The optimistic brain: Trait optimism mediates the influence of resting-state brain activity and connectivity on anxiety in late adolescence
Funding information: American CMB Distinguished Professorship Award of USA, Grant/Award Number: F510000/G16916411; Changjiang Scholar Professorship Award of China, Grant/Award Number: T2014190; Program for Changjiang Scholars and Innovative Research Team in University of China, Grant/Award Number: IRT16R52; National Key Technologies R&D Program of China, Grant/Award Number: 2012BAI01B03; National Natural Science Foundation of China, Grant/Award Numbers: 81621003
Abstract
As a hot research topic in the field of psychology and psychiatry, trait optimism reflects the tendency to expect positive outcomes in the future. Consistent evidence has demonstrated the role of trait optimism in reducing anxiety among different populations. However, less is known about the neural bases of trait optimism and the underlying mechanisms for how trait optimism protects against anxiety in the healthy brain. In this investigation, we examined these issues in 231 healthy adolescent students by assessing resting-state brain activity (i.e., fractional amplitude of low-frequency fluctuations, fALFF) and connectivity (i.e., resting-state functional connectivity, RSFC). Whole-brain correlation analyses revealed that higher levels of trait optimism were linked with decreased fALFF in the right orbitofrontal cortex (OFC) and increased RSFC between the right OFC and left supplementary motor cortex (SMC). Mediation analyses further showed that trait optimism mediated the influence of the right OFC activity and the OFC-SMC connectivity on anxiety. Our results remained significant even after excluding the impact of head motion, positive and negative affect and depression. Taken together, this study reveals that fALFF and RSFC are functional neural markers of trait optimism and provides a brain-personality-symptom pathway for protection against anxiety in which fALFF and RSFC affect anxiety through trait optimism.
1 INTRODUCTION
Anxiety is a common negative state occurring in our daily lives. It reflects a complex cognitive and affective change in response to potential threats in the future (Grupe, & Nitschke, 2013). Given the role of anxiety in the development of physical illnesses and psychiatric disorders (Bower et al., 2010; Brown, Chorpita, & Barlow, 1998; Rawson, Bloomer, & Kendall, 1994; Zinbarg, & Barlow, 1996), exploring factors for reducing anxiety is one main goal in the field of psychology, psychiatry and psychoradiology. With the success of positive psychology over the past two decades, more and more investigators have looked to find positive individual traits that protect against anxiety (Duckworth, Steen, & Seligman, 2005; Park, & Peterson, 2009). As one of these character strengths, trait optimism refers to an individual's general expectation regarding future positive outcomes (Carver, & Scheier, 2014; Carver, Scheier, & Segerstrom, 2010). Converging evidence has indicated that trait optimism plays an essential role in an individual's physical and mental health (Alarcon, Bowling, & Khazon, 2013; Andersson, 1996). In particular, trait optimism is related to engagement coping, emotional regulation and resilience against stressful life events (Carver et al., 2014; Carver et al., 2010; Nes, & Segerstrom, 2006; Wu et al., 2013). These psychological constructs have been found to be the crucial prerequisites for reducing anxiety (Cisler, Olatunji, Feldner, & Forsyth, 2010; Hjemdal et al., 2011; Kennedy, Duff, Evans, & Beedie, 2003; Ng, Ang, & Ho, 2012). Evidence from numerous empirical investigations has confirmed that trait optimism can predict anxious symptoms among both clinical and healthy populations (e.g., Dooley, Fitzgerald, & Giollabhui, 2015; Griva, & Anagnostopoulos, 2010; Morton, Mergler, & Boman, 2014; Rajandram et al., 2011; Schweizer, Beck-Seyffer, & Schneider, 1999; Sheridan, Boman, Mergler, & Furlong, 2015; Siddique et al., 2006; Yu et al., 2015; Zenger et al., 2010). In the current study, we used resting-state functional magnetic resonance imaging (RS-fMRI) to examine the functional brain bases of trait optimism and then explore how trait optimism reduces anxiety in the brain. In brief, we used a multi-dimensional method (i.e., a brain-personality-symptom method) to investigate the associations among resting-state brain activity and connectivity, trait optimism and anxiety in a large sample of healthy adolescent students (N = 231).
Although the protective effect of trait optimism on anxiety has been established, the neural bases of trait optimism are not fully understood. Evidence from the limited literature has suggested that the function and structure of the prefrontal cortex (PFC) are crucial for trait optimism (Beer & Hughes, 2010; Dolcos et al., 2016; Grimm et al., 2009; Moran et al., 2006; Pauly, Finkelmeyer, Schneider, & Habel, 2013; Sharot, Korn, & Dolan, 2011; Sharot, Riccardi, Raio, & Phelps, 2007). For instance, two studies based on task-related fMRI reported that when participants imagined future positive events, there was increased activity in PFC regions including the orbitofrontal cortex (OFC), the medial PFC, the superior/inferior frontal gyrus and the anterior cingulate cortex (Sharot et al., 2011; Sharot et al., 2007). Similarly, increased activity of PFC regions (e.g., OFC, medial PFC and anterior cingulate cortex) has been observed when individuals make positive evaluations of their personalities or skills (Beer et al., 2010; Moran et al., 2006; Pauly et al., 2013). Moreover, evidence from a voxel-based morphometry study has revealed an association between individual differences in trait optimism and OFC gray matter volume, providing direct evidence for the structural brain basis of trait optimism (Dolcos et al., 2016). Notably, previous researchers have mostly used task-related fMRI designs but not task-free designs (e.g., structural MRI and RS-fMRI) to investigate the neurobiological basis regarding an individual's tendency for optimism. However, trait optimism is a personality trait with stable individual differences among people (Carver et al., 2014; Carver et al., 2010). Thus, the task-free designs may have an advantage in examining the brain bases of trait optimism because the stable individual differences in personality might be more clearly manifested in the overall brain structure and function under task-free conditions (DeYoung, 2010; Mar, Spreng, & DeYoung, 2013).
