Abnormal neural functions associated with motor inhibition deficits in schizophrenia and bipolar disorder
Funding information: National Institute of Mental Health, Grant/Award Number: F32MH112334; U.S. Department of Veterans Affairs, Grant/Award Number: I01CX000227; National Institutes of Health
Abstract
Deficits in response inhibition have been observed in schizophrenia and bipolar disorder; however, the neural origins of the abnormalities and their relevance to genetic liability for psychosis are unknown. We used a stop-signal task to examine motor inhibition and associated neural processes in schizophrenia patients (n = 57), bipolar disorder patients (n = 21), first-degree biological relatives of patients with schizophrenia (n = 34), and healthy controls (n = 56). Schizophrenia patients demonstrated motor control deficits reflected in longer stop-signal reaction times and elongated reaction times. With the possibility of needing to inhibit a button press, both schizophrenia and bipolar disorder patients showed diminished reductions of the P300 brain response and only the healthy controls demonstrated adjustments in response execution time, as measured by response-locked lateralized readiness potentials. Schizotypal traits in the biological relatives were associated with less P300 modulation consistent with the motor-related anomalies being associated with subtle schizophrenia-spectrum symptomatology in family members. The two patient groups had elongated response selection processes as manifest in the delayed onset of the stimulus-locked lateralized readiness potential. The bipolar disorder group was unique in showing significantly diminished neural responses to the stop-signal to inhibit a response. Antipsychotic medication dosage was related to worse motor inhibition, thus motor inhibition deficits in schizophrenia may be partially explained by the effect of pharmacological agents. Failed modulation of brain processes in relation to response inhibition probability and the lengthening of motor response selection appear to be transdiagnostic abnormalities spanning schizophrenia and bipolar disorder.
1 INTRODUCTION
Schizophrenia and bipolar disorder are characterized by poor response inhibition (Clementz, 1998; Lima, Peckham, & Johnson, 2018) which may reflect neural processes underlying core dysfunctions in the disorders (Kopf et al., 2018; Thakkar, Schall, Logan, & Park, 2015). Because response inhibition is a manifestation of cognitive control over behavioral response tendencies, it is essential for adapting behavior to changing environments (Verbruggen & Logan, 2008). The stop-signal task (SST) is a useful probe of the ability to inhibit an already initiated motor response (Barch, Braver, Carter, Poldrack, & Robbins, 2009; Verbruggen & Logan, 2008), and deficits in response inhibition have been reported in schizophrenia and bipolar disorder using the SST (Barch et al., 2009; Hughes, Fulham, Johnston, & Michie, 2012; Kopf et al., 2018). Poor response inhibition may also mark genetic liability for schizophrenia (Clementz, 1998). As schizophrenia is a heritable and polygenic disorder which shares genetic liability with bipolar disorder (Green et al., 2010; International Schizophrenia Consortium et al., 2009; Lichtenstein et al., 2009; Williams et al., 2011), investigations of response inhibition in bipolar disorder, as well as first-degree biological relatives of individuals with schizophrenia, are necessary to understand the relevance of inhibitory deficits to transdiagnostic mechanisms and disease specific genetic liability.
Aron (2011) described two different response inhibition processes, reactive inhibition and proactive inhibition. Reactive inhibition refers to the process of stopping an already initiated motor response as measured by stop-signal reaction time (SSRT) where longer SSRTs correspond to reactive inhibition deficit. Proactive inhibition refers to the process of preparing to inhibit one's response prior to the presentation of a stop-signal and deficits are demonstrated by elongated reaction time (RT). Studies of response inhibition that have included SSTs provide evidence consistent with reactive inhibition deficits and neural abnormalities in schizophrenia and bipolar disorder, but intact performance in relatives (Fortgang, Hultman, van Erp, & Cannon, 2016; Hughes et al., 2012; Leibenluft et al., 2007; Strakowski et al., 2009; Weathers et al., 2012) (see supplemental materials for more detailed discussion of these studies). Patients with schizophrenia have additionally demonstrated deficits in proactive inhibition, with inconsistent behavioral results in first-degree biological relatives of schizophrenia patients despite both groups demonstrating neural abnormalities to motor-response cues (Vink, Ramsey, Raemaekers, & Kahn, 2006; Zandbelt, van Buuren, Kahn, & Vink, 2011).
To date, most investigations of the neural correlates of response inhibition in schizophrenia and bipolar disorder have used functional magnetic resonance imaging (fMRI). Thus, the timing of inhibition-related neural activity in relation to response inhibition deficits is largely unknown, and what neural abnormalities are a cause or consequence of poor motor response control is yet to be determined. In this study, we examined the neural correlates of response inhibition in individuals with schizophrenia and bipolar disorder, biological first-degree relatives of individuals with schizophrenia, and controls using select event-related potentials (ERPs) derived from electroencephalograms (EEGs) recorded during an SST that manipulated the likelihood of the need to inhibit an already initiated motor response (Vink et al., 2005). Our aim was to examine deficits in behavioral response inhibition, as well as the timing and nature of neural activity associated with stimulus processing and response preparation to more precisely determine whether the abnormalities were specific to schizophrenia as compared to bipolar disorder, when they occurred relative to responses or inhibition, and whether similar abnormalities were present in relatives of individuals with schizophrenia.
To investigate neural events involved in response inhibition and their time courses, we examined the lateralized readiness potential (LRP), P300, and N2 ERP components that are typically elicited by the SST. The LRP captures motor cortex activity related to response preparation and execution. Specifically, LRPs reflect the difference in EEG signal between electrodes located above the motor strip on opposite cerebral hemispheres (Coles, 1989). Activity over one hemisphere that exceeds that of the opposite hemisphere is reflected in the amplitude of the LRP and is thought to be related to preparation of motor activity (Smulders & Miller, 2011). LRPs can be stimulus-locked (S-LRPs) or response-locked (R-LRPs), meaning that either the stimulus onset or the response onset, respectively, is the anchoring point for averaging LRP waveforms. S-LRP onset is thought to be related to response preparation/activation. R-LRP onset is thought to be related to response execution. Recent work provides evidence that the S-LRP additionally reflects stimulus–response translation processes central to the SST (Hughes, Fulham, & Michie, 2016).
The P300 component is a positive deflection that is thought to be related to stimulus novelty, task relevance, and attention (Polich, 2007). Previous literature has shown that P300s during successful inhibition trials are larger and earlier compared to failed inhibition trial P300s (Hughes et al., 2012; Kok, Ramautar, De Ruiter, Band, & Ridderinkhof, 2004; Ramautar, Kok, & Ridderinkhof, 2004; Wessel & Aron, 2015) and that P300 latencies for successful inhibition trials are correlated with SSRT (Wessel & Aron, 2015). The P300 is also thought to reflect processing of the GO stimulus and the component decreases in amplitude as the probability of a stop-signal increases (Ramautar et al., 2004).
