The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain
Funding information: National Health and Medical Research Council of Australia, Grant/Award Number: G160279; National Health and Medical Research Council of Australia, Grant/Award Number: 1091415; Human Capital and Mobility, Grant/Award Number: CHRX-CT94-0432; Training and Mobility of Researchers, Grant/Award Number: ERB-FMRXCT970160
Abstract
Recurrent thalamocortical connections are integral to the generation of brain rhythms and it is thought that the inhibitory action of the thalamic reticular nucleus is critical in setting these rhythms. Our work and others' has suggested that chronic pain that develops following nerve injury, that is, neuropathic pain, results from altered thalamocortical rhythm, although whether this dysrhythmia is associated with thalamic inhibitory function remains unknown. In this investigation, we used electroencephalography and magnetic resonance spectroscopy to investigate cortical power and thalamic GABAergic concentration in 20 patients with neuropathic pain and 20 pain-free controls. First, we found thalamocortical dysrhythmia in chronic orofacial neuropathic pain; patients displayed greater power than controls over the 4–25 Hz frequency range, most marked in the theta and low alpha bands. Furthermore, sensorimotor cortex displayed a strong positive correlation between cortical power and pain intensity. Interestingly, we found no difference in thalamic GABA concentration between pain subjects and control subjects. However, we demonstrated significant linear relationships between thalamic GABA concentration and enhanced cortical power in pain subjects but not controls. Whilst the difference in relationship between thalamic GABA concentration and resting brain rhythm between chronic pain and control subjects does not prove a cause and effect link, it is consistent with a role for thalamic inhibitory neurotransmitter release, possibly from the thalamic reticular nucleus, in altered brain rhythms in individuals with chronic neuropathic pain.
1 INTRODUCTION
There is a body of evidence that the thalamus plays a key role in the development or maintenance of some forms of chronic pain. Direct electrical stimulation of the thalamus can evoke pain (Lenz et al., 1993); lesions in the somatosensory thalamus can lead to persistent pain (Kim, Greenspan, Coghill, Ohara, & Lenz, 2007); and there are abnormal firing patterns of thalamic neurons in the pain state (Gerke, Duggan, Xu, & Siddall, 2003; Lenz, Kwan, Dostrovsky, & Tasker, 1989). Furthermore, the thalamus and its recurrent connections with the cortex play an integral role in the generation and sustenance of the brain rhythms that underlie brain function (Buzsaki, 2006; Hughes & Crunelli, 2005). Given that our perceptions arise from on-going activity within recurrent thalamocortical circuits, that is, thalamocortical rhythm, it has been proposed than an aberration in this circuitry, that is, thalamocortical dysrhythmia, is a characteristic of several central nervous system disorders, and may be critical for the development and maintenance of some forms of chronic pain (Jones, 2010; Sarnthein, Stern, Aufenberg, Rousson, & Jeanmonod, 2006; Walton & Llinás, 2010).
A number of investigations have shown that chronic neuropathic pain, that is, chronic pain that arises from damage to the nervous system, is associated with thalamocortical dysrhythmia (Llinás, Ribary, Jeanmonod, Kronberg, & Mitra, 1999; Sarnthein et al., 2006; Schulman, Zonenshayn, Ramirez, Ribary, & Llinas, 2005; Stern, Jeanmonod, & Sarnthein, 2006). While these previous studies used both electroencephalography and magnetoencephalography techniques to explore resting brain rhythms in individuals with neuropathic pain in different body locations, they consistently found increases in resting low-frequency cortical power in individuals with neuropathic pain. Similarly, we used resting-state functional magnetic resonance imaging to demonstrate significantly increased infra-slow oscillation power (0.03–0.06 Hz) within the ascending pain pathway, including the thalamus, in individuals with chronic orofacial neuropathic pain (Alshelh et al., 2016). We demonstrated that increased infra-slow oscillations were temporally coupled across specific regions in the ascending pain pathway, including the ventroposterior medial thalamus, the thalamic reticular nucleus (TRN), and the orofacial representation in the primary somatosensory cortex.