As one of the most important psychoradiology techniques, RS-fMRI can detect intrinsic brain activity (i.e., low-frequency fluctuations) and is widely employed to investigate the neural substrates underlying human personality and behaviors (Biswal, 2012; DeYoung, 2010; Mar et al., 2013). Prior evidence has shown that intrinsic brain activity at rest is reliably linked with task-induced brain activity (Tavor et al., 2016). As a reliable and popular measure of low-frequency fluctuations, the fractional amplitude of low-frequency fluctuations (fALFF) reflects the regional properties of spontaneous brain activity (Zou et al., 2008). This measure has been widely used to investigate the neural correlates of human personality traits (e.g., Kong et al., 2017; Kong et al., 2015; Kunisato et al., 2011; Wang et al., 2017b; Wang et al., 2017d). As another popular measure for low-frequency fluctuations correlations, resting-state functional connectivity (RSFC) reflects the synchronization among different brain regions (Fox & Raichle, 2007). Increasing evidence has suggested that RSFC is a good neural marker of personality traits (e.g., Adelstein et al., 2011; Pan et al., 2016; Sampaio et al., 2014; Takeuchi et al., 2013; Wang et al., 2017c). In summary, fALFF and RSFC are suitable for examining the neural bases of trait optimism.
To our knowledge, only two studies have used fALFF and RSFC via RS-fMRI to explore the neural correlates of trait optimism. First, Wu et al. (2015) reported an association between individual differences in trait optimism and the fALFF in the medial PFC. However, the sample size of this study was quite small, and the sex ratio was disproportionate (46 female and 4 male). Moreover, the statistical analyses were conducted for a given mask including several brain regions but not for the whole-brain mask. These limitations might limit the generalizability of their findings. Second, through an analysis of RSFC, Ran et al. (2017) observed that individual differences in trait optimism were correlated with the RSFC between the ventral medial PFC and inferior frontal gyrus. It is worth noting that the RSFC analysis used in this study depended on a region of interest (ROI; i.e., ventral medial PFC), which was selected from prior studies but not identified in a single cohort of participants. In addition, to our knowledge, no study has investigated the neural bases of trait optimism in young participants (e.g., adolescents). Considering that the brains of the adolescents are undergoing functional and structural reformation (Blakemore & Robbins, 2012; Crone & Dahl, 2012), it is necessary and valuable to probe the association between trait optimism and the brain in adolescents. Therefore, in the present study, we first used whole-brain correlation analyses to identify the brain regions related to trait optimism and then explored whether these regions interacted with other regions in the brain to predict trait optimism. In particular, the RSFC analysis used in the current study was a complementary approach to that of previous study by Ran et al. (2017). Moreover, we focused on a large sample of adolescents to ensure adequate statistical power for the whole-brain analyses. Given previous neural findings regarding trait optimism (Beer et al., 2010; Dolcos et al., 2016; Grimm et al., 2009; Moran et al., 2006; Pauly et al., 2013; Ran et al., 2017; Sharot et al., 2011; Sharot et al., 2007; Wu et al., 2015), the fALFF in the PFC regions (e.g., the OFC, the medial PFC, the superior/inferior frontal gyrus and the anterior cingulate cortex) might be associated with individual differences in trait optimism. Considering the RSFC findings of trait optimism that were reported in previous study (Ran et al., 2017), we further hypothesized that the RSFC in the PFC regions might be related to trait optimism.
The PFC is also consistently regarded as the core brain region for processing an individual's anxious symptoms (Bishop, 2007; Grupe et al., 2013). For example, a large number of fMRI studies have revealed that the anxiety levels of individuals with anxiety disorder were associated with the function of PFC regions such as the OFC, the medial PFC, the lateral PFC and the anterior cingulate cortex (Brühl, Delsignore, Komossa, & Weidt, 2014a; Ding et al., 2011; Etkin & Wager, 2007; Qiu et al., 2015; Zhang et al., 2015). The role of PFC function in predicting anxiety has also been reported in subclinical or healthy populations (Kim et al., 2011; Tian et al., 2016; Xue, Lee, & Guo, 2017). Furthermore, the structural variances of the PFC regions (e.g., the OFC, the medial PFC, the lateral PFC and the anterior cingulate cortex) are found to be linked with anxious symptoms among both clinical and healthy samples (Blackmon et al., 2011; Brühl et al., 2014b; Ducharme et al., 2014; Shang et al., 2014; Spampinato, Wood, De Simone, & Grafman, 2009; Syal et al., 2012; Talati et al., 2013). Finally, some evidence has shown that cognitive behavioral therapy can change PFC function and structure, which, in turn, reduces anxious symptoms in patients with anxiety disorder (Kircher et al., 2013; Klumpp, Fitzgerald, & Phan, 2013; Steiger et al., 2017). Given these findings and the predictive ability of trait optimism for anxiety, trait optimism might mediate the influence of PFC regions (e.g., the OFC, the medial PFC and anterior cingulate cortex) on anxiety. Alternatively, the PFC regions might mediate the association between trait optimism and anxiety.
To investigate these questions, standard measurements of trait optimism and anxiety were administered to all participants. Next, whole-brain correlation analyses were performed to identify the resting-state brain region(s) and connectivity linked with trait optimism. Mediation analyses were then conducted to examine the nature of the associations between resting-state brain activity and connectivity, trait optimism and anxiety. Finally, to test the specificity of the results, positive and negative affect and depression were controlled for.