Finally, the N2 component precedes the P300 and has been found to be particularly important to stop-signal processing (van Boxtel, van der Molen, Jennings, & Brunia, 2001). N2s during failures of inhibition are later and larger than during successful inhibition of responses (Kok et al., 2004; Ramautar et al., 2004). More generally, Folstein and Van Petten (2008) have proposed that N2 can be elicited by novel/mismatched stimuli, conditions that require cognitive control, and while orienting visual attention. Examination of LRP, P300, and N2 ERP components across SST task conditions in this study allowed us to identify important component processes to impaired response inhibition in schizophrenia, when the abnormalities occur relative to stimuli and responses, and determine whether these impaired processes are similarly present in individuals with bipolar disorder and first-degree relatives of individuals with schizophrenia.
Based on previous literature we hypothesized that individuals with schizophrenia would have reactive inhibition deficits, as indexed by longer SSRTs than controls (Hoptman et al., 2018; Hughes et al., 2012). With regard to aberrant neural responses during the SST, we hypothesized that individuals with schizophrenia would show smaller P300 amplitudes (Hoptman et al., 2018; Hughes et al., 2012), smaller response-related ERP amplitudes (Luck et al., 2009), and difficulties in appropriate response selection (Luck et al., 2009) compared to controls. Finally, given that Vink et al. (2006) reported intact proactive inhibition behavioral performance but aberrant associated neural activity in first-degree relatives of individuals with schizophrenia, we hypothesized that proactive inhibition would be preserved in relatives (as indexed by SSRT similar to controls) but that EEG neural indices associated with proactive inhibition would show abnormalities. We included contrasts with individuals with bipolar disorder to appraise the diagnostic specificity of behavioral and neural abnormalities elicited by the SST. We also examined whether gradations of psychotic symptomatology were related to response inhibition abnormalities.
2 MATERIALS AND METHODS
2.1 Participants
Participants were enrolled in a family study of severe psychopathology at the Minneapolis VA Health Care System. Table 1 summarizes demographic, cognitive, and clinical variables for all groups of participants. Recruitment procedures have been reported previously (Goghari, Macdonald, & Sponheim, 2014). Exclusion criteria were age younger than 18 or older than 60, non-native English speakers, mental retardation, current alcohol or substance dependence, central nervous system condition, history of electroconvulsive therapy, and history of head injury with substantial loss of consciousness or skull fracture. An additional exclusion criterion for controls was a family history of psychosis or bipolar disorder. All participants completed a Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002), and controls and relatives completed a modified version of the Structured Interview for Schizotypy (Kendler, Lieberman, & Walsh, 1989). The relative group consisted of first-degree biological relatives of schizophrenia patients who participated in the larger family study. The schizophrenia group included 57 individuals, 52 of whom met criteria for schizophrenia, and five of whom met criteria for schizoaffective disorder-depressive type. All participants in the bipolar disorder group (n = 21) met criteria for bipolar I disorder. Controls (n = 56) had no history of psychotic or bipolar disorders, no Cluster A personality disorders, and no current major depressive disorder or alcohol or substance abuse. Relatives (n = 34) had no current alcohol or substance abuse. One relative had a history of substance induced psychotic disorder, one was diagnosed with a bipolar disorder, one had a Cluster A personality disorder, and one had both a bipolar and Cluster A personality disorder. A minimum of two trained diagnosticians (advanced doctoral students in clinical psychology, postdoctoral researchers, or licensed psychologists) reached consensus on all diagnoses, which were based on the DSM-IV-TR criteria (DSM-IV-TR, 2000). Current symptomatology was assessed using the Brief Psychiatric Rating Scale (BPRS; Ventura, Nuechterlein, Subotnik, Gutkind, & Gilbert, 2000) and schizotypal characteristics were measured with the Schizotypal Personality Questionnaire (SPQ; Raine, 1991). BPRS symptom dimension scores (Wilson & Sponheim, 2014) and SPQ factor scores (Calkins, Curtis, Grove, & Iacono, 2004) were computed. Antipsychotic medication dosages were converted to chlorpromazine equivalent dosages (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010) for all individuals in the patient groups who had complete medication information. Participant handedness was evaluated between groups by comparing the sum of scores on the Edinburgh Handedness Inventory (Oldfield, 1971) and the number of participants that had left hand, mixed handedness, or right hand preference for writing. Study procedures were reviewed, approved, and monitored by IRBs at the Minneapolis VA Medical Center and the University of Minnesota.
| Schizophrenia | Bipolar disorder | Control | Relative | Group statistic | |
|---|---|---|---|---|---|
| na | 57 | 21 | 56 | 34 | |
| Age | 38.7 (11.8)b ,c | 45.0 (11.8) | 45.3 (11.4) | 46.7 (9.4) | F(3, 164) = 5.03** |
| Males:females | 44:13b ,c | 16:5c | 29:27 | 16:18 | χ2(3, 168) = 13.13** |
| Years of education | 13.6 (2.5)b | 14.5 (2.6) | 15.3 (2.0) | 14.7 (2.1) | F(3, 163) = 5.30** |
| Parental education | 5.0 (1.2) | 5.1 (1.0) | 4.5 (1.4) | 4.5 (1.4) | F(3, 155) = 2.10 |
| Estimated IQ | 95.1 (16.7)b ,c | 101.7 (15.2) | 109.1 (14.9) | 109.1 (15.6) | F(3, 163) = 9.20** |
| Handedness scale sum | 41.1 (9.8) | 44.2 (6.3) | 40.7 (10.4) | 42.9 (10.5) | F(3, 160) = 0.84 |
| Writing handedness (left:mixed:right) | 9:0:48 | 0:1:20 | 8:0:48 | 5:0:25 | χ2(6, 164) = 10.39 |
| Medication (CPZ equivalent values) | 564.4 (394.2) | 227.5 (286.1) | N/A | N/A | |
| BPRS | |||||
| Total score | 46.9 (12.0)b ,c ,d | 40.1 (9.8)b ,c | 29.1 (5.0) | 33.0 (6.7) | F(3, 161) = 41.04** |
| Positive symptoms | 2.6 (1.3)b ,c ,d | 1.4 (0.7) | 1.1 (0.2) | 1.2 (0.4) | F(3, 161) = 34.34** |
