Passive perceptual learning modulates motor inhibitory control in superior frontal regions
Abstract
Response inhibition is of vital importance in the context of controlling inappropriate responses. The role of perceptual processes during inhibitory control has attracted increased interest. Yet, we are far from an understanding of the mechanisms. One candidate mechanism by which perceptual processes may affect response inhibition refers to “gain control” that is closely linked to the signal-to-noise ratio of incoming information. A means to modulate the signal-to-noise ratio and gain control mechanisms is perceptual learning. In the current study, we examine the impact of perceptual learning (i.e., passive repetitive sensory stimulation) on response inhibition combining EEG signal decomposition with source localization analyses. A tactile GO/NOGO paradigm was conducted to measure action restraint as one subcomponent of response inhibition. We show that passive perceptual learning modulates response inhibition processes. In particular, perceptual learning attenuates the detrimental effect of response automation during inhibitory control. Temporally decomposed EEG data show that stimulus-related and not response selection processes during conflict monitoring are linked to these effects. The superior and middle frontal gyrus (BA6), as well as the motor cortex (BA4), are associated with the effects of perceptual learning on response inhibition. Reliable neurophysiological effects were not evident on the basis of standard ERPs, which has important methodological implications for perceptual learning research. The results detail how lower level sensory plasticity protocols affect higher-order cognitive control functions in frontal cortical structures.
1 INTRODUCTION
Response inhibition, as a core feature of executive functioning, is of vital importance in the context of controlling dominant, inappropriate responses (Diamond, 2013; Miyake & Friedman, 2012). Even though attentional and perceptual processes play a major role when inhibitory control has to be exerted (Boehler et al., 2009; Chmielewski, Mückschel, Stock, & Beste, 2015; Huster, Westerhausen, Pantev, & Konrad, 2010; Raud & Huster, 2017; Shedden & Reid, 2001; Stock, Popescu, Neuhaus, & Beste, 2016; Verbruggen, Liefooghe, & Vandierendonck, 2006), we are far from an understanding how modulations of perceptual processes affect response inhibition. For better comprehension of this interrelation, we investigate action restraint (withholding a predominant response) as one subcomponent of response inhibition (Bari & Robbins, 2013; Schachar et al., 2007; Sebastian et al., 2013) that is usually measured by means of a GO/NOGO task (Diamond, 2013; Eagle, Bari, & Robbins, 2008; Sebastian et al., 2013; Sebastian, Forstmann, & Matzke, 2018). A candidate mechanism by which perceptual processes may affect response inhibition refers to “gain control.” Gain control mechanisms relate sensory input to the corresponding (motor) output (Aston-Jones & Cohen, 2005; S.-C. Li & Rieckmann, 2014; Servan-Schreiber, Printz, & Cohen, 1990). Gain control critically depends on the signal-to-noise ratio of sensory information. When signal-to-noise ratio is high, the input has a strong effect on output activation (Aston-Jones & Cohen, 2005; Servan-Schreiber et al., 1990). Just recently it has been shown that gain control principles and neurobiological factors modulating signal-to-noise ratio of information processing in neural circuits affect response inhibition processes in superior frontal cortices (Adelhöfer, Mückschel, Teufert, Ziemssen, & Beste, 2019). An important mechanism by which gain control related mechanisms can be modulated is perceptual learning (B. Dosher & Lu, 2017).
Perceptual learning can be induced by passive repetitive sensory stimulation (Beste & Dinse, 2013; Freyer, Reinacher, Nolte, Dinse, & Ritter, 2012; Parianen Lesemann, Reuter, & Godde, 2015; Seitz & Dinse, 2007). The mechanism behind this type of learning is likely based on changes in synaptic transmission (Beste & Dinse, 2013) and is also associated with cortical reorganization processes (Pleger et al., 2003). Studies investigating the effects of repetitive sensory stimulation have been conducted in various modalities, yet the tactile modality has been studied most extensively (Beste & Dinse, 2013). Repetitive tactile stimulation protocols enhance spatial tactile acuity at the stimulation site (Beste & Dinse, 2013; Dinse, Kattenstroth, Lenz, Tegenthoff, & Wolf, 2017; Godde, Stauffenberg, Spengler, & Dinse, 2000; Ragert, Vandermeeren, Camus, & Cohen, 2008; Rocchi et al., 2017). Interestingly, it has been demonstrated that perceptual learning enhances the stimulus-related signal and the representation of the relevant signal in neural circuits (B. A. Dosher & Lu, 1998; Gold, Bennett, & Sekuler, 1999). Therefore, and because the efficiency of the stimulus to trigger a specific output is increased by perceptual learning (Gold et al., 1999), it is conceivable that perceptual learning facilitates behavioral control (Beste, Wascher, Güntürkün, & Dinse, 2011). Importantly, increasing the strength of stimulus representations reduces automated response tendencies in response inhibition tasks and makes it easier to inhibit a response (Cavina-Pratesi, Bricolo, Prior, & Marzi, 2001; Chmielewski & Beste, 2016; Dippel, Mückschel, Ziemssen, & Beste, 2017; Fiedler, Schröter, & Ulrich, 2011; Gondan, Götze, & Greenlee, 2010). Therefore, we hypothesize that response inhibition performance in a tactile GO/NOGO task is enhanced through the use of a tactile perceptual learning protocol. This hypothesis is also reasonable on the basis of neuroanatomical considerations: The somatosensory cortex is connected to motor areas and the supplementary motor cortex (SMA) (Ackerley & Kavounoudias, 2015; Borich, Brodie, Gray, Ionta, & Boyd, 2015; Chouinard & Paus, 2006). The latter is part of a response inhibition network (Sebastian et al., 2018). Furthermore, motor control is modulated by changes of somatosensory input (Borich et al., 2015) and it has already been shown that repetitive tactile and electrical stimulation improves motor performance (Kalisch, Tegenthoff, & Dinse, 2008; Kalisch, Tegenthoff, & Dinse, 2010). Since somatosensory perceptual learning modulates processing in somatosensory cortices (Dinse, Ragert, Pleger, Schwenkreis, & Tegenthoff, 2003), it seems plausible to assume that perceptual modulation by a tactile stimulation protocol is able to modulate motor response inhibition processes that are dependent on prefrontal structures (Bari & Robbins, 2013).