2 METHODS
2.1 Participants
Two hundred and thirty-four healthy high school students (52.1% females, age = 18.60 ± 0.78 years) participated in this study as a part of an ongoing project aimed at exploring the neural mechanisms underlying mental health, academic achievement and social cognition among adolescents in Chengdu, China (Wang et al., 2018; Wang et al., 2017a; Wang et al., 2017b; Wang et al., 2017c; Wang et al., 2017d; Wang et al., 2017e). All participants were right-handed as determined by a self-report using the Edinburgh Handedness Inventory (Oldfield, 1971). None of participants reported a history of psychiatric or neurological illnesses. Three participants were excluded because of an abnormal brain structure (e.g., unusual cyst). The ethical approval of this study was granted by the local research ethics committee of West China Hospital, Sichuan University. Written informed consents were obtained from all participants and their parents before the experiments.
2.2 Behavioral tests
2.2.1 Revised life orientation test (LOT-R)
The LOT-R was used to evaluate an individual's trait optimism (Scheier, Carver, & Bridges, 1994). It is a unidimensional scale that consists of six items such as “I am always optimistic about my future” (positively worded item) and “I hardly ever expect things to go my way” (negatively worded item). Participants were instructed to indicate the degree of agreement on each item, which ranged from 1 (strongly disagree) to 5 (strongly agree). To obtain the score for LOT-R, we first reverse-scored the negatively worded items and then summed the ratings of all items. Higher scores indicate higher levels of optimism. Previous investigations have suggested that the LOT-R shows excellent validity and reliability in different samples of participants (Mehrabian & Ljunggren, 1997; Scheier et al., 1994). This test has been repeatedly used in Chinese populations (e.g., Lai, Cheung, Lee, & Yu, 1998; Lai & Yue, 2000; Yang, Wei, Wang, & Qiu, 2013; Yu et al., 2015). In our dataset, the LOT-R showed an adequate internal consistency (Cronbach's α = 0.74).
To confirm that the single-factor structure of the LOT-R that was reported in prior studies could be used in our sample, a confirmatory factor analysis was conducted with AMOS software (Version 22.0). In this analysis, the model's goodness-of-fit was assessed using four indices (Hu & Bentler, 1999; Kline, 2015): the comparative fit index (CFI) and the non-normed fit index (NNFI), with values above 0.95 indicating a good fit; the root mean square error of approximation (RMSEA) and the standardized root mean square residual (SRMR), with values below 0.06 indicating an excellent fit. The results showed that the single-factor model fit well with our data (χ2 (9) = 15.50, p < .001; CFI = 0.98, NNFI = 0.95, RMSEA = 0.056, SRMR = 0.037), with the factor loadings ranging from 0.52 to 0.83. Thus, the factor structure of our data was the same as the original LOT-R (Scheier et al., 1994).
2.2.2 Trait anxiety inventory (TAI)
Participants' anxiety levels were assessed using the TAI (Spielberger, 1983). The scale contains one dimension, with 20 items ranging from 1 (not at all) to 4 (very much so). Respondents were asked to indicate how they felt about each statement, such as “I feel nervous and restless” (positively worded item) and “I make decisions easily” (negatively worded item). After reverse-scored the negatively worded items, the score for the TAI was computed by summing the ratings of all items, with higher scores indicating greater anxiety. The Chinese version of the TAI has demonstrated satisfactory validity and reliability in adolescents and adults (Shek, 1988; Tian et al., 2016). The scale exhibited good internal reliability in our dataset (Cronbach's α = 0.88).
2.2.3 Positive and negative affect schedule (PANAS)
The PANAS was employed to measure the levels of participants' positive and negative affect (Watson, Clark, & Tellegen, 1988). It is comprised of 20 emotional words, with 10 items for positive affect (e.g., “inspire”) and 10 items for negative affect (e.g., “scared”). The scoring for each item ranges from 1 (not at all) to 5 (extremely). Respondents were required to rate how often they have felt specific affections during a long period time. The scores for positive affect and negative affect were calculated separately, with higher scores suggesting higher levels of the corresponding affection. The reliability and validity of the Chinese version of PANAS have been established among different populations (e.g., Chen, 2004; Kang et al., 2003; Wang, Shi, & Li, 2009). In our dataset, Cronbach's α for positive affect and negative affect were 0.86 and 0.84, respectively, showing satisfactory internal consistency.
2.2.4 Beck depression inventory (BDI)
The 21-item BDI was used to evaluate participants' depressive symptoms (Beck et al., 1961). It is a widely-used self-report questionnaire that measures the severity of depressive symptoms in the past week. Participants were asked to answer each item, which has four possible answers and ranges from 0 (“never”) to 3 (“very heavy”). The score for BDI was obtained by summing the ratings of all items, with higher scores indicating greater depression. The psychometric properties of BDI have been well established in clinical and non-clinical samples (Beck, Steer, & Carbin, 1988). This scale has also been repeatedly used in Chinese populations (e.g., Li et al., 2014; Liu et al., 2016a; Liu et al., 2016b). In our dataset, Cronbach's α for BDI was 0.85, showing satisfactory internal consistency.
2.3 RS-fMRI data acquisition and preprocessing
2.3.1 Data acquisition
For each participant, a total of 480 s RS-fMRI scanning was performed using a Siemens-Trio Erlangen 3.0 T MRI system, which is located at West China Hospital of Sichuan University. We used an echo-planar imaging sequence to obtain the RS-fMRI data, and the scanning parameters were as follows: repetition time, 2,000 ms; echo time, 30 ms; 240 volumes; voxel size, 3.75 × 3.75 × 5 mm3; 30 slices; slice thickness, 5 mm; interslice gap, 0 mm; matrix, 64 × 64; flip angle, 90°; field of view, 240 × 240 mm. In addition, T1-weighted anatomical images were acquired with the following parameters: inversion time, 900 ms; echo time, 2.26 ms; repetition time, 1,900 ms; 176 slices; voxel size, 1 × 1 × 1 mm3; flip angle, 9°; matrix, 256 × 256.