We also previously showed that neuropathic pain is associated with significantly reduced on-going activity in the region of the TRN (Gustin et al., 2014; Henderson et al., 2013). The TRN surrounds the rostral and lateral surface of the dorsal thalamus, contains exclusively GABAergic neurons and, via extensive inhibitory outputs, modulates all incoming sensory information on its way to the cerebral cortex (Buzsaki, 2006; Guillery, Feig, & Lozsádi, 1998; Krause, Hoffmann, & Hajos, 2003; McAlonan, Cavanaugh, & Wurtz, 2008; Pinault, 2004; Zikopoulos & Barbas, 2006). The TRN therefore plays a critical role in controlling the firing patterns of ventroposterior thalamic neurons and is thought to play a critical role in controlling thalamocortical rhythm (Fuentealba & Steriade, 2005). Indeed, preclinical studies have shown that inhibition of the TRN increases power in the same low-frequency range as that seen in individuals with neuropathic pain (Marini, Giglio, Macchi, & Mancia, 1995; Marini, Ceccarelli, & Mancia, 2002). This is consistent with our previous finding of significantly reduced thalamic (TRN) GABAergic content in individuals with neuropathic pain (Gustin et al., 2014; Henderson et al., 2013) and raises the prospect that following nerve injury, reduced GABAergic output from the TRN results in thalamocortical dysrhythmia.
In this study, we used electroencephalography and magnetic resonance spectroscopy to determine the relationship between thalamocortical rhythm and thalamic GABA concentration in individuals with chronic orofacial neuropathic pain compared to healthy pain-free controls. We hypothesise that individuals with chronic orofacial neuropathic pain would (a) display altered thalamocortical rhythm characterized by increased low frequency power, (b) display reduced thalamic GABA content, and (c) show a magnitude of thalamocortical dysrhythmia correlated with a reduction in thalamic GABA.
2 MATERIALS AND METHODS
2.1 Participants
Twenty subjects with chronic orofacial neuropathic pain (7 males; mean ± SEM age 50.1 ± 4.4 years), and 20 healthy pain-free control subjects (7 males, mean age 42.2 ± 2.9 years) were recruited. The two groups were gender-matched and there was no significant difference in age between the groups (t test; p > .05). Subjects with chronic (>3 months duration) orofacial neuropathic pain were referred by an oral maxillofacial surgeon (author E.R.V.) after a diagnosis of post-traumatic trigeminal neuropathy (Nurmikko & Eldridge, 2001). Standard MRI safety contraindications (cardiac pacemaker, pregnancy, metal implants) and EEG safety considerations (history of head injury or seizures) were applied and potential participants were also excluded if they suffered from psychiatric or neurological disorders.
As well as giving information on their relevant clinical history, each chronic pain subject kept a pain diary during the seven days prior to or following the EEG/MRI session. They recorded the intensity of their on-going pain three times a day using a 10 cm horizontal visual analogue scale (VAS), with 0 indicating “no pain” and 10 indicating “the most intense imaginable pain.” These pain intensity scores were then averaged over the 7-day period to create a mean pain intensity score. Each chronic pain subject also outlined the location of their on-going pain on a standard drawing of the head, listed their current medication use and completed the McGill Pain Questionnaire (Melzack, 1975), which directs respondents to select sensory, emotional, and cognitive qualitative descriptors of their pain. Informed written consent was obtained for all procedures according to the Declaration of Helsinki and the study was approved by our local Institutional Human Research Ethics Committees. A subset of the neuroimaging data (not spectroscopy or EEG data) from 11 of the 20 chronic pain subjects was used in a previous investigation (Alshelh et al., 2016).