To examine underlying neurophysiological processes, we recorded the electrophysiological signal (EEG) and modulations at the level of functional neuroanatomical structures were identified using source localization analysis (sLORETA). In terms of EEG correlates, especially the NOGO-N2/P3 event-related potential (ERP) time windows are important. The NOGO-N2/P3 ERPs reflect different response inhibition subprocesses as premotor inhibition, conflict monitoring or response-related evaluation are important to consider (Falkenstein, Hoormann, & Hohnsbein, 1999; Huster, Enriquez-Geppert, Lavallee, Falkenstein, & Herrmann, 2013). Yet, especially in the N2 time range, perceptual and response selection processes have been found to be intermingled (Chmielewski, Mückschel, & Beste, 2018; Folstein & Van Petten, 2008; Larson, Clayson, & Clawson, 2014; Mückschel, Dippel, & Beste, 2017). It has been suggested that the N2 component reflects a composition of different coding levels: one coding level refers to perceptual processes, the other to response selection processes (Chmielewski et al., 2018; Folstein & Van Petten, 2008; Mückschel, Dippel, & Beste, 2017). Since we are specifically modulating perceptual processes by passive sensory stimulation, it is likely that no reliable effects are observed in standard ERPs, which reflect various signals from different sources (Huster, Plis, & Calhoun, 2015; Nunez et al., 1997; Stock, Gohil, Huster, & Beste, 2017). Rather, effects are likely to be confined to the perceptual coding level involved during conflict monitoring in response inhibition. To disentangle coding levels during response inhibition, we use residue iteration decomposition (RIDE) (Chmielewski et al., 2018; Mückschel, Dippel, & Beste, 2017; Ouyang, Herzmann, Zhou, & Sommer, 2011; Ouyang, Sommer, & Zhou, 2015a, 2015b). This procedure decomposes the EEG signal into different clusters: Stimulus-related processes like perception or attention are reflected by the S-cluster (Ouyang et al., 2011; Ouyang et al., 2015b). Processes associated with response selection are reflected by the C-cluster (Ouyang, Hildebrandt, Sommer, & Zhou, 2017; Verleger, Metzner, Ouyang, Śmigasiewicz, & Zhou, 2014). We hypothesize that neurophysiological effects manifest in the S-cluster. However, it cannot a-priori be ruled out that effects are also evident in the C-cluster. C-cluster effects have already been found in studies investigating the influence of perceptual processes on response inhibition performance (Friedrich, Mückschel, & Beste, 2017a, 2017b). Yet, in these studies manipulation of perceptual processes was also linked to changes in response assignments so that C-cluster effects may have occurred because the C-cluster is rather involved in stimulus–response transition processes (Bluschke, Chmielewski, Mückschel, Roessner, & Beste, 2017; Ouyang et al., 2017; Verleger et al., 2014).
On a functional neuroanatomical level, we hypothesize prefrontal and premotor areas of the frontal cortex to be associated with the effect of perceptual learning on response inhibition since they are frequently shown to be involved in response inhibition processes (Aron, Robbins, & Poldrack, 2004; Bari & Robbins, 2013). For example, the superior frontal gyrus, including the supplementary motor area (SMA), has been shown to modulate task performance in cognitive and motor control tasks (W. Li et al., 2013) and has already been linked to S-cluster modulations in the context of cognitive control (Mückschel, Chmielewski, Ziemssen, & Beste, 2017). Furthermore, the medial prefrontal gyrus has been linked to GO/NOGO task performance deficits (Bari & Robbins, 2013). It is also plausible that the SMA and pre-SMA are of relevance since they constitute core structures of response inhibition (Bari & Robbins, 2013; Chen, Muggleton, Tzeng, Hung, & Juan, 2009; C. R. Li, Huang, Constable, & Sinha, 2006; Swick, Ashley, & Turken, 2011) and especially the SMA is linked to somatosensory areas (Borich et al., 2015). Because of its relevance in regard to movement selection (Rushworth, Johansen-Berg, Göbel, & Devlin, 2003) and cognitive aspects of motor control (Tanaka, Honda, & Sadato, 2005) activity in the premotor cortex is likely to be modulated as well. Moreover, the dorsal part of the premotor area, which is closely connected with prefrontal areas, has been associated with linking arbitrary cues to specific responses (Chouinard & Paus, 2006; Chouinard & Paus, 2010) and is therefore expected to be involved in the modulation of response inhibition.
2 MATERIALS AND METHODS
2.1 Participants
The study tests an interaction between the time point of task execution (before or after perceptual learning) and group (stimulation or no-stimulation group): that is, a group which received perceptual learning should reveal modulations of response inhibition after the perceptual learning protocol has been commenced. A group receiving no such protocol should reveal no modulations in response inhibition performance. The study design hence comprises a between-subject factor and a within-subject factor. We conservatively considered a smaller effect size of 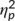 = .10 to be detectable with a power of at least 95%. The power analysis using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) revealed that a total sample size of N = 32 is required for that. Importantly, the size of the actually observed effect (see Section 3) was larger (i.e.,
= .10 to be detectable with a power of at least 95%. The power analysis using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) revealed that a total sample size of N = 32 is required for that. Importantly, the size of the actually observed effect (see Section 3) was larger (i.e., 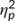 > .198), which underlines that the study was sufficiently powered.
> .198), which underlines that the study was sufficiently powered.
N = 34 participants (24 females) between 19 and 30 years took part in the experiment. N = 17 participants (12 females) received repetitive tactile stimulation (mean age = 24.2; SEM = 0.75) and the other half (mean age = 24.3; SEM = 0.8) did not receive it. All participants were right-handed and had no psychiatric or neurological disorder. Participants were pseudorandomly assigned to the experimental groups; that is, it was ensured that the distribution of sexes is equal in each group. In advance, written informed consent was obtained by the participants in accordance with the Helsinki Declaration of 1975, as revised in 2008. All methods were performed in accordance with the relevant guidelines and regulations. The local ethics committee of the Medical Faculty of the TU Dresden approved the study.
2.2 Somatosensory response inhibition task
Since perceptual learning processes are best studied in the somatosensory domain, we examined response inhibition using a somatosensory response inhibition task developed by our group (Friedrich et al., 2017b). During the task, small electromagnetic stimulators were used for vibrotactile stimulation (Dancer Design; for more detailed information see http://www.dancerdesign.co.uk). A “main module” (Neurocore; http://www.neurocore.de/) was used to control the stimulators that deliver the stimulation. The stimulation setup is described in Figure 1 and identical to previous studies (Friedrich et al., 2017b).