2.3.2 Data preprocessing
The Data Processing Assistant for Resting-State fMRI (DPARSF; Yan, Wang, Zuo, & Zang, 2016), which employs the statistical parametric mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm), was used to preprocess the image data. The preprocessing was conducted with the following steps. The first ten images were discarded to allow for participants familiarization and fMRI signal stabilization. The remaining 230 images were corrected for temporal shifts between slices, realigned to the middle volume, and unwrapped to correct for susceptibility-by-movement interaction. Next, by using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) in SPM8 (Ashburner, 2007), each image volume was spatially normalized to the Montreal Neurological Institute 152-brain template, with a resolution of 3 × 3 × 3 mm3 (Wang et al., 2017b). The images were then spatially smoothed with an 8-mm FWHM Gaussian kernel and linear trends were subsequently removed. Finally, to remove the effects of very-low-frequency drifts and high-frequency noises, all images were filtered using a temporal bandpass filter (0.01 ∼ 0.08 Hz, only for RSFC but not for fALFF).
2.3.3 Quality control
During the process of scanning, each participant was asked to keep still and remain relaxed, not open his/her eyes and not think of anything deliberately. Foam pads and earplugs were employed to reduce head motion and scanning noise. Before the data preprocessing, a medical radiologist who was blind to the present study visually inspected the data for image quality. Given that the translational or rotational parameters were less than ±1.5 mm or ±1.5° and the mean framewise displacement (FD) values did not exceed 0.30, no participants were excluded during preprocessing. To rule out physiological noise (e.g., fluctuations caused by motions or respiratory and cardiac cycles), we regressed out nuisance signals including global mean signal, white matter signal, cerebrospinal fluid signal and six head motion parameters before bandpass filtering (Hallquist, Hwang, & Luna, 2013).
2.4 Data analyses
2.4.1 fALFF-behavior correlation analyses
Following the methods proposed by Zou et al. (2008), the time courses in each voxel were transferred into the frequency domain. After calculating the square root of each frequency in the power spectrum, the mean square root was computed across a low-frequency range (0.01–0.08 Hz). The fALFF was then computed as a fraction, with amplitudes at a low-frequency range (0.01–0.08 Hz) dividing by the amplitudes across the whole frequency range (0.01–0.25 Hz). To exclude the global influences of variability between individuals, the normalized score of fALFF (i.e., z-fALFF) was finally obtained. These computations were conducted using DPARSF toolbox (Yan et al., 2016). To identify the brain regions where spontaneous activity related to trait optimism, we carried out a whole-brain correlation analysis between the LOT-R scores and fALFF of each voxel in the brain, with age and gender as the controlling variables. To infer the regions of significance, we used the Gaussian random field approach (Worsley, Evans, Marrett, & Neelin, 1992) with the following settings: p < .05 at cluster level and p < .001 at voxel level; 61 × 73 × 61 dimension; 70,831 voxels in the mask; the estimated size of spatial smoothness was (10.59, 11.15, and 10.44 mm); at least 44 voxels or 1,188 mm3. We performed these analyses with REST software (Song et al., 2011).
2.4.2 RSFC-behavior correlation analyses
We performed RSFC-behavior correlation analyses to investigate whether the clusters identified through the fALFF-behavior correlation analyses interplayed with other regions to explain trait optimism. To do so, seed ROIs were created using the clusters with a significant relation to trait optimism. For each subject, we first averaged the time series of all voxels in each seed. Then, we performed correlation analyses between the mean time series in each seed and that of other voxels in the brain, and participant-level correlation maps were obtained. For standardization purposes, the correlation maps were normalized to z maps. In the group-level analyses, we conducted correlation analyses between z maps and LOT-R scores to detect the association between RSFC and trait optimism, with age and gender as controlling variables. For multiple comparisons correction, we used the Gaussian random field approach (Worsley et al., 1992) with the following settings: p < .05 at cluster level and p < .001 at voxel level; 61 × 73 × 61 dimension; 70,831 voxels in the mask; the estimated size of spatial smoothness was (13.43, 14.01, and 13.99 mm]; at least 59 voxels or 1,593 mm3. The analyses above were performed using REST software (Song et al., 2011).
2.4.3 Prediction analyses
To examine the robustness of the brain-optimism relation, we implemented a machine learning approach via balanced cross-validation using linear regression (Evans et al., 2015; Kong et al., 2017; Qin et al., 2014; Supekar et al., 2013; Wang et al., 2017a; Wang et al., 2017b). In the regression model, the dependent variable was the LOT-R score, the independent variables were the fALFF or RSFC values of different regions obtained from the voxel-wise fALFF and RSFC analyses. First, we divided the data by four-fold to ensure that the independent variable and dependent variable distributions across folds were balanced (Evans et al., 2015; Kong et al., 2017; Qin et al., 2014; Supekar et al., 2013; Wang et al., 2017a; Wang et al., 2017b). We then used three folds to build a linear regression model and left out the fourth fold. Next, we employed this model to predict the fourth fold data. After repeating this procedure four times, we obtained a final r(predicted, observed) (i.e., the average association between the observed data and the predicted data). To assess the statistical significance of the model, a nonparametric testing method was applied that generated 1,000 surrogate datasets to estimate the null hypothesis that there were no associations between trait optimism and fALFF or RSFC. By counting the number of r(predicted, observed)i values greater than r(predicted, observed) and then dividing that count by the number of Di datasets (1,000), the statistical significance (p-value) for the model was obtained (Evans et al., 2015; Kong et al., 2017; Qin et al., 2014; Supekar et al., 2013; Wang et al., 2017a; Wang et al., 2017b).