| Negative symptoms | 2.3 (1.3)b ,c ,d | 1.4 (0.7) | 1.2 (0.3) | 1.3 (0.5) | F(3, 161) = 18.39** |
| Disorganization | 2.0 (0.7)b ,c | 1.7 (0.6)b | 1.3 (0.3) | 1.5 (0.4) | F(3, 161) = 15.18** |
| Mania | 1.3 (0.7) | 1.6 (0.7)b ,c | 1.1 (0.2) | 1.2 (0.4) | F(3, 161) = 4.72** |
| Depression | 2.0 (1.0)d | 2.7 (1.3)b | 1.6 (0.7) | 2.0 (0.9) | F(3, 161) = 6.11** |
| SPQ | |||||
| Total score | 34.8 (14.9)b ,c ,d | 21.2 (13.3)b ,c | 10.0 (9.2) | 9.5 (8.8) | F(3, 150) = 46.02** |
| Interpersonal factor | 16.5 (6.7)b ,c ,d | 11.0 (6.9)b ,c | 5.5 (5.2) | 5.5 (5.2) | F(3, 150) = 35.46** |
| Cognitive-perceptual factor | 14.7 (8.2)b ,c ,d | 6.8 (6.0)b ,c | 2.7 (3.2) | 2.3 (2.7) | F(3, 150) = 49.22** |
| Disorganization factor | 7.7 (4.5)b ,c | 5.3 (4.3)b | 2.7 (3.4) | 2.6 (3.0) | F(3, 150) = 17.66** |
| Go Only median RT (ms) | 366 (72.5)b ,c ,d | 334 (38.7) | 322 (46.9) | 326 (42.8) | F(3, 159) = 5.38** |
| Go/Stop median RT (ms) | 416 (87.0)b ,c | 401 (62.3) | 386 (59.1) | 392 (77.7) | |
| SSRT (ms) | 242 (40.9)b ,c ,d | 225 (26.0) | 233 (31.5) | 229 (32.7) | F(3, 144) = 4.69** |
| Adjusted P(r) | 0.61 (.09)b ,c ,d | 0.57 (.08) | 0.57 (.10) | 0.57 (.13) | F(3, 144) = 3.43* |
- Note: Adjusted P(r) is the probability of responding in the presence of a stop signal adjusted for rate of response omissions in the High Stop Probability condition. Due to unavailability of data, five schizophrenia patients, 2 bipolar disorder patients, 4 controls, and 1 relative were excluded from measures of parental education; 1 schizophrenia patient was excluded from years of education and Estimated IQ analyses; 1 schizophrenia patient and 2 healthy controls were excluded from all BPRS analyses; and 10 participants with schizophrenia and 6 relatives were excluded from SPQ score comparisons. Parental education was measured on a seven point scale to reflect highest degree of education (1 = 7th grade education or less, 2 = 7th–9th grade, 3 = 10th–12th, but not graduated, 4 = completed high school education, 5 = partial college completion, 6 = completion of a 4 year college/university program, and 7 = completion of a graduate degree). Four schizophrenia relatives were missing handedness information. Fifty-four schizophrenia and nine bipolar disorder patients were taking antipsychotics. Of which, six schizophrenia patients and three bipolar disorder patients were missing dosage information. Seven schizophrenia patients, one bipolar disorder patient, three healthy controls, and four relatives were excluded from analyses of SSRT and adjusted P(r) because they did not have calculable SSRTs.
- a This is the total sample size used for all analyses unless otherwise noted.
- b Different from the control group mean, p < .05.
- c Different from the Schizophrenia Relative Group mean, p < .05.
- d Different from the Bipolar Disorder Group mean, p < .05.
- * p < .05.
- ** p < .01.
2.2 Stop-signal task
The SST used to measure motor inhibition in this study replicated the task used by Vink et al. (2005). Figure 1 depicts the task, including the GO and STOP stimuli, as well as Go and Stop trials. This experiment had five blocks of 160 trials with rest periods between the blocks. Each block consisted of a Go Only condition containing 20 Go trials, followed by a Go/Stop condition of 120 trials that included 24 Stop trials interspersed among 96 Go trials, which was followed by another Go Only condition containing 20 Go trials. During the Go/Stop condition, trials were presented pseudorandomly with at least two, but no more than six, Go trials separating each Stop trial. The probability of a Stop trial within the Go/Stop condition increased as the number of consecutive Go trials increased, which allowed us to examine adjustments in response latency and modulation of neural activity as a function of Stop trial probability. The SST was preceded by a 20 Go trial-training period during which no EEG data were recorded.
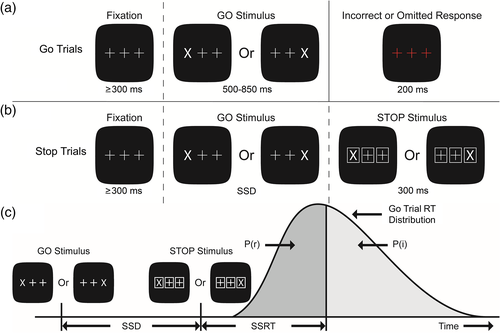
See Figure 1 for depiction of the SST trial structure. During Go trials, a white X (GO stimulus) replaced either the left or the right crosshair, and participants were instructed to use their corresponding thumb to press the button on the corresponding side of the button box as quickly as possible. During Stop trials, the GO stimulus appeared, but following a variable stop-signal delay (SSD) all stimuli were surrounded by white boxes (the STOP stimulus/stop-signal). Participants were instructed to withhold their response when they encountered a stop-signal. In this way, the stop-signal interrupted preparation and execution of a button press.
Three different SSDs were calculated and adjusted for each participant according to their reaction time. The middle SSD for each participant started as the participant's mean training period reaction time minus 150 ms and was adjusted according to task performance such that participants achieved approximately 50% accuracy (see Table S1 in supplemental materials for accuracy data). Short and long SSDs are described in supplemental materials, as are additional details related to SSD adjustments based on task performance. SSRTs calculated using SSDs that result in approximately 50% stop trial accuracy are considered reliable estimates of speed of inhibition (Logan, Schachar, & Tannock, 1997). As such, mean middle SSD length was used for behavioral analysis.
SSRT, the primary SST dependent variable, represents the amount of time between the onset of a stop-signal and the completion of the inhibition process. In the rank-ordering method of calculating SSRT suggested by Band, van der Molen, and Logan (2003), accuracy (i.e., % correct) during middle SSD Stop trials is first determined. Next, reaction times (RTs) from Go trials three to six trials after a Stop trial (i.e., High Stop probability Go trials) are rank ordered from shortest to longest. The RT in this rank order list corresponding to the same percentile as accuracy is then selected, and average middle SSD is subtracted from this RT to generate SSRT. We used a modified version of the rank-ordering SSRT calculation method, similar to that used by Tannock, Schachar, Carr, Chajczyk, and Logan (1989), which allowed us to adjust the calculation to account for errors of omission (i.e., nonresponses to GO stimuli) as described in the supplemental materials.