2.2 Electroencephalography (EEG)
The EEG assessment was carried out at approximately the same time of day, in the morning, for all participants, to control for natural diurnal changes in brain rhythms (Sarnthein et al., 2006). A 32-channel EEG cap (NEURO PRAX, neuroCare Group, Germany) containing premeasured electrode sites according to the 10–20 system was fitted to each participant. Chest and eye electrodes were also fitted to monitor cardiac activity, and eye/facial muscle contractions, respectively. EEG data were acquired in a quiet, dimly-lit room for a continuous 5-min period with the subject relaxed and with their eyes closed. EEG recordings were sampled at a rate of 500 Hz and a band filter of 0.3–70 Hz with a notch filter at 50 Hz was applied.
2.3 Magnetic resonance spectroscopy (MRS)
MRS measurements were acquired immediately prior to the EEG and on the same morning. All subjects lay supine on the bed of a 3 T MRI scanner (Philips Achieva TX) with their head immobilized in a close-fitting 32-channel head coil. With each subject relaxed and at rest, a high resolution T1-weighted anatomical scan was acquired (repetition time = 5600 ms; echo time = 2.5 ms; flip angle = 8°; voxel size = 0.87 mm3). Using this T1-weighted anatomical scan as a guide, a 20 × 20 × 20 mm voxel was placed on the thalamus contralateral to the side of the on-going pain in the chronic pain subjects and on the right thalamus of the control subjects. GABA-edited MEGA-PRESS spectroscopy was then performed on this voxel (repetition time = 2000 ms; echo time = 68 ms; 1024 data points) (Mullins et al., 2014). One hundred averages were acquired with the MEGA-PRESS editing pulse centered at 1.9 parts per million (ppm) (“on” spectra) and 100 averages were acquired with the pulse centered at 7.46 ppm (“off” spectra). Following this, a single-voxel short-echo PRESS sequence was performed in the same voxel location in order to measure concentration of other metabolites, including creatine for the relative quantification of thalamic GABA (repetition time = 2000 ms; echo time = 28 ms; 1024 data points).
2.4 Data analysis
2.4.1 Electroencephalography
Using SPM12 software (Wellcome Trust Centre for Neuroimaging, University College London), EEG data were referenced to the average reference montage; filtered 0.5–30 Hz; downsampled to 200 Hz; and manually inspected for artefacts in 2-s epochs (Boord et al., 2008; Jensen et al., 2013; Stern et al., 2006). Channels or trials of data were removed if the underlying EEG signal contained artefacts such that the underlying signal was not clear. Noise was found across multiple datasets at frequencies <4 Hz, and for this reason we discarded these data for all subjects. The remaining EEG signal between 4 and 25 Hz was then Fourier time–frequency transformed and power values at each frequency were determined. These power values were logarithmically transformed (log 10) to achieve normality. Power values at each frequency were then plotted for the control and chronic pain groups and significant differences in power between groups over the entire frequency range, 4–25 Hz, and between groups within the theta (4–8 Hz), alpha (9–12 Hz), and beta (13–25 Hz) ranges were determined (p < .05, one-way ANOVA, one-tailed).
To explore the spatial distribution of these power differences, we compared the logged power of the chronic pain subjects with that of controls at each frequency across each EEG channel (two-sample t tests). We plotted the t values at each frequency for each of the 27 EEG channels. We also calculated t values for power differences between controls and chronic pain subjects in the theta, alpha, and beta ranges for each EEG electrode, plotted these values onto a surface representation of the head, and determined electrodes with significant power differences (two-sample t tests). In addition to power differences between control and chronic pain groups, we explored linear relationships between power at each EEG electrode and an individual's on-going pain intensity (Pearson's correlation coefficient) and pain duration (Spearman's rank correlation coefficient). The rho values of these relationships for each electrode were then plotted onto a surface representation of the head and any significant relationships determined (p < .05).