2.4.4 Mediation analyses
To verify if the resting-state brain activity and connectivity affected anxiety through trait optimism, we carried out mediation analyses using the PROCESS macro in SPSS (Hayes, 2013). In the mediation model, we treated trait optimism as the mediator variable, anxiety as the dependent variable and the fALFF or RSFC of brain regions as the independent variable. Trait optimism can be considered a mediator if the influence of the fALFF or RSFC in brain regions on anxiety is reduced significantly when including trait optimism as a mediator variable in the model. The significance of the mediating effect was assessed using a bootstrapping method with 5000 iterations. If a 95% confidence interval (CI) does not contain zero, then the mediating effect is significant. To investigate another hypothesis that the association between trait optimism and anxiety could be mediated by resting-state activity and connectivity, we performed another mediation analysis with the fALFF or RSFC of brain regions as the mediator variable, anxiety as the dependent variable and trait optimism as the independent variable.
3 RESULTS
3.1 The neural correlates of trait optimism
The descriptive statistics for age and behavioral variables are listed in Table 1. The skewness and kurtosis values for all behavioral variables (except for depression) were acceptable for the normality assumption (Marcoulides & Hershberger, 1997), with a range between −1 and +1. No gender differences were observed in trait optimism [t (229) = 1.45, p = .15]. There was no significant correlation between trait optimism and age (r = −.10, p = .12). We then investigated the neural substrates underlying trait optimism.
| Variable | Mean | SD | Range | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Age | 18.48 | 0.54 | 16–20 | 0.50 | 1.71 |
| Trait optimism | 22.59 | 3.01 | 12–29 | −0.36 | 0.37 |
| Trait anxiety | 39.42 | 7.16 | 21–61 | 0.14 | 0.09 |
| Positive affect | 33.65 | 5.79 | 17–50 | −0.05 | −0.28 |
| Negative affect | 22.84 | 5.54 | 10–41 | 0.70 | 0.77 |
| Depression | 6.91 | 6.55 | 0–42 | 2.01 | 6.08 |
- Abbreviations: N = number; SD = standard deviation.
To reveal the relationship between spontaneous brain activity and trait optimism, we correlated individuals' LOT-R scores with the fALFF of each voxel in the brain. After adjusting for gender and age, trait optimism was negatively related to the fALFF in the right OFC (see Figure 1 and Table 2). We found no other significant associations in this analysis. We then performed prediction analyses to test the robustness of the relation between spontaneous brain activity and trait optimism. After adjusting for gender and age, the fALFF in the right OFC significantly predicted trait optimism [r(predicted, observed) = −.32, p < .001].

Brain region linked with trait optimism after adjusting for age and gender. (a) Trait optimism was negatively associated with the fALFF in the right OFC. (b) Scatter plots showing the association between trait optimism and the fALFF in the right OFC (r = −.34, p < .001). OFC, orbitofrontal cortex; fALFF, fractional amplitude of low-frequency fluctuations [Color figure can be viewed at wileyonlinelibrary.com]
| Peak MNI coordinate | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Peak z score | Cluster size (mm3) |
| Correlation with fALFF | ||||||
| Right OFC | 11 | 18 | 51 | −9 | −4.3 | 1296 |
| Correlation with RSFC | ||||||
| Left SMC | 6 | −12 | −15 | 66 | 4.1 | 1728 |
- The threshold for significant regions was set at p < .05 at the cluster level and p < .001 at the voxel level (Gaussian random field approach; for the regions of fALFF analyses: cluster size ≥44 voxels, 1,188 mm3; for the regions of RSFC analyses: cluster size ≥59 voxels, 1,593 mm3).
- Abbreviations: OFC, orbitofrontal cortex; SMC, supplementary motor cortex; BA, Brodmann's area; MNI = Montreal Neurological Institute. fALFF, fractional amplitude of low-frequency fluctuations; RSFC, resting-state functional connectivity.
To explore whether the identified right OFC interacted with other regions to predict trait optimism, we carried out a correlation analysis between RSFC strength and trait optimism with gender and age as controlling variables. We found that trait optimism was positively related to the RSFC strength between the right OFC and left supplementary motor cortex (SMC; see Figure 2 and Table 2). We found no other significant associations in this analysis. We then performed prediction analyses to test the robustness of the relation between RSFC and trait optimism. After adjusting for gender and age, the OFC-SMC connectivity significantly predicted trait optimism [r(predicted, observed) = .24, p < .001].
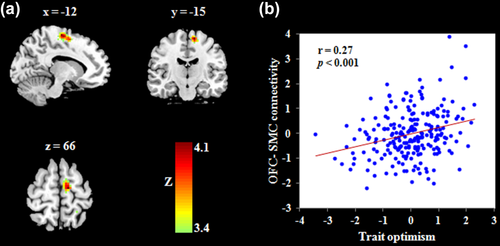
Functional connectivity linked with trait optimism after adjusting for age and gender. (a) Trait optimism was positively associated with the connectivity between right OFC and left SMC. (b) Scatter plots showing the association between trait optimism and the strength of OFC-SMC connectivity (r = .27, p < .001). OFC, orbitofrontal cortex; SMC, supplementary motor cortex [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Trait optimism mediates the effect of resting-state brain activity and connectivity on anxiety
To investigate the brain-optimism mechanisms that protect an individual's anxious symptoms, the TAI was administered. First, we replicated the significant association of trait optimism and anxiety (r = −.35, p < .001) after controlling for gender and age. Second, we investigated whether the resting-state brain activity and connectivity related to trait optimism could predict anxiety. The results revealed that after adjusting for gender and age, the fALFF in the right OFC (r = .20, p = .002) and the OFC-SMC connectivity (r = −.21, p < .001) were significantly associated with anxiety. These results indicated that there were close relationships between trait optimism, anxiety and resting-state brain activity and connectivity, but the nature of the relationships remained unknown.