2.3 EEG acquisition and preprocessing
EEG data were recorded using a Biosemi Active-Two system sampling at 1024 Hz and Ag/AgCl electrode arrays in an elastic cap. Ninety-seven participants had 64-channel electrode recordings and 71 had 128-channel electrode recordings, but electrodes common to both montages were used for data analysis of ERPs. A single earlobe reference signal was used for recording and then data were re-referenced to a linked earlobe signal. Data were processed with a .5 Hz high-pass filter to remove large DC offset voltages and down-sampled to 256 Hz using anti-aliasing resample function after low-pass filtering with 256 Hz cut-off frequency. Epochs with prominent low and high frequency noise were identified via visual inspection and removed. The remaining epochs were decomposed with the fast independent component analysis (ICA) algorithm (Hyvärinen & Oja, 2000) with data dimensionality being reduced through principle component analysis (PCA). The number of data dimensions was estimated using a Bayesian model order selection method based on maximum likelihood of the eigenvalues of EEG data (Rajan & Rayner, 1997). Ocular, heart, and muscular artifact ICs were identified based on an inspection of scalp topography, power spectrum, and time series displays of ICs. EEG data were reconstituted after removal of noise ICs. Reconstituted EEG data were average head re-referenced, divided into epochs, and averaged according to the conditions of interest to form ERPs. Exact time windows for quantifying ERP components were determined through inspection of grand averages for each condition derived from all participants.
2.4 GO stimulus ERPs
Data were epoched from −500 to 1,200 ms relative to the onset of GO stimuli. Only correct trial EEG data were included to form Go trial ERPs. A −200 to 0 ms prestimulus baseline correction was performed and a 15 Hz low pass Butterworth filter was applied on the trial level signals before averaging Go trial ERPs for each participant. P300 components were analyzed for Go trials during both the Go Only and Go/Stop conditions, along with Go trials with a Low Stop probability (the first two Go trials after the occurrence of a Stop trail) and Go trials with High probability for a stop-signal (Go trials three to six trials after the occurrence of a stop-signal). The time windows used for characterizing the P300 varied by electrode in order to capture the full extent of the component at each site (300–500 ms at Fz, 160–500 ms at Cz, and 220–500 ms at Pz). P300 latency was determined by locating the local peak maxima of the average waveforms within these designated time windows (i.e., the maximum amplitude where there is a smaller amplitude both preceding and following the peak). Participants with less than 30 trials for any Go condition were excluded from analysis (this applied to one participant with bipolar disorder).
2.5 Lateralized readiness potential analysis
To measure preparatory motor cortical activity, we resolved lateralized readiness potentials (LRPs) using the same technique as reported in Coles (1989). LRPs were calculated for each participant in response to Go trials during both the Go Only and Go/Stop conditions. We did not calculate LRPs for Stop trials since there were not enough trials per hand to form reliable LRPs for successful and unsuccessful Stop trials. See supplemental material for additional details of LRP computation.
Mean amplitude and LRP onset measures were calculated for comparison of LRPs between groups and conditions. LRP onset latencies were determined as the time point at which the amplitude reached 50% of its local peak amplitude (Kiesel, Miller, Jolicoeur, & Brisson, 2008). Stimulus-locked LRPs (S-LRPs), which were averaged with the onset of the Go stimulus at 0 ms, represent motor response preparation relative to the onset of a stimulus. Response-locked LRPs (R-LRPs), which were averaged such that the participant's button press was located at 0 ms for each trial prior to averaging, represent motor response preparation relative to response termination. Both S-LRPs and R-LRPs were analyzed because examination of the onset latencies associated with these different averaging methods allow us to determine the onset of response preparation and the duration of response execution (starting with response preparation), respectively (Kappenman et al., 2016; Luck et al., 2009). S-LRPs were computed with −200 to 0 ms prestimulus baseline correction for each trial before averaging. Time windows for S-LRP mean amplitude calculation, local minimum detection, and onset latency estimation were 100 ms to 450 ms poststimulus. R-LRPs were computed with −700 to −500 ms pre-response baseline correction for each trial before averaging. The time window was −300 ms pre-response to 100 ms post-response for mean amplitude calculation, local minimum detection, and onset latency estimation. Trials where a participant had a RT that was less than 200 ms were not included in LRP analysis.
2.6 STOP stimulus ERPs
Data were epoched from −200 to 800 ms relative to STOP stimuli. There was a −200 to 0 ms prestimulus baseline correction and a 15 Hz low pass Butterworth filter was applied on the trial level signals before averaging for each participant. Distortion in Stop trial ERPs caused by overlap between GO stimuli ERPs and STOP stimuli ERPs was removed via the Adjacent Response (ADJAR) filter method level 1 (Woldorff, 1993). See supplemental material for more information about the ADJAR procedure. Participants with fewer than 20 trials on either the Stop Success (successful inhibition) or Stop Failure (unsuccessful inhibition) trials were excluded from analysis (see Table 2 for the number of participants excluded from each group). N2 components were examined 175–350 ms post-STOP stimulus at the Fz and Cz electrodes. Stop-signal P300 components were examined 275–650 ms post-STOP stimulus at Fz, Cz, and Pz. N2 and P300 latencies were determined by locating the local peak minima and maxima, respectively, on the average waveforms within the designated time windows. See supplemental materials for additional details regarding STOP stimuli ERP analysis.
| Schizophrenia | Bipolar disorder | Control | Relative | Group statistic | Group × condition statistic | ||
|---|---|---|---|---|---|---|---|
| Mean amplitude (μV) | |||||||
| Go Only P300 | Fz | .15 (.60) | .30 (.68) | .21 (.76) | .45 (.75) | F(3, 159) = 1.31 | F(3, 159) = 10.22** |
| Cz | .84 (.62)a ,b | .69 (.51)a ,b | 1.23 (.66) | 1.25 (.79) | |||
| Pz | .77 (.57)a | .48 (.58)a ,b | 1.08 (.50) | .93 (.56) | |||
| Go/Stop P300 | Fz | .03 (.58)a | −.11 (.65) | −.18 (.73) | −.01 (.67) | ||
| Cz | .62 (.53) | .33 (.55) | .48 (.55) | .54 (.73) | |||
| Pz | .65 (.50) | .37 (.57) | .76 (.58) | .69 (.53) | |||
| Onset latency (ms) | |||||||
| Go Only S-LRP | 201 (53)b | 207 (55)b | 187 (46) | 168 (37) | F(3, 142) = 3.96** | F(3, 142) = 0.77 | |
| Go/Stop S-LRP | 217 (66)a ,b | 227 (64) | 189 (48) | 190 (56) | |||
| Go Only R-LRP | −127 (51) | −106 (48) | −118 (40) | −131 (55) | F(3, 141) = 0.28 | F(3, 141) = 3.22* | |
| Go/Stop R-LRP | −117 (55) | −129 (48) | −142 (64) | −120 (62) | |||
| Peak amplitude (μV) | |||||||
| Stop Success N2 | Fz | −1.43 (1.53)c | −.42 (1.16)a | −1.35 (1.31) | −1.28 (1.95) | F(3, 128) = 1.18 | F(3, 128) = 2.92* |
| Cz | −2.16 (1.69) | −2.32 (1.57) | −2.45 (1.81) | −2.45 (1.90) | |||
| Stop Failure N2 | Fz | −2.08 (1.93) | −1.67 (1.46) | −2.19 (1.60) | −2.65 (2.30) | ||
| Cz | −2.80 (2.01) | −2.89 (1.45) | −3.26 (1.79) | −3.73 (2.48) |
- Note: Participants were excluded from S-LRP and R-LRP onset latency analyses if they did not have: (1) more than 40 trials for each hand, (2) S-LRP signal to noise ratios greater than one, (3) a detectable local peak, or (4) calculable onset latency for both the Go Only and Go/Stop conditions. Participants were also excluded from S-LRP latency analyses if S-LRP onset did not occur after average RT, and from R-LRP latency analyses if R-LRP onset did not occur after average stimulus presentation. In sum, this led to the exclusion of six schizophrenia patients, two patients with bipolar disorder, five healthy controls, and four schizophrenia relatives from S-LRP latency analyses and six schizophrenia patients, two patients with bipolar disorder, seven healthy controls, and three schizophrenia relatives from R-LRP latency analyses. For Stop analyses, stop trial omissions was an additional covariate. Eleven schizophrenia patients, 4 bipolar disorder patients, 11 healthy controls, and 4 relatives were excluded from Stop peak amplitude analyses because they had fewer than 20 trials in either the Stop Failure or Stop Success conditions.