2.4.2 Magnetic resonance spectroscopy
The acquired spectra were analysed with the Java-based magnetic resonance users' interface version 3 (jMRUI 3.0, MRUI Consortium). First, the dominant water peak was removed using the Hankel Lanczos Singular Values Decomposition Filter tool, then the “on” and “off” spectral subsets were summed. The 68 ms “on” and “off” subspectra were then subtracted, resulting in GABA-edited difference spectra to measure GABA at the resonance of 3.01 ppm. GABA concentration was quantified using AMARES, an Advanced Method for Accurate, Robust and Efficient Spectral fitting (Vanhamme, van den Boogaart, & Van Huffel, 1997). Peak fitting for GABA was performed after manually defining the center frequency and line width of the GABA peak, and modelling the GABA peak as a Gaussian singlet. GABA concentration was expressed relative to creatine concentration (GABA:Cr) as per recent technical guidelines (Mullins et al., 2014). After the same process for removal of the dominant water peak as for the GABA concentration, creatine concentration was quantified using QUEST, a time-domain algorithm that fits a weighted metabolite basis set to the spectra acquired (Ratiney et al., 2005). Creatine concentration was measured at 3.02 ppm. Significant differences between chronic pain and control groups were then determined (p < .05, one-tailed two-sample t test). For the chronic pain group, significant linear relationships between thalamic GABA:Cr and pain intensity and duration were determined (p < .05).
2.4.3 Electroencephalography versus magnetic resonance spectroscopy
To explore the relationship between thalamic GABA levels and EEG power, we performed regression analyses of linear relationships between total theta, alpha and beta power and an individual's thalamic GABA:Cr levels (p < .05). In addition, to explore regional relationships, we performed regression analyses of linear relationships between power at each of the 27 EEG electrodes and an individual's thalamic GABA:Cr levels. The rho values of these relationships for each electrode were then plotted onto a surface representation of the head for the control and chronic pain groups and any significant relationships determined (p < .05).
3 RESULTS
3.1 Participants
The pain subjects' mean (± SEM) pain intensity over a week was 3.7 ± 0.4 and their median duration of pain was 66 months (range 4–302 months). Chronic pain subjects reported on-going pain encompassing the maxillary and mandibular distributions of the trigeminal nerve (Figure 1a). Fifteen of the chronic pain subjects reported left-sided pain, and five reported right-sided pain. (Two subjects reported bilateral pain; however, the pain was only mild on one side therefore the predominantly painful side was used here.) Chronic pain subjects most often described their on-going pain as “shooting,” “throbbing,” or “nagging.” Eighteen of the 20 participants in the patient group were taking some medication for pain. See Table 1 for other patient characteristics and Figure 1a for the distribution of patients' pain.
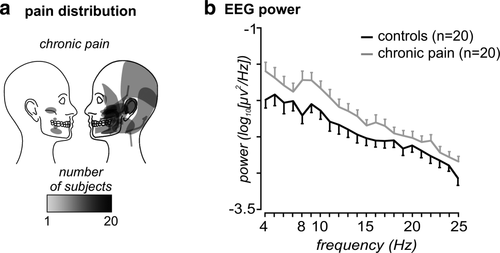
Pain distribution and EEG power: (a) Individual pain distribution patterns in 20 chronic orofacial pain subjects. (b) Plots of mean (±SEM) resting EEG power between 4 and 25 Hz in controls (black) and chronic orofacial pain (grey) subjects, over all 27 cortical EEG electrodes. Note that overall power is greater in chronic pain subjects than in controls over most frequencies