To examine whether the relationships between anxiety and resting-state brain activity or connectivity could be explained by trait optimism, we carried out mediation analyses with gender and age as covariates. At the regional level, after adding trait optimism as a mediator, the fALFF in the right OFC were no longer related to anxiety (Figure 3a). The 5,000 bootstrap simulations further suggested that trait optimism had a significant mediating effect on the relationship between the fALFF in the right OFC and anxiety (indirect effect = 0.11, 95% CI = [0.05, 0.20], p < .05). At the connectivity level, after adding trait optimism as a mediator, the OFC-SMC connectivity was no longer related to anxiety (Figure 3b). The 5,000 bootstrap simulations further showed that trait optimism had a significant mediating effect on the relationship between OFC-SMC connectivity and anxiety (indirect effect = −0.09, 95% CI = [−0.17, −0.04], p < .05). These results suggested that the resting-state brain activity and connectivity affected anxiety through trait optimism.
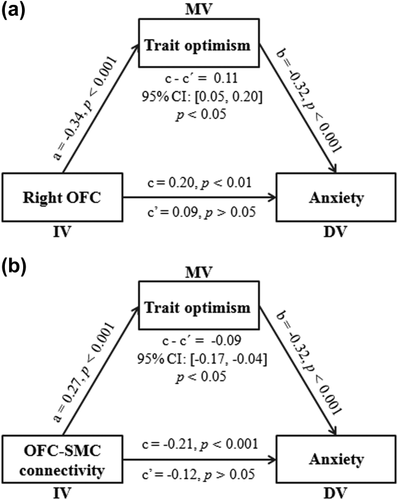
Trait optimism mediates the influence of the right OFC and OFC-SMC connectivity on anxiety. The depicted diagram shows that the fALFF of the right OFC (a) and OFC-SMC connectivity (b) affect anxiety through trait optimism after controlling for age and gender. Standardized regression coefficients are present in the path diagram. IV, independent variable; MV, mediator variable; DV, dependent variable. OFC, orbitofrontal cortex; SMC, supplementary motor cortex
In addition, we performed another two mediation analyses to confirm the directionality of the associations between resting-state brain activity or connectivity, trait optimism and anxiety. In these analyses, the dependent variable was anxiety, the independent variable was trait optimism, the mediator variable was either the fALFF in the right OFC or the OFC-SMC connectivity, and the controlling variables were gender and age. We found that the association between trait optimism and anxiety was not mediated by the fALFF in the right OFC (indirect effect = −0.03, 95% CI = [−0.09, 0.01], p > .05) or the OFC-SMC connectivity (indirect effect = −0.03, 95% CI = [−0.08, 0.01], p > .05). Therefore, our findings indicated that there might be only one brain-optimism mechanism for protecting against anxiety, in which anxiety is affected by OFC spontaneous activity and OFC-SMC connectivity through trait optimism.
3.3 Supplementary analyses
First, participants' head motions during scanning have been regarded to be a reliable trait that can affect fMRI data (Kong et al., 2014). Thus, we computed the mean FD as an index of head motion (Van Dijk, Sabuncu, & Buckner, 2012) and found no association between FD and trait optimism (r = .01, p = .881). Moreover, FD was not correlated with the fALFF in the right OFC (r = −.10, p = .123) and the OFC-SMC connectivity (r = .11, p = .084). We then verified whether our results could be affected by head motion. After including FD as another covariate, trait optimism was still related to the fALFF in the OFC (see Supporting Information Figure S1 and Table S1) and OFC-SMC connectivity (see Supporting Information Figure S2 and Table S1). Furthermore, mediation analyses showed that after controlling for gender, age and FD, the mediating effects of trait optimism were still significant for the association between the right OFC activity and anxiety (indirect effect = 0.11, 95% CI = [0.05, 0.20], p < .05; Supporting Information Figure S3a) and for the association between OFC-SMC connectivity and anxiety (indirect effect = −0.09, 95% CI = [−0.16, −0.04], p < .05; Supporting Information Figure S3b). In summary, our results were independent of head motion.
Second, positive and negative affect has been found to be associated with trait optimism (e.g., Scheier et al., 1994), anxiety (e.g., Watson et al., 1988) and intrinsic brain activity (e.g., Kong et al., 2015). Thus, we checked whether positive and negative affect could influence our results. The results showed that positive affect was significantly correlated with the fALFF in the right OFC (r = −0.13, p = .046) and the OFC-SMC connectivity (r = .17, p = .011). However, negative affect was not correlated with the fALFF in the right OFC (r = .04, p = .581) and the OFC-SMC connectivity (r = −.06, p = .336). We then investigated the effect of positive and negative affect on the neural substrates of trait optimism. After controlling for positive affect and negative affect, trait optimism was still related to the fALFF in the right OFC (r = −.32, p < .001) and the OFC-SMC connectivity (r = .23, p < .001). Furthermore, we explored whether positive and negative affect could influence the mediating effect of trait optimism on the relation between resting-state brain activity and connectivity and anxiety. After adjusting for positive affect and negative affect, the mediating effects of trait optimism were still significant for the association between the right OFC activity and anxiety (indirect effect = 0.05, 95% CI = [0.01, 0.10], p < .05) and for the association between OFC-SMC connectivity and anxiety (indirect effect = −0.04, 95% CI = [−0.08, −0.01], p < .05). Gender and age were controlled for in these analyses. Therefore, our findings were specific to trait optimism to some degree, although positive and negative affect has some effects on the optimism-brain association.