- a Different from the Control Group mean, p < .05.
- b Different from the Schizophrenia Relative Group mean, p < .05.
- c Different from the Bipolar Disorder Group mean, p < .05.
- * p < .05.
- ** p < .01.
2.7 Statistical analysis
To investigate group and task manipulation effects on behavioral and neurophysiological variables we conducted several mixed analyses of covariance (ANCOVAs) that included the within-subjects factor of task condition (e.g., Go Only, Go/Stop), between-subjects factors of group (four levels: schizophrenia, bipolar disorder, relatives of schizophrenia patients, controls) and sex (two levels), and the covariate of age (groups varied in age and sex; means and other data displayed in figures and tables show unadjusted values). An additional within-subjects factor of electrode was used in examination of the neurophysiological variables, apart from LRP analyses. The electrode selection derived from evidence of where the components were largest and previous electrode sites where motor inhibition effects were observed. Electrode site was used as a factor for the sake of being congruent with previous literature. The electrodes used for the electrode site factor for P300 (Fz, Cz, and Pz) were chosen because these were the electrodes used for the electrode factor in several studies which characterized the P300 component during the stop-signal paradigm (Kok et al., 2004; Ramautar et al., 2004; Ramautar, Kok, & Ridderinkhof, 2006). Fz and Cz electrodes were examined for the N2 component because inhibition-related N2 has historically been characterized as a frontocentral component (Hughes et al., 2012; Kok et al., 2004; Ramautar et al., 2006; van Boxtel et al., 2001).
ANCOVAs that yielded significant main or interaction effects involving group were followed by paired comparison tests to investigate group differences underlying the effect, and associations were subsequently examined between ERP, behavioral, and clinical indices by computing Pearson product–moment correlations. When statistical tests yielded group differences or interactions implicating the schizophrenia group, Pearson product–moment correlations were used to investigate the relationship between the dependent variable and antipsychotic medication dosage. We also used Pearson product–moment correlations to explore the relationship between neural indices that showed significant group effects and behavioral measures in order to test their relevance to performance on the SST. The reported correlations are not corrected for multiple comparisons. See supplemental material for additional details regarding statistical analyses.
3 RESULTS
3.1 Behavioral indices of motor response and inhibition
3.1.1 Stop-signal reaction time (SSRT)
Indices reflecting motor behavior and inhibition during the SST are in Table 1 and Table S1. An ANCOVA revealed that there were group differences in the ability to inhibit an activated motor response. The schizophrenia group had longer SSRTs than every other group, indicating slowed reactive inhibition (i.e., inhibition of an already initiated motor response). The schizophrenia group also showed a higher adjusted probability of responding (p[r]) than all other groups, reflecting higher rates of errors of omission (see supplemental materials). For schizophrenia patients, antipsychotic dosage as measured by chlorpromazine (CPZ) equivalent dosages was significantly correlated with SSRT such that higher dosages were associated with longer SSRTs, r(42) = .48, p = .001. Antipsychotic dosage was not significantly correlated with adjusted probability of responding in individuals with schizophrenia, r(42) = .27, p = .089.
3.1.2 Go trial reaction time
Analyses of median RTs for button presses during the Go Only and Go/Stop conditions yielded main effects of group (F[3, 159] = 5.38, p = .001), condition (F[1, 159] = 7.01, p = .009), and age (F[1, 159] = 21.95, p < .0005). There was no group × condition interaction, F(3, 159) = 0.69, p = .562. Follow-up paired comparisons revealed that the schizophrenia group had longer RTs for the Go Only condition than each of the three other groups. In the Go/Stop condition, individuals with schizophrenia showed longer RTs than controls and relatives of schizophrenia patients, but not bipolar disorder patients. Importantly, SSRT was computed by subtracting the stop-signal delay (SSD) which had been individually adjusted for each participant according to their RT. This correction prevented SSRT from contamination due to slowing of RT (see supplemental materials for more details). Antipsychotic dosages were not significantly correlated with RT in either the Go Only condition (r[48] = .20, p = .184) or Go/Stop condition (r[48] = .25, p = .085) in the schizophrenia group. There were no significant correlations between antipsychotic medication and changes in RT between both sets of conditions (Go Only to Go/Stop: r[48] = .16, p = .283; Go Low to Go High: r[48] = .02, p = .915).
3.1.3 Summary of behavioral findings
Schizophrenia patients demonstrated longer SSRTs and higher adjusted response probability, suggesting slowed and ineffective reactive inhibition. Because SSRT and response probability demonstrated significant and trending associations with medication dosage, analyses of correlations between these behavioral indices and ERP indices included CPZ equivalents as a control variable. The lack of group × condition interaction for Go trial RT suggested there was no behavioral deficit in proactive inhibition in the patient groups, nor the relatives group.
3.2 Neurophysiological indicators of motor responses and inhibition
3.2.1 Go stimulus P300: Processing of a motor response cue given possible need for inhibition
To better understand the neural activity associated with motor inhibition we examined ERP responses to Go trials within the context of the Go Only condition and the Go/Stop condition (see Figure 2). The P300 component was the most evident ERP that varied depending on the possibility of a stop-signal being presented. Table 2 presents means, standard deviations, and statistics for the P300 indices. Analyses of P300 mean amplitude yielded main effects of condition (F[1, 159] = 6.91, p = .009) and electrode (F[1.4, 228.9] = 27.89, p < .0005), as well as group × condition (F[3, 159] = 10.22, p < .0005), electrode × age (F[1.4, 228.9] = 9.87, p < .0005), condition × electrode (F[1.6, 260.2] = 5.34, p = .009), and condition × electrode × group (F[1.6, 260.2] = 3.62, p = .004) interactions.
