, L. V.; Konstantinova, L. S.; Rozhkov, V. Yu.; Lebedev, O. V.; Epishina, M. A.; Makhova, N. N.; Ovchinnikov, I. V.; Khmelnitskii, L. I. In Proceeding of 29th International Annual Conference of ICT, June 30–July 3, Karlsruhe, Germany, 1998, 55/1–55/10.
- 23(a) Kots, A. Y.; Batog, L. V.; Betin, V. L.; Rozhkov, V. Yu.; Ukraintsev, K. E.; Khropov, Yu. V.; Epishina, M. A.; Sheremetev, A. B.; Makhova, N. N.; Bulargina, T. V. Russian Patent 2151799, 1999; (b) Sheremetev, A. B.; Betin, V. L.; Yudin, I. L.; Kulagina, V. O.; Khropov, Yu. V.; Bulargina, T. V.; Kots, A. Y. Russian Patent 2167161, 2000.
- 24 Kharitonova, O. V.; Sheremetev, A. B.; Novikova, T. S. In Vth All-Union Symp Chem Nitrogen Contain Heterocycl Compd Abst, Chernogolovka, 1991, p. 227 (in Russian).
- 25
Sheremetev, A. B.;
Kharitonova, O. V.
Mendeleev Commun
1992, 2(4), 157–158.
10.1070/MC1992v002n04ABEH000183 Google Scholar
- 26 Sheremetev, A. B.; Kharitonova, O. V.; Novikova, T. S.; Khmelnitskii, L. I.; USSR Patent SU 1803407, 1993; Chem Abstr 1994, 120, P191744z.
- 27
Sheremetev, A. B.;
Kulagina, V. O.;
Yudin, I. L.;
Kuzmina, N. E.
Mendeleev Commun
2001, 11(3), 112–114.
10.1070/mc2001v011n03ABEH001424 Google Scholar
- 28 Epishina, M. A.; Makhova, N. N.; Batog, L. V.; Konstantinova, L. S.; Khmelnitskii, L. I. Mendeleev Commun 4(3), 1994, 102–103.
- 29
Eman, V. E.;
Sukhanov, M. S.;
Lebedev, O. V.;
Batog, L. V.;
Konstantinova, L. S.;
Rozhkov, V. Yu.;
Khmelnitskii, L. I.
Mendeleev Commun
1996, 6(2), 66–67.
10.1070/MC1996v006n02ABEH000583 Google Scholar
- 30
Batog, L. V.;
Konstantinova, L. S.;
Eman, V. E.;
Sukhanov, M. S.;
Batsanov, A. S.;
Struchkov, Yu. T.;
Lebedev, O. V.;
Khmelnitskii, L. I.
Khim Geterotsikl Soedin
1996, 406–408 (in Russian);
Chem Heterocycl Compd
1996, 32, 352–354.
10.1007/BF01169258 Google Scholar
- 31
Batog, L. V.;
Konstantinova, L. S.;
Lebedev, O. V.;
Khmelnitskii, L. I.
Mendeleev Commun
1996, 6(5), 193–195.
10.1070/MC1996v006n05ABEH000641 Google Scholar
- 32 Batog, L. V.; Rozhkov, V. Yu.; Konstantinova, L. S.; Eman, V. E.; Dekaprilevich, M. O.; Struchkov, Yu. T.; Semenov, S. E.; Lebedev, O. V.; Khmelnitskii, L. I. Izv Akad Nauk, Ser Khim 1996, 1250–1254 (in Russian).
- 33
Eman, V. A.;
Sukhanov, M. S.;
Lebedev, O. V.;
Batog, L. V.;
Konstantinova, L. S.;
Rozhkov, V. Yu.;
Dekaprilevich, M. O.;
Struchkov, Yu. T.;
Khmelnitskii, L. I.
Mendeleev Commun
1997, 7(1), 5–7.
10.1070/mc1997v007n01ABEH000672 Google Scholar
- 34 Novikova, T. S.; Melnikova, T. M.; Kharitonova, O. V.; Kulagina, V. O.; Aleksandrova, N. S.; Sheremetev, A. B.; Pivina, T. S.; Khmelnitskii, L. I.; Novikov, S. S. Mendeleev Commun 1994, 4(4), 138–140.
- 35 Sheremetev, A. B.; Kharitonova, O. V.; Mantseva, E. V.; Kulagina, V. O.; Shatunova, E. V.; Aleksandrova, N. S.; Melnikova, T. M.; Ivanova, E. A.; Dmitriev, D. E.; Eman, V. A.; Yudin, I. L.; Kuzmin, V. S.; Strelenko, Yu. A.; Novikova, T. S.; Lebedev, O. V.; Khmelnitskii, L. I. Zh Org Khim 1999, 35, 1555–1566 (in Russian); Russ J Org Chem 1999, 35, 1525–1535.