The right thumb was stimulated since it is easiest to avoid contact of the stimulation device with the table or response device. 70% of trials presented GO stimuli and 30% presented NOGO stimuli. This ratio fosters a response tendency taxing inhibitory control processes (Dippel et al., 2017; Helton, 2009; Stevenson, Russell, & Helton, 2011). Frequencies of 40 and 150 Hz (duration of 100 ms) were utilized as GO or NOGO stimuli since they are clearly separable (Friedrich et al., 2017a). These frequencies were chosen because stimulation in the range from 10 to 50 Hz is predominantly activating primary sensory cortex (Chung et al., 2013; Francis et al., 2000; Harrington & Hunter Downs III, 2001), while the 100–400 Hz stimulation range activates the secondary somatosensory cortex (Chung et al., 2013; Francis et al., 2000; Kalberlah, Villringer, & Pleger, 2013). The experiment consisted of four blocks and participants received 204 trials per block (i.e., 832 trials in total). In two blocks, the low frequency served as GO and the high frequency as NOGO stimulus. In the other two blocks this was vice versa. The order of blocks presenting different GO and NOGO stimulus frequencies was counterbalanced across subjects. The data were analyzed without considering the frequency used to present GO and NOGO trials, since we had no a-priori hypothesis that the frequency used to present GO or NOGO stimuli interacts with the perceptual learning protocol; that is, the low and high frequency GO trials were pooled afterwards. The same was done for NOGO trials. The amplitude of the tactile stimuli was the same for all participants. Participants were asked to respond to GO stimuli with the index finger of their right hand as fast as possible and to inhibit their response in NOGO trials. The trial sequence in each block was pseudorandomized to eliminate sequence effects and the time between trials was jittered between 700 and 1,100 ms to avoid stimulus onset predictability. A GO trial was classified as correct when the button press occurred within 100–1,000 ms after GO stimulus presentation. For a correct NOGO trial, no response should occur in that interval. Prior to the experiment, participants were familiarized with the stimuli and received practice blocks to make sure that the procedure was understood.
2.3 Perceptual learning protocol
Due to the high spatial specificity of tactile perceptual learning effects (Ragert et al., 2008), the stimulation site during the perceptual learning protocol was the thumb, which was also used as stimulation site during the GO/NOGO task. Also, the same electromagnetic stimulators were used. The perceptual learning protocol consisted of a 20 Hz stimulation delivered for 1 s followed by an interval of 5 s with no stimulation. This sequence was repeated for period of 40 min. The protocol was chosen since it has been shown to facilitate tactile discrimination for at least 24 hr when applied for 20 min (Ragert et al., 2008) and was also used in other studies (Dinse et al., 2017; Freyer et al., 2012; Freyer, Becker, Dinse, & Ritter, 2013). Similar effects were also demonstrated in other sensory domains (Beste & Dinse, 2013). To ensure sufficient aftereffects, stimulation duration was set to 40 min as done in studies applying visual stimulation protocols (Beste et al., 2011). To avoid that subjects attended the stimulation, they watched a nature documentary during stimulation. Participants who did not receive tactile stimulation also watched the documentary while the stimulation device was still attached to the thumb so that the set up was identical between the two groups. This procedure was chosen because it ensures controlled conditions between the testings and also ensured that the participants being stimulated did not actively attend the tactile stimulation protocol. It was considered the methodologically most appropriate alternative to have exact the same set up for both groups except of varying the administration of the stimulation protocol. A control stimulation at the same hand was excluded since it could still have interfered with the relevant stimulation site due to potentially overlapping cortical representations (Ackerley & Kavounoudias, 2015; Kalberlah et al., 2013; Sanchez Panchuelo, Besle, Schluppeck, Humberstone, & Francis, 2018). Conducting the control stimulation on the other hand was also not an option because transcallosal connections between homologous somatosensory regions as well as nonhomologous regions (Tamè, Braun, Holmes, Farnè, & Pavani, 2016) could have a potential effect on the results. After watching the documentary with or without stimulation, participants performed the tactile GO/NOGO task again.
2.4 EEG recording and analysis
EEG recording was done using 60 passive Ag/AgCl ring electrodes arranged at equidistant positions. Electrodes were connected to a QuickAmp amplifier (Brain Products Inc.) and the EEG data was preprocessed with BrainVision Analyzer (Brain Products Inc.). The coordinates for the ground and reference electrodes were theta = 58, phi = 78 and theta = 90, phi = 90, respectively. EEG data were sampled at a rate of 500 Hz. The recording bandwidth was 0.5–80 Hz and electrode impedances were kept below 5 kΩ. The IIR band-pass filter was set to 0.5–20 Hz with a slope of 48 dB/oct. Afterwards, a manual raw data inspection was conducted to discard infrequent technical or muscular artifacts. This was followed by an independent component analysis (ICA; infomax algorithm) performed for all blocks to detect regularly occurring artifacts like blinks or lateral eye movements. Independent components clearly reflecting such artifacts were removed. Then, the data was segmented according to the different experimental conditions. Only trials with correct responses were used for further data analysis steps. Criteria were a correct button press within 100–1,000 ms after GO stimulus presentation and no button press within this 1,000 ms time period in NOGO trials. In an automated artifact rejection procedure, trials with a maximal value difference of 200 μV in a 200 ms period were discarded. Further rejection criteria were amplitudes below −200 μV and above 200 μV, as well as below 0.5 μV in a 100 ms interval. On average, 11% of all GO trials and 18% of all NOGO trials were rejected either manually or by automated artifact rejection. Of originally 582 GO trials 516 remained and of originally 250 NOGO trials 204 remained (on average).
On average, 13% of all trials were rejected either manually or by automated artifact rejection. Of initially 832 trials, 720 remained. Thus, the remaining number of trials is sufficient for a reliable data analysis.
Subsequently, a current source density (CSD) transformation was applied with 4 splines and 10 polynomials. By conducting a CSD transformation the reference potential is removed and a reference-free evaluation of the data is obtained leading to amplitude values in μV/m2. Since the CSD transformation works as a spatial filter (Nunez & Pilgreen, 1991; Tenke & Kayser, 2012), it fosters the identification of relevant electrode sites for data quantification. Following this, a baseline correction from −200 to 0 was conducted with time point 0 representing the time of GO/NOGO stimulus presentation. In the next step, an averaging of different conditions (stimulation/no-stimulation group, first or second time point of task execution and GO/NOGO trials) was performed on the single-subject level. Then, the ERP amplitudes were quantified at the single subject level. The time windows and electrode sites for data quantification were chosen on the basis of a visual inspection of corresponding ERP scalp topography maps. This choice of electrodes and time windows was validated using a statistical method (Mückschel, Stock, & Beste, 2014): Within each of the search intervals (see below), the peak amplitude was extracted for all electrode sites. Each electrode was subsequently compared against the average of all other electrodes using Bonferroni-correction for multiple comparisons (critical threshold p = .0007). Only electrodes that showed significantly larger mean amplitudes (i.e., negative for N-potentials and positive for the P-potentials) than the remaining electrodes were selected. The identified electrode sites matched those determined in the visual inspection of the data. We quantified the P2 in the time window between 170 and 200 ms in GO and NOGO trials for the “stimulation group” and 210–240 ms in GO and NOGO trials for the “no-stimulation group.” The mean amplitude in the N2 time window was quantified at electrode Cz in the time range from 350 to 380 ms in GO trials for both groups and time points. This time window might seem late for this component, yet the N2 component can occur between 200 and 400 ms after stimulus onset (Albares, Lio, & Boulinguez, 2015) and the chosen time window is still within this range. In NOGO trials, the time window from 260 to 290 ms was used for the “stimulation group” for both time points. In the “no stimulation group” the time range between 305 and 335 ms was quantified for the first time point and 280–310 ms for the second time point. The mean amplitude of the P3 component was also quantified at electrode Cz in the time period from 470 to 510 ms in GO trials for both time points and groups. In NOGO trials, the time window from 360 to 410 ms was quantified in the “stimulation group” for both time points as well as the “no stimulation group” at the second time point. The time range between 440 and 500 ms was used for the first time point in the “no stimulation group.”