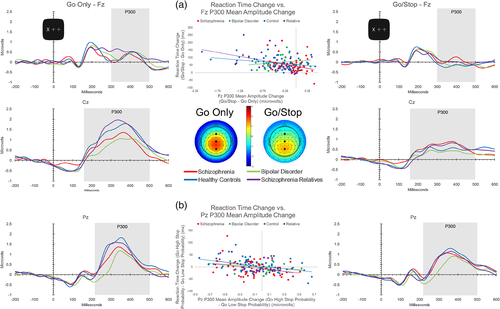
Paired comparisons revealed that the three-way interaction derived from the schizophrenia group having smaller Go Only condition P300s compared to healthy controls at Cz and Pz and relatives at Cz. Go Only P300 mean amplitudes at these electrodes failed to be related to antipsychotic medication dosages (Cz: r[48] = −.11, p = .45; Pz: r[48] = .03, p = .84). The bipolar disorder group also had smaller Go Only condition P300s at Cz and Pz compared to both the healthy control and relative groups.
All groups demonstrated a significant decrease in P300 amplitude from the Go Only to Go/Stop condition at each electrode except the schizophrenia group at Fz and the bipolar disorder group at Pz (change in P300 amplitude between conditions at Fz was unrelated to antipsychotic medication dosage in schizophrenia patients, r[48] = −.05, p = .753). The schizophrenia group had larger P300 amplitudes compared to healthy controls at Fz during the Go/Stop condition. See supplemental material for additional Go stimulus P300 amplitude and latency results.
Consistent with P300 reductions indexing the possible need to inhibit a motor response, increases in RT from Go Only to Go/Stop conditions were associated with decreases in P300 amplitude from Go Only to Go/Stop conditions at Fz for schizophrenia, control, and relative groups (see Figure 2, Panel a). The relationship remained in the schizophrenia group after controlling for antipsychotic dosage, r(45) = −.32, p = .029. The association was absent in the bipolar disorder group. Within the Go/Stop condition, increases in RT from trials of Low to High Stop probability were associated with reductions in P300 amplitudes at Pz from Low to High Stop probability for all groups but the bipolar disorder group, also suggesting that the brain response was related to probability of inhibition (see Panel (b) of Figure 2). Partial correlations considering variance associated with antipsychotic dosage resulted in the association for the schizophrenia group becoming a statistical trend, r(45) = −.26, p = .074. See supplemental materials for Low and High Stop probability P300 amplitude and latency values (Table S3) and additional Low versus High Stop probability ERP analyses. SSRT was not associated with changes in P300 amplitude between conditions at any electrode.
3.2.2 Summary of P300 findings
Both patient groups demonstrated P300 abnormalities, as well as abnormal P300 modulation as a function of stop-signal probability. On average, schizophrenia patients failed to modulate the P300 over the frontal electrode in response to the possibility of needing to inhibit a response. Decreases in P300 amplitude were correlated with increases in RT as the likelihood of a stop-signal increased. Bipolar disorder patients failed to exhibit a relationship between P300 and increased reaction time corresponding to the possibility of response inhibition.
3.2.3 Stimulus-locked LRP (S-LRP) onset latency: Amount of time to complete response selection and begin motor preparation
To investigate neural activity related to the preparation and execution of motor behavior we examined the LRP, which is a negative voltage potential immediately prior to a motor response reflecting asymmetry of movement-related neural activity across motor cortices of the two cerebral hemispheres. We first examined the onset of S-LRPs to characterize the amount of time between presentation of the GO stimulus and initiation of response preparation (see Figure 3 Panels a and b, and Table 2). An analysis comparing S-LRP onset latencies between the Go Only and Go/Stop conditions revealed main effects of group (F[3, 142] = 3.96, p = .010), sex (F[1, 142] = 4.52, p = .035), and age (F[1, 142] = 19.00, p < .0005). The schizophrenia group had later S-LRP onset latencies than the controls and relatives consistent with slower response selection/activation. The bipolar disorder group had later S-LRP onset latencies compared to the relative group. Examining the relationship of antipsychotic dosage to S-LRP onset latency for the schizophrenia group revealed minimal relationship between these variables in the Go Only (r[42] = .04, p = .818) and Go/Stop (r[42] = .21, p = .187) conditions. There were no main or interaction effects for S-LRP mean amplitude for the analysis comparing Go Only and Go/Stop conditions (see supplemental text and Table S2).

Correlations between S-LRP onset latencies and behavioral measures were examined to explore the relationship between processes leading up to the activation of the response process (S-LRP onset latency) and behavioral measures of response inhibition. Later S-LRP onset latencies were associated with longer SSRTs in the schizophrenia group for both the Go Only (r[46] = .39, p = .008) and Go/Stop (r[46] = .39, p = .008) conditions (these relationships dropped to marginal and trend significance levels, respectively, when controlling for antipsychotic medications; see supplemental materials for details). There was a similar association between S-LRP onset latency and SSRT in the control group for the Go Only condition, r(48) = .33, p = .022 (see Figure 3). These relationships suggest that the motor inhibition deficits observed in the schizophrenia group may be partially explained by delays in processes that are common to both reaction time tasks and motor inhibition (e.g., stimulus processing).
Later S-LRP onset latencies were also associated with longer RTs for trials in the Go Only condition for the schizophrenia patients (r[51] = .36, p = .009) and bipolar disorder patients (r[19] = .70, p = .001). In the schizophrenia group, the relationship between Go Only S-LRP onset latency and Go Only RT remained significant after controlling for antipsychotic medication, r(39) = .36, p = .022. A similar relationship was evident for S-LRP onset latency and RT for the Go/Stop condition in the bipolar disorder group, r(19) = .53, p = .019. These associations suggest that prolonged response selection and activation processes contributed to RT slowing in individuals with schizophrenia.
3.2.4 Response-locked LRP (R-LRP) onset latency: Amount of time to complete response execution
We also examined the LRP locked to the button press, the R-LRP, to characterize the amount of time required to execute a motor response. See Figure 3 Panel d for depiction of R-LRPs. An analysis of R-LRP onset latencies for Go Only and Go/Stop conditions yielded a condition × group effect (F[3, 141] = 3.22, p = .025) and a main effect of age (F[1, 141] = 4.28, p = .040). Follow-up analyses revealed that only the control group demonstrated an earlier R-LRP onset latency during the Go/Stop compared to the Go Only condition, reflecting that a possible need for motor inhibition was associated with a greater amount of time to execute a motor response. The schizophrenia, bipolar disorder, and relatives groups failed to show significant alterations in R-LRP onset latency in response to the possibility of needing to inhibit a motor response. Antipsychotic medication dosages failed to be associated with change in R-LRP onset latency in schizophrenia, r(42) = −.056, p = .726.