Third, some evidence has suggested that TAI is not a clean measure of anxiety because several items of TAI are more relevant to depression than to anxiety (Nitschke et al., 2001; Watson, Stanton, & Clark, 2017). Thus, we used BDI to test whether depression could influence our results. Behaviorally, we found that depression was significantly correlated with trait optimism (r = −.30, p < .001) and anxiety (r = .46, p < .001). Neurally, depression was significantly correlated with the fALFF in the right OFC (r = .23, p < .001) and the OFC-SMC connectivity (r = −.13, p = .042). Importantly, after controlling for depression, trait optimism was still correlated with anxiety (r = −.24, p < .001), as well as the fALFF in the right OFC (r = −.27, p < .001) and the OFC-SMC connectivity (r = .23, p < .001). Moreover, after controlling for depression, anxiety was still correlated with the fALFF in the right OFC (r = .13, p = .043) and the OFC-SMC connectivity (r = −0.19, p = .003). Finally, after controlling for depression, the mediating effects of trait optimism were still significant for the association between the right OFC activity and anxiety (indirect effect = 0.06, 95% CI = [0.02, 0.12], p < .05) and for the association between OFC-SMC connectivity and anxiety (indirect effect = −0.05, 95% CI = [−0.10, −0.01], p < .05). In summary, our findings have the specific nature to some degree, although depression can reduce the effect sizes of the results.
4 DISCUSSION
The aim of this study was to investigate the neurobiological correlates of trait optimism and their relations to anxiety in healthy adolescents using fALFF and RSFC obtained via RS-fMRI. Confirming our first hypothesis, the whole-brain correlation analyses revealed that higher trait optimism was linked to lower fALFF in the right OFC, which is not reported in previous fALFF study regarding trait optimism (i.e., Wu et al., 2015). Moreover, we found that trait optimism was positively associated with the RSFC between the right OFC and left SMC, which adds new evidence for the RSFC underlying trait optimism beyond the findings revealed by prior study (i.e., Ran et al., 2017). Furthermore, trait optimism mediated the influence of right OFC activity and OFC-SMC connectivity on anxiety. These results remained even when excluding the impact of head motion, positive and negative affect and depression. Overall, our study provides novel evidence that OFC spontaneous activity and OFC-SMC connectivity are linked to trait optimism and offers an underlying mechanism for reducing anxiety in which resting-state brain activity and connectivity affect anxiety through trait optimism.
First, we observed an association between enhanced fALFF in the right OFC and lower trait optimism. This negative association fits well with many investigations that have revealed increased fALFF in the OFC in low-optimism-related psychopathologies such as anxiety disorder (Qiu et al., 2015), bipolar disorder (Xu et al., 2014), posttraumatic stress disorder (Yin et al., 2011) and major depression disorder (Liu et al., 2014). This finding is also consistent with previous studies based on healthy populations reporting a relationship of enhanced fALFF in the OFC and lower levels of trait hope (Wang et al., 2017b), life satisfaction (Kong et al., 2015) and emotion regulation (Pan et al., 2014), which are constructs highly related to trait optimism (Alarcon et al., 2013; Carver et al., 2014; Carver et al., 2010). Enhanced fALFF in the OFC may develop as a compensatory (but not sufficiently efficient) mechanism for those difficulties or outcomes induced by a reduction in optimism or lack of optimism among low-optimistic participants. In fact, many previous studies have demonstrated the compensatory mechanism to counteract functional or structural decrease or defects in the brain (e.g., Bing et al., 2013; Li et al., 2015; Orr et al., 2013; Sun et al., 2016; Yang et al., 2011). In addition, our finding regarding the association between trait optimism and OFC spontaneous activity may considerably advance prior structural and functional MRI findings linking the OFC and an individual's tendency for optimism (Beer et al., 2010; Dolcos et al., 2016; Moran et al., 2006; Pauly et al., 2013; Sharot et al., 2007). Specifically, trait optimism has been found to be associated with activity in the OFC/ventral medial PFC when individuals make positive evaluations of their future, personality and skills (Beer et al., 2010; Moran et al., 2006; Pauly et al., 2013; Sharot et al., 2007). Evidence from a voxel-based morphometry study has revealed that the gray matter volume in the OFC can predict individual differences in trait optimism (Dolcos et al., 2016). In addition, we found that only right OFC but not left OFC was associated with trait optimism. A longstanding literature has suggested dichotomous lateralization in the PFC as a function of either approach/avoidance motivation or emotional valence, with the left PFC being related to approach tendency and positive affect, and the right PFC to avoidance tendency and negative affect (e.g., Harmon-Jones, Gable, & Peterson, 2010; Hecht, 2013; Heller, Nitschke, & Miller, 1998). However, the findings from many recent studies are inconsistent with this traditional hypothesis (see Miller et al., 2013, a systematic review). There may be a more complex nature of PFC activation and lateralization (Miller et al., 2013). For example, the right OFC but not the left OFC has been found to be involved in processing positive information, such as life satisfaction (Kong et al., 2015) and emotion regulation (Pan et al., 2014). In summary, our finding may add the new evidence for the right OFC in processing approach- and positive-related information.
Moreover, the present study showed that trait optimism was positively related to the RSFC between the right OFC and left SMC. This finding is in line with two task-related fMRI studies revealing enhanced activity in the left SMC but not the right SMC when participants make positive evaluations of their personality versus others' personalities (Moran et al., 2006), and when participants think about their positive traits in the past, the present and the future (D'Argembeau et al., 2010). Thus, the left SMC may play a key role in processing self-related positive evaluation, which is conceptually linked with trait optimism. Broadly, the SMC has also been considered to be a critical node of the brain networks underlying future reward processing (Dalley, Everitt, & Robbins, 2011) and emotion regulation (Kohn et al., 2014), which seem to be indispensable components for the concept of trait optimism (Carver et al., 2014; Carver et al., 2010). Furthermore, the SMC is generally known to be implicated in motor-related behaviors (Nachev, Kennard, & Husain, 2008). In particular, this region has been found to be linked with physical activity (Erickson et al., 2010; Voelcker-Rehage & Niemann, 2013), which is a reliable factor for predicting trait optimism (Kavussanu & Mcauley, 1995). In short, the association between trait optimism and OFC-SMC connectivity might reflect the role of SMC in positive self-evaluations, future reward processing, emotion regulation and physical activity, which may contribute to optimistic individuals' positive cognitive style.