2.5 Residue iteration decomposition
The RIDE algorithm uses single-trial EEG data and was performed with MATLAB (MATLAB 12.0; Mathworks Inc.). We applied the RIDE toolbox (available on http://cns.hkbu.edu.hk/RIDE.htm) following previous work (Mückschel, Chmielewski, et al., 2017; Ouyang et al., 2011; Verleger et al., 2014). For mathematical details of that method please refer to previous literature on the method (Ouyang et al., 2015a). In principle, the RIDE algorithm decomposes ERP components and minimizes the residual error arising from temporal (i.e., latency) variability in single trials (Ouyang et al., 2015a). The RIDE decomposition is performed for each electrode separately without taking scalp distributions or waveforms into account. Therefore, the CSD transformation can be applied without biasing the results. The RIDE algorithm has the advantage of decomposing ERP signals into clusters that can be linked to stimulus onset (S-cluster) or reaction times (R-cluster). Since no reaction time measures can be recorded in correct NOGO trials, the response-related R-cluster is not considered in GO/NOGO paradigms (Ouyang, Schacht, Zhou, & Sommer, 2013). Furthermore, a third cluster (C-cluster) can be extracted. It has a variable latency and is temporally located between stimulus and response. The C-cluster waveform is initially estimated and then iteratively improved. A time window function initially estimates C-cluster latency and a self-optimizing iteration scheme is employed to enhance C-cluster latency estimation. This process works by removing the S-cluster and re-estimating the C-cluster latency by mean of a template matching approach. It is essential to predefine the time windows in which the clusters are expected to occur. Therefore, it is necessary to adjust the values to the data under investigation (Ouyang et al., 2015a). Further information is available in Ouyang et al. (Ouyang et al., 2011, 2015a, 2015b). The time window for the S-cluster was set to −200 to 600 ms and for the C-cluster to 150–800 ms around stimulus onset. These time windows are comparable to those used in previous studies (Friedrich et al., 2017a, 2017b).
The S-cluster was quantified at electrode Cz in the time window between 350 and 380 ms in GO trials for both time points and groups. In NOGO trials, the time range from 260 to 290 ms was quantified within the “stimulation group” for both time points. In the “no stimulation group” the time window from 300 to 340 ms was chosen for both time points. Furthermore, the S-cluster was quantified between 170 and 200 ms in GO and NOGO trials for the “stimulation group” and 210–240 ms in GO and NOGO trials for the “no stimulation group.” The C-cluster was quantified between 510 and 540 ms in GO trials for the “stimulation group” for both time points. In the “no stimulation group” the time range was set to 550–580 ms for both time points. In NOGO trials, the time window in the “stimulation group” was quantified from 380 to 400 ms for both time points and in the “no stimulation group” the time period from 420 to 440 ms was chosen for both time points. The selection of time windows and electrode sites was performed and validated using the same procedure as used for the standard ERP data.
2.6 Source localization analysis
Source localization analysis was performed using sLORETA (standardized low resolution brain electromagnetic tomography) (Pascual-Marqui, 2002). sLORETA has the advantage of providing a single solution to the inverse problem (Marco-Pallarés, Grau, & Ruffini, 2005; Pascual-Marqui, 2002; Sekihara, Sahani, & Nagarajan, 2005). First, the intracerebral volume is split into 6,239 voxels that have a spatial resolution of 5 mm. By means of a realistic three-shell spherical head model, the standardized current density is then calculated for each voxel (Fuchs, Kastner, Wagner, Hawes, & Ebersole, 2002) based on an MNI152 template (Mazziotta et al., 2001). sLORETA yields reliable results without localization bias as demonstrated mathematically (Sekihara et al., 2005). Sources found by sLORETA have also been validated by further EEG/fMRI and neuronavigated EEG/TMS studies (Dippel & Beste, 2015; Sekihara et al., 2005). To compare voxel-based sLORETA images across conditions and groups, the sLORETA-built-in voxel-wise randomization tests with 2,500 permutations were applied which rests on statistical nonparametric mapping (SnPM). Voxels demonstrating significant differences (p < .01, corrected for multiple comparisons) between calculated contrasts were shown in the MNI brain.
2.7 Statistical analysis
Behavioral data (i.e., hits and reaction times in GO trials, as well as false alarms in NOGO trials) were analyzed by means of mixed-effects analysis of variance (ANOVAs). “Time point” (first/second time point of task execution) was used as a within-subject factor. “Group” (stimulation/no stimulation) was set as a between-subject factor. Mixed-effects ANOVAs were also used to analyze neurophysiological data and “trial type” was added as another within-subject factor. All tests were Greenhouse–Geisser corrected and Bonferroni correction was conducted for all post hoc tests. The mean and SEM are given for the descriptive statistics.
3 RESULTS
3.1 Behavioral data
A mixed-effects ANOVA of hit rates revealed a significant main effect of “time point” (F(1,32) = 7.1; p = .012; 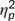 = .182) with higher hit rates at the first (99% ± 0.2) than at the second time point of task execution (98.1% ± 0.4). No other main or interaction effect was significant (all F ≤ 1; p ≥ .335). Analyzing hit reaction times demonstrated a significant main effect of “time point” (F(1,32) = 75.3; p < .001;
= .182) with higher hit rates at the first (99% ± 0.2) than at the second time point of task execution (98.1% ± 0.4). No other main or interaction effect was significant (all F ≤ 1; p ≥ .335). Analyzing hit reaction times demonstrated a significant main effect of “time point” (F(1,32) = 75.3; p < .001; 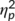 = .702) wither faster responses at the second (381 ms ± 9) than at the first time point of task execution (434 ms ± 12). No further main or interaction effect was significant (all F ≤ 0.7; p ≥ .411).