- 36 Sheremetev, A. B.; Makhova, N. N.; Friedrichsen, W. Adv Heterocycl Chem 2001, 78, 65–188.
- 37
Recently we reported the metal ion template cyclization to synthesis of stable complexes (M = Cu, Co, Ni), which can be defined by formula:
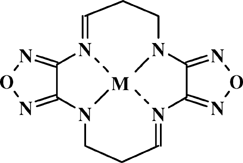
- 37 See Sheremetev, A. B. Broydo, P. I. In Vth All-Union Symp Chem Nitrogen Contain Heterocycl Compd Abst, Chernogolovka, 1991, p. 226 (in Russian).
- 38 Semenov, S. E.; Churakov, A. M.; Ioffe, S. L.; Vinogradova, E. A.; Zlotin, S. G.; Lukyanov, O. A. Izv Akad Nauk SSSR, Ser Khim 1991, 1940–1941 (in Russian); Bull Acad Sci USSR, Div Chem Sci 1991, 1727.
- 39 Our initial communication [25] described the reaction of bis(aminofurazanylic) ether of diethylene glicol with DBI; intramolecular product was main (63%).
- 40 In contrast, E and Z-isomers of azobenzene crowns may be easily assigned by 1H NMR [13].
- 41 In an earlier paper [42], the term “trans/cis position” refers to the position of furazan rings relative one to the other, the term “configuration” to Z, E geometry of furazan rings relative to NN bond, and the term “conformation” to geometry around the single bonds.
- 42 Chertanova, L.; Pascard, C.; Sheremetev, A. Supramolec Chem 1993, 3, 71–78.
- 43 Klyne, W.; Prelog, V. Experientia 1960, 16, 521–523.
- 44 Beal, R. W.; Incarvito, C. D.; Rhatigan, B. J.; Rheingold, A. L.; Brill, T. B. Propellants, Explosives, Pyrotechnics 2000, 25, 277–283.
- 45 Zelenin, A. K.; Trudell, M. L. J Heterocycl Chem 1998, 35, 151–155.
- 46 For review of the nitrofurazan chemistry, see A. B. Sheremetev, Ross Khim Zh (Zh. Ross. Khim. Ob-va im. D. I. Mendeleeva) 1997, 41(2), 43–54 (in Russian); Mendelev Chem J (Engl. Transl.) 1997, 41(2), 62–80.
- 47(a) Strelenko, Yu. A.; Sheremetev, A. B.; Khmelnitskii, L. I. Khim Geterotsikl Soedin 1992, 927–930; Chem Heterocycl Compd (Engl. Transl.) 1992, 28; (b) Dmitriev, D. E.; Strelenko, Yu. A.; Sheremetev, A. B. Izv AN, Ser Khim 2002, 277–282; Russ Chem Bull 2002, 51(2), 290–296.
- 48 It seems that in this case we have encountered a specific impact of the furazan ring that is a strong electron acceptor. In 1H and 13C NMR spectra all signals are broadened what makes detailed research impossible (for an example, see Sheremetev, A. B., Mantseva, E. V., Dmitriev, D. D., Izv AN, Ser Khim 2001, (12), 2366–2367; Russ Chem Bull (Engl. Transl.) 2001, 50, 2479–2480). At lower temperatures solubility drops drastically thereby hampering low-temperature examinations.
- 49
Paton, R. M.
In Comprehensive Heterocyclic Chemistry;
A. R. Katritzky;
C. W. Rees;
E. F. V. Scriven (Eds.);
Pergamon Press: Oxford,
1996; Vol. 4, pp. 229–265.
10.1016/B978-008096518-5.00083-6 Google Scholar
- 50 For reviews of dinitramide salts, see Lukyanov, O. A.; Tartakovskii, V. A. Ross Khim Zh (Zh Ross Khim Ob-va im. D. I. Mendeleeva) 1997, 41(2), 5–13 (in Russian).
- 51 Batsanov, A. S.; Struchkov, Yu. T. Zh Strukt Khim 1985, 26, 65–68 (in Russian).
- 52 Averkiev, B. B.; Antipin, M. Yu.; Sheremetev, A. B.; Timofeeva, T. V. Acta Cryst, Sect C, 2003, C59, 383–387.
- 53 Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. J Chem Soc, Perkin Trans 2 1987, S1–S19.
- 54 Zefirov, Yu. V.; Zorkii, P. M. Usp Khim 1995, 64, 446–461 (in Russian); Russ Chem Rev 1995, 64.
- 55 Sheldrick, G. M. SHELXTL-97, Version 5.10, Bruker AXS Inc., Madison, WI.




