Novel 2-phosphabicyclo[2.2.2]oct-5-ene derivatives and their use in phosphinylations†
Corresponding Author
György Keglevich
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, HungarySearch for more papers by this authorJános Kovács
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorTamás Körtvélyesi
Department of Physical Chemistry, University of Szeged, 6701 Szeged, Hungary
Search for more papers by this authorGyula Parlagh
Department of Physical Chemistry, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorTímea Imre
Hungarian Academy of Sciences, Chemical Research Center, 1525 Budapest, Hungary
Search for more papers by this authorKrisztina Ludányi
Hungarian Academy of Sciences, Chemical Research Center, 1525 Budapest, Hungary
Search for more papers by this authorLászló Hegedűs
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorMiklós Hanusz
Chinoin Company Ltd. (member of Sanofi-Synthelabo Group), 1045 Budapest, Hungary
Search for more papers by this authorKálmán Simon
Chinoin Company Ltd. (member of Sanofi-Synthelabo Group), 1045 Budapest, Hungary
Search for more papers by this authorAndrea Márton
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorGyörgy Marosi
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorLászló Tőke
Research Group of the Hungarian Academy of Sciences at the Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorCorresponding Author
György Keglevich
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, HungarySearch for more papers by this authorJános Kovács
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorTamás Körtvélyesi
Department of Physical Chemistry, University of Szeged, 6701 Szeged, Hungary
Search for more papers by this authorGyula Parlagh
Department of Physical Chemistry, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorTímea Imre
Hungarian Academy of Sciences, Chemical Research Center, 1525 Budapest, Hungary
Search for more papers by this authorKrisztina Ludányi
Hungarian Academy of Sciences, Chemical Research Center, 1525 Budapest, Hungary
Search for more papers by this authorLászló Hegedűs
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorMiklós Hanusz
Chinoin Company Ltd. (member of Sanofi-Synthelabo Group), 1045 Budapest, Hungary
Search for more papers by this authorKálmán Simon
Chinoin Company Ltd. (member of Sanofi-Synthelabo Group), 1045 Budapest, Hungary
Search for more papers by this authorAndrea Márton
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorGyörgy Marosi
Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorLászló Tőke
Research Group of the Hungarian Academy of Sciences at the Department of Organic Chemical Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
Search for more papers by this authorThe article is dedicated to Professor Dr. SÁNDOR ANTUS (University of Debrecen, Hungary) on the occasion of his sixtieth birthday.
Abstract
The [4 + 2] cycloaddition of the double-bond isomers (A and B) of dihydrophosphinine oxide 1 afforded novel phosphabicyclo[2.2.2]oct-5-ene derivates (2–4), formation of which was justified by PM3 semiempirical calculations. The compounds of dimer type (2–4) were utilized in the UV light-mediated fragmentation-related phosphinylation of nucleophiles, especially in that of alcohols. To explore the role of structural modifications on the fragmentation ability, disulfide 5, phosphabicyclooctane 7 obtained by the hydrogenation of 2, and the adduct of dihydrophosphinine oxide 1 with benzoquinone (7) were also synthesized and tested in fragmentation. © 2004 Wiley Periodicals, Inc. Heteroatom Chem 15:97–106, 2004; Published online in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/hc.10221
REFERENCES
- 1 Heydt, H. In Multiple Bonds and Low Coordination in Phosphorus Chemistry: Methylene(imino, oxo, thioxo, and selenoxo)phosphoranes; M. Regitz; O. J. Scherer (Eds.); G. Thieme Verlag: Stuttgart, 1990; Ch. E2, p. 381.
- 2 Keglevich, Gy. Curr Org Chem 2002, 6, 891.
- 3 Keglevich, Gy.; Steinhauser, K.; Ludányi, K.; Tőke, L. J Organomet Chem 1998, 570, 49.
- 4 Keglevich, Gy.; Szelke, H.; Nagy, Z.; Dobó, A.; Novák, T.; Tőke, L. J Chem Res (S) 1999, 580.
- 5 Quin, L. D.; Tang, J.-S.; Quin, G. S.; Keglevich, Gy. Heteroat Chem 1993, 4, 189.
- 6 Quin, L. D.; Tang, J.-S.; Keglevich, Gy. Heteroat Chem 1991, 2, 283.
- 7 Keglevich, Gy.; Szelke, H.; Dobó, A.; Nagy, Z.; Tőke, L. Synth Commun 2001, 31, 1737.
- 8 Jankowski, S.; Rudzinski, J.; Szelke, H.; Keglevich, Gy. J Organomet Chem 2000, 595, 109.
- 9 Keglevich, Gy.; Szelke, H.; Tamás, A. M.; Harmat, V.; Ludányi, K.; Vaskó, Á. Gy.; Tőke, L. Heteroat Chem 2002, 13, 626.
- 10 Keglevich, Gy.; Vaskó, Á. Gy.; Dobó, A.; Ludányi, K.; Tőke, L. J Chem Soc, Perkin Trans 1 2001, 1062.
- 11 Keglevich, Gy.; Kovács, J.; Ludányi, K.; Tőke, L. Het Commun 2002, 8, 31.
- 12 Stewart, J. J. P. MOPAC93 (Revision V. 2); Fujitsu Ltd, Tokyo, 1995.
- 13 Stewart, J. J. P. J Comput Chem 1989, 10, 209.
- 14 Stewart, J. J. P. J Comput Chem 1989, 10, 221.
- 15 Moedritzer, K. Synth React Inorg Met-Org Chem 1974, 4, 119.
- 16 Lewis, E. S.; McCortney, B. A. Can J Chem 1986, 64, 1156.
- 17 Keglevich, Gy.; Steinhauser, K.; Keserű, Gy. M.; Böcskei, Zs.; Újszászy, K.; Marosi, Gy.; Ravadits, I.; Tőke, L. J Organomet Chem 1999, 579, 182.
- 18 Keglevich, Gy.; Androsits, B.; Tőke, L. J Org Chem 1988, 53, 4106.
- 19
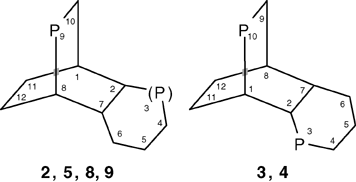
According to the IUPAC nomenclature, the following numbering was used for naming the products:
- 20 teXsan for Windows, version 1.06; Molecular Structure Corporation, The Woodlands, Texas, 1997–1999.
- 21 Sheldrick, G. M. SHELXL-97; University of Göttingen, Germany, 1997.
- 22 Hyperchem, Release 7.0; Hypercube, Inc., 2002.
- 23 Schaftenaar, G.; Noordik, J. H. J Comput-Aided Mol Des 2000, 14, 123.