= .702) wither faster responses at the second (381 ms ± 9) than at the first time point of task execution (434 ms ± 12). No further main or interaction effect was significant (all F ≤ 0.7; p ≥ .411).
The analysis of false alarm rates (i.e., responding in NOGO trials although no response is required) as the most important indicator of response inhibition performance showed a significant main effect of “time point” (F(1,32) = 31.2; p < .001;  = .493). More inhibition errors were committed at the second time point of task execution (8.8% ± 1) than at the first (6.7% ± 1). There was no main effect “group” (F(1,32) = 0.2; p = .674;
= .493). More inhibition errors were committed at the second time point of task execution (8.8% ± 1) than at the first (6.7% ± 1). There was no main effect “group” (F(1,32) = 0.2; p = .674; 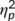 = .006). Importantly, a significant interaction effect of “time point x group” was obtained (F(1,32) = 7.9; p = .008;
= .006). Importantly, a significant interaction effect of “time point x group” was obtained (F(1,32) = 7.9; p = .008; 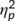 = .198). A post hoc power calculation revealed a power of 99% for that effect. Post hoc paired t tests were conducted showing that within the “no stimulation group” false alarm rates at the first (5.8% ± 1.1) and the second time point of task execution (8.9% ± 1.4) differed significantly (t(16) = −6.29; p < .001). In the “stimulation group” there was only a marginally significant difference between the task performance at the first (7.7% ± 1.6) and the second time point of task execution (8.7% ± 1.6) (t(16) = −1.86; p = .082). Importantly, an independent samples t test showed that the difference between the false alarm rates at the first and the second time point of task execution was significantly larger in the “no stimulation group” (3.1% ± 0.5) than in the “stimulation group” (1% ± 0.5) (t(32) = 2.8; p = .008). This shows that the extent of deterioration of inhibition performance between testing time points is significantly larger in the group that did not receive tactile stimulation.
= .198). A post hoc power calculation revealed a power of 99% for that effect. Post hoc paired t tests were conducted showing that within the “no stimulation group” false alarm rates at the first (5.8% ± 1.1) and the second time point of task execution (8.9% ± 1.4) differed significantly (t(16) = −6.29; p < .001). In the “stimulation group” there was only a marginally significant difference between the task performance at the first (7.7% ± 1.6) and the second time point of task execution (8.7% ± 1.6) (t(16) = −1.86; p = .082). Importantly, an independent samples t test showed that the difference between the false alarm rates at the first and the second time point of task execution was significantly larger in the “no stimulation group” (3.1% ± 0.5) than in the “stimulation group” (1% ± 0.5) (t(32) = 2.8; p = .008). This shows that the extent of deterioration of inhibition performance between testing time points is significantly larger in the group that did not receive tactile stimulation.
Details can be found in Table 1.
| Group | Time point of task execution | Hit rates | Hit reaction times | False alarms |
|---|---|---|---|---|
| Stimulation group | First | 98% | 424 ms | 7.7% |
| Second | 99% | 373 ms | 8.7% | |
| No stimulation group | First | 98% | 444 ms | 5.8% |
| Second | 99% | 388 ms | 8.9% |
- Note: Interaction effects. Mean (M) hit rates, hit reaction times, and false alarm rates.
3.2 Standard event-related potentials (ERP components)
The standard ERPs are shown in Figure 2.
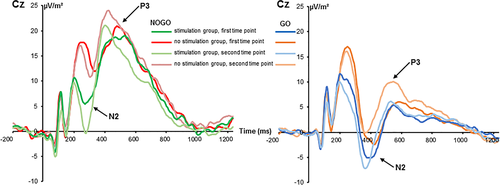
The mixed-effects ANOVA for the N2 component revealed a main effect of “time point” (F(1,32) = 5.09; p = .031; 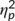 = .137) with a larger (i.e., less negative) amplitude at the first (2.97 μV/m2 ± 1.96) than at the second time point (1.42 μV/m2 ± 1.7). The main effect of “trial type” was also significant (F(1,32) = 28.05; p < .001;
= .137) with a larger (i.e., less negative) amplitude at the first (2.97 μV/m2 ± 1.96) than at the second time point (1.42 μV/m2 ± 1.7). The main effect of “trial type” was also significant (F(1,32) = 28.05; p < .001; 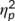 = .467) and the N2 was more negative in GO (−3.13 μV/m2 ± 1.67) than in NOGO trials (7.51 μV/m2 ± 2.39). These effects are in contrast to the literature (Huster et al., 2013), where the N2 is usually more negative in NOGO than GO trials. However, this may reflect an effect of the repeated measures design, intraindividual variability in the data and specific effects of the stimulation protocol on a subset of processes intermingled in the N2 time window. The latter is also suggested by the RIDE data analysis. There was a significant interaction of “time point × group” (F(1,32) = 6.91; p = .013;
= .467) and the N2 was more negative in GO (−3.13 μV/m2 ± 1.67) than in NOGO trials (7.51 μV/m2 ± 2.39). These effects are in contrast to the literature (Huster et al., 2013), where the N2 is usually more negative in NOGO than GO trials. However, this may reflect an effect of the repeated measures design, intraindividual variability in the data and specific effects of the stimulation protocol on a subset of processes intermingled in the N2 time window. The latter is also suggested by the RIDE data analysis. There was a significant interaction of “time point × group” (F(1,32) = 6.91; p = .013; 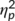 = .178). Post hoc independent samples t tests showed that groups did not differ at the first time point (t(32) = 1.27; p = .213) but at the second time point (t(32) = 2.53; p = .016). The N2 was more negative in the “stimulation group” (−2.88 μV/m2 ± 2.55) than in the “no stimulation group” (5.72 μV/m2 ± 2.24). A significant interaction of “time point × trial type” was obtained (F(1,32) = 6.73; p = .014;
= .178). Post hoc independent samples t tests showed that groups did not differ at the first time point (t(32) = 1.27; p = .213) but at the second time point (t(32) = 2.53; p = .016). The N2 was more negative in the “stimulation group” (−2.88 μV/m2 ± 2.55) than in the “no stimulation group” (5.72 μV/m2 ± 2.24). A significant interaction of “time point × trial type” was obtained (F(1,32) = 6.73; p = .014; 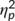 = .174). Post hoc paired t-tests revealed that the first and the second time point were not significantly different in GO trials (t(33) = −0.02; p = .984) but in NOGO trials (t(33) = 3.11; p = .004). The amplitude at the first time point was more positive (9.07 μV/m2 ± 2.60) than at the second time point (5.95 μV/m2 ± 2.42). Importantly, no other main or interaction effect was significant (all F ≤ 3.55; p ≥ .069), including an interaction “time point × trial type × group” (all F ≤ 0.99; p ≥ .549). That interaction, however, is important given the behavioral data. To examine this obvious lack of a differential effect of time point and trial type in further detail, we conducted a Bayesian analysis of that particular interaction using the method by Masson (2011). This means, we calculated the probability of the null hypothesis being true given the data p(H0/D). According to Raftery (1995), values higher 0.5 indicate that the null hypothesis is more likely to be true than the alternative hypothesis. For the interaction, we calculated a probability of p(H0/D) = 0.83. These additional results provide positive evidence in favor of the null hypothesis.