3.2.5 Summary of LRP findings
Both patient groups demonstrated delayed response preparation, as indexed by S-LRP onset latency. Later response preparation was related to longer SSRT in individuals with schizophrenia and controls, and to longer RTs in both patient groups. Only the control group modulated response execution time as a function of the possibility of needing to inhibit a motor response.
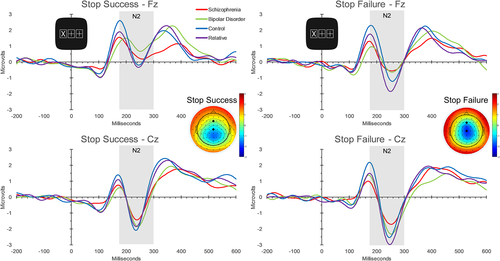
3.2.6 Stop-signal ERPs: Processing a signal to stop a motor response
We also investigated how individuals processed the stop-signal by analyzing the frontal midline N2 and P300 ERPs. An ANCOVA of N2 amplitude revealed main effects of condition (Stop Success vs. Stop Failure; F[1, 128] = 22.82, p < .0005), electrode (Fz vs. Cz; F[1, 128] = 6.49, p = .012), and Stop trial omissions (F[1, 128] = 12.20, p = .001), and interactions of group × condition (F[3, 128] = 2.92, p = .037), condition × age (F[1, 128] = 6.02, p = .015) and electrode × Stop trial omissions (F[1, 128] = 7.08, p = .009). The bipolar disorder group showed smaller (i.e., less negative) N2 amplitudes than the schizophrenia and control groups at Fz during trials when there was a successful response inhibition, suggesting anomalous processing of the stop-signal. See Table 2 for N2 amplitudes and Figure 4 for Stop trial waveforms. There were no significant main effects or interactions involving group in analyses of N2 local peak latency and P300 mean amplitude and local peak latency. See supplemental material for results involving N2 latency and P300 responses to stop-signals.
3.3 Correlations between ERPs and clinical variables
To explore the significance of the SST behavioral and ERP abnormalities with respect to psychopathology we tested for relationships between the SST indices and measures of symptoms and cognition. Correlations were selectively computed for indices showing group effects. Total score on a questionnaire-based assessment of schizotypal traits (SPQ) was correlated with P300 and SSRT indices. Specifically, more schizotypal traits was associated with smaller decreases from Low to High Stop probability trials in P300 amplitude at Pz, r(30) = .52, p = .003, in biological relatives of schizophrenia patients, which is broadly consistent with the effect wherein schizophrenia patients did not demonstrate modulation of the P300 based on stop probability. In controls, schizotypal traits and SSRT were correlated, r(53) = .29, p = .038, such that greater schizotypal traits was associated with longer SSRT. Thus, across groups there were indications that schizotypal traits corresponded to abnormalities in response inhibition. Interestingly, greater symptomatology as measured by the total score on the BPRS was associated with shorter SSRT in bipolar disorder patients, r(20) = −.51, p = .022, which was the opposite direction of the association with schizotypy in controls.
4 DISCUSSION
4.1 Motor inhibition and reaction time deficits
In this study, we used an SST to examine response inhibition and associated neural processes in individuals with schizophrenia and bipolar disorder, biological first-degree relatives of individuals with schizophrenia, and controls. Behavioral results revealed impairments in reactive inhibition only in the schizophrenia group, with individuals with bipolar disorder and relatives demonstrating performance similar to controls. Results are consistent with previous findings of reactive inhibition deficits in individuals with schizophrenia (Fortgang et al., 2016; Hughes et al., 2012) but intact behavioral performance in first-degree relatives (Fortgang et al., 2016); yet our results contrast with previous findings of reactive inhibition deficits in bipolar disorder (Fortgang et al., 2016; Strakowski et al., 2009). Of note however, the significant correlation between SSRT and chlorpromazine equivalent dosages suggests that antipsychotic medication contributes to the longer SSRTs in individuals with schizophrenia in the current study, and perhaps in other studies. Additionally, only the schizophrenia patients demonstrated elongated reaction times relative to controls, consistent with the finding of an information processing inefficiency in schizophrenia patients (Dickinson, Ramsey, & Gold, 2007). Inconsistent with the previous behavioral findings (Vink et al., 2006; Zandbelt et al., 2011), the schizophrenia and relatives groups demonstrated increases in RT similar to controls as stop-signal probability increased.
4.2 Delayed response selection and behavioral correlates
Increased S-LRP onset latency in individuals with schizophrenia and bipolar disorder replicates previous literature examining the LRP in schizophrenia and is consistent with delayed response selection and preparation processes in schizophrenia (Kappenman et al., 2012, 2016; Karayanidis et al., 2006; Luck et al., 2009). The relationship between S-LRP onset latency and RT in the patient groups in this study has been demonstrated previously in both controls and individuals with schizophrenia (Kappenman et al., 2016; Luck et al., 2009). The significant, positive correlations between S-LRP onset latency, RT, and SSRT suggest that there could be a common factor or factors preceding response initiation that contribute to slow response activation (longer S-LRP onset latency), slowed response completion (RT), and delayed motor inhibition (SSRT). Thus, findings suggest that the observed deficits in motor inhibition in schizophrenia patients are in part due to slowed processes that precede motor activation. For example, there has been recent evidence to suggest that schizophrenia patients demonstrate deficient response inhibition in part due to abnormalities in stimulus processing (Hoptman et al., 2018; Matzke, Hughes, Badcock, Michie, & Heathcote, 2017). In addition, because S-LRP onset latency and antipsychotic dosage were related to SSRT but not related to each other, both processes preceding motor response initiation and antipsychotics may independently contribute to the observed motor inhibition deficits in schizophrenia patients.
4.3 Stop probability, neural modulation, and behavioral correlates
Both patient groups demonstrated abnormalities in P300 modulation in response to the possibility of a need to inhibit a response, consistent with individuals affected by these disorders showing impairments in modulating cognitive control in preparation for potential response inhibition (i.e., proactive inhibition). Reductions in P300 amplitude were associated with increased reaction time as a function of stop-signal probability, supporting the notion that response ambiguity after a Go stimulus impacts the P300 component. This is consistent with the idea that P300 is sensitive to the behavioral relevance and saliency of stimuli (Duncan-Johnson & Donchin, 1977; Hajcak, MacNamara, & Olvet, 2010). Indeed, Ramautar et al. (2004) reported larger Go stimulus P300 amplitude when stop-signals were less likely. Abnormalities in modulating P300 as a function of the possibility of needing to inhibit a response in both patient groups suggests that there are abnormalities in updating the changed context surrounding Go stimuli in individuals with schizophrenia and bipolar disorder.
In first-degree biological relatives of patients with schizophrenia, greater schizotypal symptomology was associated with less responsive neural activity (P300 modulation) to changes in the probability for response inhibition. This suggests that neural abnormalities evident during the potential need for response inhibition, and thus proactive inhibition, are tied to subtle psychotic symptomatology. Furthermore, given that the association is only present in relatives and that the distribution of schizotypal characteristics is similar in relatives and controls, genetic liability for schizophrenia may be mediating the relationship between schizotypal symptoms and P300 modulation related to response inhibition probability.