Importantly, we found that trait optimism mediated the influence of the fALFF in the right OFC and OFC-SMC connectivity on anxiety. Previous investigations have consistently suggested that trait optimism is strongly associated with anxiety among different populations (Dooley et al., 2015; Griva et al., 2010; Morton et al., 2014; Rajandram et al., 2011; Schweizer et al., 1999; Sheridan et al., 2015; Siddique et al., 2006; Yu et al., 2015; Zenger et al., 2010). In our dataset, we confirmed the association between trait optimism and anxiety (r = −0.34, p < .001). Furthermore, our study revealed that trait optimism could explain additional variances in anxiety even when adjusting for gender, age, head motion, positive and negative affect and depression (△R2 = 1.1%, partial r = −.15, p = .027). According to the guidelines for what constitutes small (r = .10), medium (r = .30), and large (r = .50) effect sizes (Cohen, 1988), trait optimism has a small-to-medium predictive ability for anxiety even after controlling for gender, age, head motion, positive and negative affect and depression. Therefore, trait optimism might be an essential factor in protecting against anxiety. On the other hand, we found that the OFC spontaneous activity and OFC-SMC connectivity could predict individual differences in anxiety. The associations between anxiety and OFC structure and function have been well established in previous investigations (Blackmon et al., 2011; Brühl et al., 2014a; Etkin et al., 2007; Qiu et al., 2015; Talati et al., 2013; Tian et al., 2016; Xue, Lee, & Guo, 2017). In particular, higher spontaneous activity in the OFC has been found to be linked with higher anxiety levels among healthy participants and patients with anxiety disorder (Qiu et al., 2015; Tian et al., 2016; Xue, Lee, & Guo, 2017), which fits well with our findings. In addition, evidence from a body of studies has indicated that the functioning of the SMC plays a critical role in an individual's anxious symptoms (e.g., Andreescu et al., 2009; Fonzo et al., 2015; Klumpp et al., 2014; Lang & Mcteague, 2009). Given that the SMC and OFC are two core brain regions in the processing of emotional information and regulation (Etkin, Egner, & Kalisch, 2011; Kohn et al., 2014), SMC-OFC connectivity might affect anxiety through changes in emotion-related processing. In brief, our findings substantiate the idea that trait optimism can serve as a potential mechanism that explains the impact of OFC spontaneous activity and OFC-SMC connectivity on anxiety.
The present study has several limitations that deserve consideration in future research. First, all behavioral measures used in this study were based on self-reports, although the reliability and validity of the measures were adequate. Future research should consider using other methods to reduce the influence of response bias. In particular, the TAI used in this study is not a pure measure of anxiety (Nitschke et al., 2001; Watson et al., 2017). Future researchers are encouraged to use other measures of anxiety to verify our findings. Second, this study only observed OFC and OFC-SMC connectivity related to trait optimism but failed to find an association between trait optimism and other brain regions (e.g., medial PFC, superior/inferior frontal gyrus and anterior cingulate cortex), which has been reported in previous studies (Beer et al., 2010; Grimm et al., 2009; Moran et al., 2006; Pauly et al., 2013; Ran et al., 2017; Sharot et al., 2011; Sharot et al., 2007; Wu et al., 2015). Considering that only fALFF and RSFC were used as the measures of brain function in our study, future investigations should also examine the neural bases of trait optimism by utilizing other measures of brain function (e.g., task-related brain activity) and structure (e.g., regional gray matter volume). Third, the participants in this study were a sample of healthy adolescents within a narrow age range, which may limit the generalizability of the findings. Future studies are necessary to extend our study to include more diverse populations, such as adults, children and the elderly. Fourth, the RS-fMRI used in this study has quite low temporal resolution and limited spatial resolution. Future researchers may consider using other imaging techniques (e.g., electroencephalogram and single-unit recording) to investigate the neurobiological basis of trait optimism.
In conclusion, the current study offers direct evidence for functional neural markers of trait optimism by revealing that OFC spontaneous activity and OFC-SMC connectivity were associated with trait optimism. Furthermore, the current study provides an underlying brain-personality-symptom pathway for protecting against anxiety in which OFC spontaneous activity and OFC-SMC connectivity affect anxiety through trait optimism. Finally, the present study may invite further work to explore how to develop behavioral (Malouff & Schutte, 2017) and neural (Scheinost et al., 2013) trainings for optimism to alleviate anxiety and promote well-being among adolescent students. This study may also provide the evidence to the developing psychoradiology (https://radiopaedia.org/articles/psychoradiology), a new field of radiology aiming to explore the neural mechanisms of neurocogntive dysfunction in the patients with psychiatric disorder (Kressel, 2017; Lui, Zhou, Sweeney, & Gong, 2016; Sun, 2017).
ACKNOWLEDGMENT
This study was funded by the National Natural Science Foundation (Grant Nos. 81621003, 81220108013, 81227002, 81030027), the National Key Technologies R&D Program (Program No. 2012BAI01B03) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China. Dr. Gong would also like to acknowledge the support from his Changjiang Scholar Professorship Award (Award No. T2014190) of China and the American CMB Distinguished Professorship Award (Award No.F510000/G16916411) administered by the Institute of International Education, USA.
CONFLICTS OF INTEREST
The authors declare no competing interests.