= .174). Post hoc paired t-tests revealed that the first and the second time point were not significantly different in GO trials (t(33) = −0.02; p = .984) but in NOGO trials (t(33) = 3.11; p = .004). The amplitude at the first time point was more positive (9.07 μV/m2 ± 2.60) than at the second time point (5.95 μV/m2 ± 2.42). Importantly, no other main or interaction effect was significant (all F ≤ 3.55; p ≥ .069), including an interaction “time point × trial type × group” (all F ≤ 0.99; p ≥ .549). That interaction, however, is important given the behavioral data. To examine this obvious lack of a differential effect of time point and trial type in further detail, we conducted a Bayesian analysis of that particular interaction using the method by Masson (2011). This means, we calculated the probability of the null hypothesis being true given the data p(H0/D). According to Raftery (1995), values higher 0.5 indicate that the null hypothesis is more likely to be true than the alternative hypothesis. For the interaction, we calculated a probability of p(H0/D) = 0.83. These additional results provide positive evidence in favor of the null hypothesis.
The mixed-effects ANOVA of the P2 component revealed no significant main or interaction effects (all F ≤ 2.7; p ≥ .110) and also the factor “group” was not significant (F(1,32) = 3.7; p = .064; 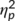 = .103).
= .103).
The mixed-effects ANOVA for the P3 component showed a main effect of “time point” (F(1,32) = 12.01; p = .002; 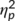 = .273) with a larger P3 amplitude at the second (14.44 μV/m2 ± 1.93) than at the first time point of task execution (11.06 μV/m2 ± 1.86). Furthermore, a main effect of “trial type” was obtained (F(1,32) = 38.88; p < .001;
= .273) with a larger P3 amplitude at the second (14.44 μV/m2 ± 1.93) than at the first time point of task execution (11.06 μV/m2 ± 1.86). Furthermore, a main effect of “trial type” was obtained (F(1,32) = 38.88; p < .001; 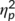 = .549) revealing larger amplitudes in NOGO (20.16 μV/m2 ± 2.66) than in GO trials (11.06 μV/m2 ± 5.34). No other main or interaction effects were significant (all F ≤ 2.73; p ≥ .109). The Bayesian analysis revealed strong support for a lack of effects, especially in the missing interaction “time point × trial type × group” (p(H0/D) = 0.85). To summarize, the standard ERP data did not parallel the observed behavioral effects.
= .549) revealing larger amplitudes in NOGO (20.16 μV/m2 ± 2.66) than in GO trials (11.06 μV/m2 ± 5.34). No other main or interaction effects were significant (all F ≤ 2.73; p ≥ .109). The Bayesian analysis revealed strong support for a lack of effects, especially in the missing interaction “time point × trial type × group” (p(H0/D) = 0.85). To summarize, the standard ERP data did not parallel the observed behavioral effects.
3.3 Residue iteration decomposition
3.3.1 S-cluster
The S-cluster is shown in Figure 3a.
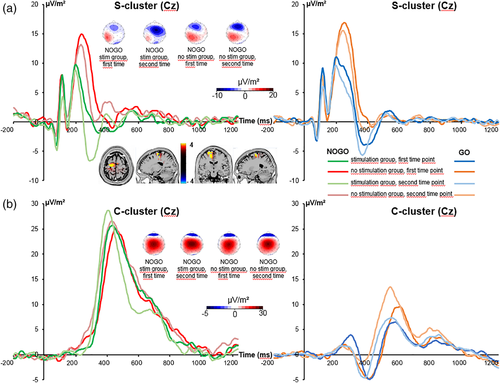
The mixed-effects ANOVA for the S-cluster in the N2 time window showed a main effect of “time point” (F(1,32) = 32.2; p < .001; 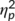 = .502) with a larger S-cluster at the second (−3.11 μV/m2 ± 1.31) than at the first time point of task execution (0.38 μV/m2 ± 1.36). Furthermore, a significant interaction of “time point × trial type” was revealed (F(1,32) = 10.22; p = .003;
= .502) with a larger S-cluster at the second (−3.11 μV/m2 ± 1.31) than at the first time point of task execution (0.38 μV/m2 ± 1.36). Furthermore, a significant interaction of “time point × trial type” was revealed (F(1,32) = 10.22; p = .003; 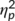 = .242). Post hoc paired t tests showed that GO (−1.44 μV/m2 ± 1.22) and NOGO trials (2.2 μV/m2 ± 1.8) differed at the first time point of task execution (t(32) = −2.6; p = .014). Yet, there was no difference at the second time point (t(32) = −0.1; p = .939). Moreover, a significant interaction of “time point × trial type × group” was obtained (F(1,32) = 5.98; p = .020;
= .242). Post hoc paired t tests showed that GO (−1.44 μV/m2 ± 1.22) and NOGO trials (2.2 μV/m2 ± 1.8) differed at the first time point of task execution (t(32) = −2.6; p = .014). Yet, there was no difference at the second time point (t(32) = −0.1; p = .939). Moreover, a significant interaction of “time point × trial type × group” was obtained (F(1,32) = 5.98; p = .020; 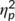 = .158). Analyzing trial types separately revealed that the interaction of “time point × group” was not significant in GO trials (F(1,32) = 0.49; p = .491;
= .158). Analyzing trial types separately revealed that the interaction of “time point × group” was not significant in GO trials (F(1,32) = 0.49; p = .491; 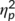 = .015), but for NOGO trials (F(1,32) = 5.16; p = .030;
= .015), but for NOGO trials (F(1,32) = 5.16; p = .030; 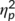 = .139). For the NOGO trials, post hoc paired t tests showed that the first and the second time point of task execution differed in the “stimulation group” (t(16) = 5.1; p < .001) as well as in the “no stimulation group” (t(16) = 2.2; p = .041). An independent samples t test revealed that the difference in S-cluster amplitudes between the second and the first time point of task execution was significantly larger in the “stimulation group” (−7.49 μV/m2 ± 1.46) than in the “no stimulation group” (−2.99 μV/m2 ± 1.34) (t(32) = 2.27; p = .030). The sLORETA analysis (refer Figure 3a) revealed that this differential effect was due to activation differences in the superior frontal gyrus (BA6), with activation extending into the middle frontal gyrus and the motor cortex (BA4). It is shown that these areas undergo stronger modulations in the stimulation group than the nonstimulation group and that the above mentioned brain regions were more strongly activated in the stimulation than the nonstimulation group. For the S-cluster data, no further main or interaction effect was significant (all F ≤ 2.96; p ≥ .095).