Neither the relative nor the patient groups exhibited the extended response process in the Go/Stop compared to the Go Only condition that was observed in controls as indexed by R-LRP onset latency. Thus, only controls adjusted the time between initiation and completion of a motor response according to the possible introduction of a stop-signal. It is possible that genetic factors related to schizophrenia alter higher-order processes governing modulation of response execution. Of note, SPQ scores in relatives and controls were virtually identical. It is possible that additional neural and/or behavioral abnormalities would have emerged in a relative sample with more schizotypal symptoms (i.e., intermediate phenotypic expression).
4.4 Current results and previous literature
Vink et al. (2006) previously used fMRI to investigate neural responses in individuals with schizophrenia, first-degree biological relatives, and controls as they completed a motor inhibition task. They reported increased striatal activity as a function of stop signal probability in controls, but not in patients or relatives. In a more recent study, this same group reported significant group differences in neural activity in the striatum, inferior frontal cortex, and temporoparietal junction in relationship to stop signal probability where individuals with schizophrenia displayed decreased activation compared to controls in these areas, and relatives demonstrated decreased striatal activation compared to controls and intermediate activation in the other regions (Zandbelt et al., 2011). Given the different approaches to analysis reported by these previous studies and the current study, care should be taken in comparing our results with those of Vink et al. (2006) and Zandbelt et al. (2011). With this consideration, results of the two studies are broadly consistent with our findings indicating abnormalities in response cue context updating in both patient groups. Specifically, the relationship between P300 and RT modulation as a function of stop trial probability, and the abnormalities in P300 modulation seen in individuals with schizophrenia suggest that some of the neural abnormalities related to proactive inhibition deficits occur at the level of processing cues for changes in response context. The lack of modulation of response execution time (i.e., R-LRP onset latency) as a function of the possible need to inhibit a response emerged as an abnormality shared by individuals with schizophrenia and first-degree relatives. Also, the correlation between S-LRP onset latency and SSRT in the schizophrenia group suggests that delays in stimulus processing may also contribute to reactive inhibition deficits in schizophrenia, highlighting additional aberrancies in neural function in schizophrenia. However, the possibility that the neural abnormalities in relatives reported by Vink et al. (2006) and Zandbelt et al. (2011) are related to schizotypal traits cannot be ruled out, and reliance on fMRI in the previous studies does not allow temporal separation of stimulus processing, response preparation, and response execution processes.
It is also interesting to consider the current results in the context of a recent transcranial magnetic stimulation (TMS) study. Dupin et al. (2018) reported decreased motor cortical excitability during a Go/NoGo task during conditions of low Go/high NoGo trial probability in controls and relatives but not individuals with schizophrenia, which suggests that patients with schizophrenia have deficits in updating probabilistic context. Dupin et al. (2018) acknowledge the potential influence of inhibitory processes on cortical excitability in the low Go trial probability Go/NoGo task such that abnormalities in proactive inhibition in schizophrenia may have contributed to the lack of response probability-dependent cortical excitability modulation. In light of their results, it is also possible that in the current study the lack of modulation of P300 and R-LRP to GO stimuli of varying Stop probability observed in patients may reflect deficits in cortical processes related to proactive inhibition.
Lastly, it is of interest to consider the findings from the present study in the context of some of the motor abnormalities that have been consistently reported in psychosis (see van Harten, Walther, Kent, Sponheim, & Mittal, 2017, for review). In their review of psychomotor slowing in schizophrenia, Morrens, Hulstijn, and Sabbe (2007) discuss the contribution of higher order processes such as cognitive control to motor function generally (Morrens et al., 2007; Ridderinkhof et al., 2004; Rushworth, Walton, Kennerley, & Bannerman, 2004; Willingham, 1998) as well as impact on psychomotor speed (Morrens et al., 2007). Additionally, neurological soft signs have been consistently reported in schizophrenia, and include the domain of sequencing of complex motor acts, which is believed to involve prefrontal cortex (Bombin, Arango, & Buchanan, 2005). Therefore, abnormalities in response inhibition, stimulus processing, and response execution modulation as a function of changed context in individuals with schizophrenia may also contribute to motor abnormalities more broadly in psychosis.
4.5 Limitations
There were several limitations in the present study. First, the sample size of the bipolar disorder group was notably smaller than the other groups. As such, it may be best to consider our results concerning individuals with bipolar disorder as preliminary pending replication with a larger sample. Second, the associations between behavioral and neural indices in this study need to be replicated in larger samples to more fully understand the relationship between SST behavioral and neural indices, and the relationships between these variables and cognitive and clinical variables. Finally, medication is a confound in the current study. While we computed correlations with chlorpromazine equivalent dosages in an effort to explore the magnitude of the relationship between variables of interest and antipsychotic medication, future work should focus on first-episode or medication-naïve populations to obviate medication confounds.
5 CONCLUSION
Overall, the results of this study suggest that schizophrenia is associated with poor regulation of motor responses in the context of the possible need for inhibition. Poor regulation is reflected in longer SSRTs as well as limited modulation of the P300 and R-LRP components. These abnormalities seen in individuals with schizophrenia suggest that some of the neural abnormalities related to response inhibition deficits occur at the level of processing response cues for which the context has changed. The correlation between S-LRP onset latency and SSRT in the schizophrenia group additionally suggests that delays in stimulus processing may also contribute to response inhibition deficits in schizophrenia.
Interestingly, the present results paint a picture of preserved behavioral response inhibition in bipolar disorder but abnormal P300 and R-LRP modulation, similar to abnormalities observed in schizophrenia patients. Bipolar patients also showed unique anomalies in frontal response inhibition processes as evidenced by significantly diminished frontal N2 responses, indicative of abnormal processing of the stop-signal. Thus, for there to be normative response inhibition in bipolar disorder, aberrant and perhaps compensatory cognitive control functions may need to be invoked.
With regard to first-degree biological relatives of schizophrenia patients, it was observed that there was a lack of modulation of response execution time, as measured by R-LRP onset latency, that was associated with the possibility of a stop-signal. This was the only abnormality observed in first-degree relatives in this study and it was shared with both patient groups. Lastly, subtle psychotic symptomatology in the form of schizotypal characteristics appears related to deviant motor inhibition processes that may reflect increased genetic liability for schizophrenia.
ACKNOWLEDGMENTS
This work was supported by funding to Scott R. Sponheim provided in part by a Merit Review Award #I01CX000227 from the U.S. Department of Veterans Affairs, Clinical Sciences Research and Development Research Program. Jerillyn S. Kent was supported by an NIMH NRSA Postdoctoral Fellowship (Award Number F32MH112334). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Veterans Affairs or the National Institutes of Health.