= .139). For the NOGO trials, post hoc paired t tests showed that the first and the second time point of task execution differed in the “stimulation group” (t(16) = 5.1; p < .001) as well as in the “no stimulation group” (t(16) = 2.2; p = .041). An independent samples t test revealed that the difference in S-cluster amplitudes between the second and the first time point of task execution was significantly larger in the “stimulation group” (−7.49 μV/m2 ± 1.46) than in the “no stimulation group” (−2.99 μV/m2 ± 1.34) (t(32) = 2.27; p = .030). The sLORETA analysis (refer Figure 3a) revealed that this differential effect was due to activation differences in the superior frontal gyrus (BA6), with activation extending into the middle frontal gyrus and the motor cortex (BA4). It is shown that these areas undergo stronger modulations in the stimulation group than the nonstimulation group and that the above mentioned brain regions were more strongly activated in the stimulation than the nonstimulation group. For the S-cluster data, no further main or interaction effect was significant (all F ≤ 2.96; p ≥ .095).
The mixed-effects ANOVA for the S-cluster in the P2 time window revealed a main effect of “trial type” (F(1,32) = 6.7; p = .015; 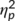 = .172) with a larger amplitude in GO (12.87 μV/m2 ± 1.73) than in NOGO trials (11.25 μV/m2 ± 1.56). No further significant main or interaction effects were achieved (all F ≤ 2.4; p ≥ .131). Also the main effect of “group” was not significant (F(1,32) = 2.9; p = .099;
= .172) with a larger amplitude in GO (12.87 μV/m2 ± 1.73) than in NOGO trials (11.25 μV/m2 ± 1.56). No further significant main or interaction effects were achieved (all F ≤ 2.4; p ≥ .131). Also the main effect of “group” was not significant (F(1,32) = 2.9; p = .099;  = .083).
= .083).
3.3.2 C-cluster
The C-cluster is shown in Figure 3b. The mixed-effects ANOVA for the C-cluster showed a significant main effect of “trial type” (F(1,32) = 34.07; p < .001; 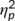 = .516) with a larger C-cluster in NOGO (25.88 μV/m2 ± 2.68) than in GO trials (8.46 μV/m2 ± 1.69). No other main or interaction effects were significant (all F ≤ 2.84; p ≥ .102). To examine this lack of a differential effect of “time point” and “trial type” in detail, we conducted a Bayesian analysis of that particular interaction using the method by Masson (2011). That is, we calculated the probability of the null hypothesis being true given the data p(H0/D). For the interaction, we calculated a probability of p(H0/D) = 0.80. These additional results provide positive evidence in favor of the null hypothesis.
= .516) with a larger C-cluster in NOGO (25.88 μV/m2 ± 2.68) than in GO trials (8.46 μV/m2 ± 1.69). No other main or interaction effects were significant (all F ≤ 2.84; p ≥ .102). To examine this lack of a differential effect of “time point” and “trial type” in detail, we conducted a Bayesian analysis of that particular interaction using the method by Masson (2011). That is, we calculated the probability of the null hypothesis being true given the data p(H0/D). For the interaction, we calculated a probability of p(H0/D) = 0.80. These additional results provide positive evidence in favor of the null hypothesis.
4 DISCUSSION
The current study aimed at investigating the role of perceptual learning in the context of response inhibition. Since perceptual learning can affect gain control mechanisms by improving the signal-to-noise ratio of sensory information, we hypothesized that response inhibition performance can be enhanced through the use of a perceptual learning protocol. Functional anatomical considerations concerning the connection of somatosensory and motor areas of the frontal cortex also provided a plausible background to examine this question. Since standard ERPs represent a combination of stimulus-related and response-related signals, the intermingled neurophysiological codes were decomposed using the RIDE algorithm. Therefore, it was possible to identify the neurophysiological effects underlying the modulation of response inhibition through the use of a perceptual learning protocol. Source localization analysis (sLORETA) was applied to reveal associated functional neuroanatomical structures.
Regarding the examined hypothesis of improved response inhibition performance through the use of a perceptual learning protocol, we found an interaction of “time point x group” on the behavioral level. It was demonstrated that the difference between the false alarm rates at the first and the second time point of task execution was larger in the group receiving no perceptual learning protocol than in the group receiving the perceptual learning protocol. Without the use of a perceptual learning protocol, a decline in inhibition performance occurs when the task is executed for the second time likely because the repetition is amplifying the automation of the response tendency triggered by more frequent GO stimuli compared to NOGO stimuli (Dippel et al., 2017). The tendency to respond to GO stimuli is assumed to automate with an increase in task experience (Bensmann, Zink, Roessner, Stock, & Beste, 2019; Dippel et al., 2017; Dippel, Chmielewski, Mückschel, & Beste, 2016; Helton, 2009). The perceptual learning protocol, however, seems to have a “protective effect” since repeated task administration that likely induces a prepotent tendency to respond did not modulate response inhibition performance. The difference in inhibition performance at the first time point (before the passive perceptual learning protocol was administered) cannot be attributed to the tactile stimulation since the protocol was only delivered after the participants initially performed the task. At the second time point of task execution, the groups showed comparable results yet the performance deterioration was significantly larger in the “no stimulation group” and the only factor that was modified between the two time points of task execution was the execution of the passive perceptual learning protocol. Therefore, we conclude that the perceptual learning protocol has a sort of a “protective” function in the way that it prevents the heavy drop in performance associated with task repetition or response automation. The reason might be the following: Gain control mechanisms are likely modulated by perceptual learning (B. Dosher & Lu, 2017). Perceptual learning likely increases the signal-to-noise ratio of incoming sensory information (B. A. Dosher & Lu, 1998; Gold et al., 1999) and strengthens the stimulus-related signal. Due to gain modulation principles, an increase in the signal-to-noise ratio by enhancing the stimulus-related signal also improves the strength of the appropriate output activation; that is, gain control becomes stronger (Aston-Jones & Cohen, 2005; Chance, Abbott, & Reyes, 2002; S.-C. Li, Lindenberger, & Sikström, 2001; S.-C. Li & Sikström, 2002; Servan-Schreiber et al., 1990). This makes it easier to perform the correct action (i.e., a fast response in GO trials and no response in NOGO trials). Probably, the automated behavior triggered by the GO stimulus is more likely to be interrupted because the behavior associated with the NOGO stimulus (i.e., no motor response) is more efficiently triggered. Therefore, the detrimental effect of response automation is attenuated. Examining the behavioral results, the question arises whether inhibitory processes are qualitatively different or whether differences between the two stimulation conditions arise from perceptual processing differences. Clearly, effects originate from different inhibitory processes because if only perceptual processes were affected, an effect would also occur in GO trials. Yet, we found no group differences in GO trials. Behavioral effects were specific for NOGO trials suggesting a modification of the inhibitory process.
Speeding of response time was accompanied by an increase in false alarm rates, which might be interpreted in terms of a speed-accuracy tradeoff. Yet, both groups become faster and produce more inhibition errors, but the crucial point is that the extent to which performance is deteriorated still differs between the groups. The detrimental influence of task repetition is obviously attenuated by the administration of the perceptual learning protocol since it is the only factor that was varied between the groups. The behavioral pattern was paralleled by a specific neurophysiological effect. The RIDE results revealed that stimulus-related processes and not response selection processes reflect behavioral results; that is, only the S-cluster in the N2 time window showed a “time point x group” interaction in NOGO trials, but not the C-cluster. A Bayesian analysis supported the absence of effects in the C-cluster. It has been suggested that conflict monitoring processes during response inhibition that are indicated by activity in the N2 time window, reflect a composition of different coding levels: one coding level refers to perceptual processes, the other to response selection processes (Chmielewski et al., 2018; Folstein & Van Petten, 2008; Mückschel, Dippel, & Beste, 2017). Especially in the N2 time range, perceptual coding levels are relevant during the inhibition of responses (Chmielewski et al., 2018; Mückschel, Dippel, & Beste, 2017) and the data suggest that perceptual learning affects response inhibition processes by modulating perceptual coding levels in the N2 time window. It is likely that the use of a perceptual learning protocol facilitates stimulus-related conflict monitoring processes during response inhibition since perceptual learning has been shown to enhance stimulus signal strength (B. A. Dosher & Lu, 1998; Gold et al., 1999). The larger S-cluster difference between the first and the second time point likely reflects the modulation of perceptual processes. Stimulus-related processes involved in conflict monitoring during response inhibition seem to become increased through perceptual learning. That is, perceptual learning intensified specific aspects of conflict processing during response inhibition. This well explains the smaller extent of response inhibition performance deterioration in the group receiving the perceptual learning protocol. The enhancement of stimulus-related processes through perceptual learning likely improves response inhibition processes.
The sLORETA data showed that S-cluster modulations were associated with activation differences in the superior and middle frontal gyrus (BA6) as well as the motor cortex (BA4). These prefrontal and motor areas have frequently been found to be associated with response inhibition processes (Bari & Robbins, 2013). Especially the medial part of BA6 (i.e., pre-SMA and SMA) has repeatedly been shown to be involved in response inhibition (Bari & Robbins, 2013; Chen et al., 2009; C. R. Li et al., 2006; Swick et al., 2011) and has also been associated with conflict monitoring processes (Rushworth, Kennerley, & Walton, 2005; Rushworth, Walton, Kennerley, & Bannerman, 2004). Conflict monitoring processes are likely to be modulated in the current study, as indicated by S-cluster amplitude modulations in the N2 time window. BA6 revealed stronger activation in the group receiving the perceptual learning protocol compared to the group receiving no such protocol. This corroborates our interpretation that perceptual learning intensified specific aspects of conflict processing during response inhibition. Interestingly, the superior frontal gyrus has already been associated with S-cluster modulations during inhibitory control (Mückschel, Dippel, & Beste, 2017) and it has been speculated that this is due direct neuroanatomical connections with sensory cortical areas. Crucially, direct neuroanatomical connections exist between somatosensory areas and the motor cortex and SMA (Ackerley & Kavounoudias, 2015; Borich et al., 2015; Chouinard & Paus, 2006). This is important since somatosensory perceptual learning likely modulates processing in somatosensory cortices (Dinse et al., 2003). However, due to the neuroanatomical connections between somatosensory areas and frontal areas of the cortical response inhibition network, it is plausible that inductions of plasticity in somatosensory areas affect response inhibition in superior frontal structures.
It is important to consider that standard ERPs did not reflect modulations explaining the behavioral data. There was no effect of perceptual learning, which was supported by a Bayesian analysis of the data. Effects are probably absent at the ERP level because especially for the N2 time window it has been shown that stimulus-related and response-related signals are intermingled (Chmielewski et al., 2018; Folstein & Van Petten, 2008; Larson et al., 2014; Mückschel, Dippel, & Beste, 2017), which was also suggested by the RIDE data analysis. Since perceptual learning seems to specifically modulate stimulus coding levels during inhibitory control it is reasonable that no effects can be observed when coding levels are not properly dissociated from each other in neurophysiological signals. This is an important, methodologically relevant result from the current study that should be considered in other studies examining the effects of perceptual learning on cognitive control processes. Regarding these methodological aspects, the variability of neurophysiological processes is important to consider. Perceptual learning protocols likely affect gain control mechanisms by improving the signal-to-noise ratio (B. Dosher & Lu, 2017). Importantly, there is a close relation between signal-to-noise ratio and variability in neural processes (Bensmann, Roessner, Stock, & Beste, 2018; Buckley & Toyoizumi, 2018; Servan-Schreiber et al., 1990; Yousif et al., 2016; Ziegler, Pedersen, Mowinckel, & Biele, 2016) that is also affecting inhibitory control (Pertermann, Mückschel, Adelhöfer, Ziemssen, & Beste, 2019). It is, therefore, important to consider intraindividual variability when being interested in the effects of protocols inducing neural plasticity on cognitive (control) processes.
To summarize, this study investigated the role of passive perceptual learning in the context of cognitive control and response inhibition in particular. We show that a perceptual learning protocol attenuates the detrimental effect of response automation during inhibitory control. Temporally decomposed EEG data show that stimulus-related and not response selection processes during conflict control are linked to these effects. The superior and middle frontal gyrus (BA6), as well as the motor cortex (BA4), are associated with the effects of perceptual learning on response inhibition. Reliable neurophysiological effects were not evident on the basis of standard ERPs, which has important methodological implications for perceptual learning research. The results detail how lower level sensory plasticity protocols affect higher-order cognitive control functions. Since it is plausible to assume that learning effects are already achieved after a shorter stimulation period, it would be of interest for future studies how a modulation of the duration of the stimulation protocol may affect results to increase the efficiency of perceptual learning.
ACKNOWLEDGMENT
This work was supported by a grant from the Deutsche Forschungsgemeinschaft FOR 2698 and BE4045/37-1.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.