| Subject | Age | Sex | Primary site of pain (if bilateral worse side is in bold) | Pain diary (ave/10) | Pain duration (months) | Medications in last 24 h |
|---|---|---|---|---|---|---|
| 01 | 67 | F | Right side, upper jaw | 4.2 | 240 | Palmitoylethanolamide, Lyrica, Efexor, Oroxine, Crestor, Ergotamine, Diphenhydramine |
| 02 | 27 | F | Left side, upper jaw | 6.9 | 29 | Nurofen PRN |
| 03 | 65 | M | Bilateral (left side lower jaw worse than right side upper jaw) | 0.4 | 26 | Endep |
| 04 | 73 | M | Right side lower jaw | 2.9 | 264 | Monopril, Aspirin PRN, Norvasc, Crestor, Lithicarb |
| 05 | 37 | F | Left lower jaw | 3.4 | 96 | Gabapentin, Endep |
| 06 | 43 | F | Bilateral (right upper and lower jaw worse than left-upper only) | 2.8 | 24 | Mobic |
| 07 | 66 | F | Left upper jaw | 3.7 | 22 | Lyrica |
| 08 | 59 | F | Left lower jaw | 7.1 | 115 | Aspirin RPN |
| 09 | 25 | M | Left side upper jaw | 3.6 | 8 | Palmitoylethanolamide, Panadeine PRN |
| 10 | 71 | F | Left side upper jaw | 5.1 | 8.5 | Mobic, Panadol PRN, Gabapentin, Nortriptyline |
| 11 | 36 | M | Left side upper jaw | - | 84 | Oxycontin, Gabapentin, Panadol PRN |
| 12 | 19 | M | Right side upper jaw | 5 | 84 | None |
| 13 | 59 | F | Left side upper jaw | 4.4 | 120 | None |
| 14 | 27 | F | Left side upper and lower jaw | 2.7 | 8 | Palmitoylethanolamide |
| 15 | 57 | F | Left side upper jaw | 2.4 | 6 | Palmitoylethanolamide, Coversyl |
| 16 | 69 | F | Left upper and lower jaw | 4.8 | 108 | Palmitoylethanolamide |
| 17 | 64 | M | Right side upper and lower jaw | 1.2 | 66 | Gabapentin, Endep, Zyloprim |
| 18 | 21 | M | Left side lower jaw | 1.9 | 4 | Palmitoylethanolamide |
| 19 | 33 | F | Left side upper jaw | 2.3 | 88 | Palmitoylethanolamide, Dienogest |
| 20 | 76 | F | Left side upper jaw | 4.8 | 302 | Palmitoylethanolamide, Panadol PRN, Prestiq, Somac, Alprazolam |
3.2 Cortical EEG power
Power spectra over the entire frequency range (4–25 Hz) for both groups are depicted in Figure 1b. Chronic pain subjects displayed significantly greater power than controls over the entire frequency range 4–25 Hz (mean ± SEM log10[µv2/Hz]: controls −2.43 ± 0.10, chronic pain −2.20 ± 0.085, p = .04), in the theta (controls −2.03 ± 0.12, chronic pain −1.73 ± 0.11, p = .04) and alpha (controls −2.20 ± 0.12, chronic pain −1.89 ± 0.10, p = .03) ranges, but the two groups were not significantly different in the beta range (controls −2.66 ± 0.09, chronic pain −2.48 ± 0.07, p = .07). In addition, chronic pain subjects exhibited greater power at the dominant frequency (controls −1.71 ± 0.14, chronic pain −1.33 ± 0.13, p = .03). Although the mean dominant frequency was lower in patients than controls, this result was not significant (patients 6.75 ± 0.58 Hz, controls 7.80 ± 0.94 Hz, p = .18). In the chronic pain group there were no significant linear relationships between pain intensity and theta power (r = .21, p = .40), alpha power (r = .25, p = .31), or beta power (r = .14, p = .56), or between pain duration and theta power (Spearman's rho = −.05, p = .85), alpha power (rho = .33, p = .15), or beta power (rho = .19, p = .42). Although only two of the chronic pain subjects were not taking any medications, they both had greater power than control subjects in the theta (−0.60, −0.86), alpha (−1.18, −1.41), and beta (−1.91, −2.02) ranges.
3.2.1 Spatial distribution of EEG power differences
Consistent with the evidence of an overall increase in power, an exploration of the difference in power between groups at each EEG electrode at each frequency revealed significant power increases in chronic pain subjects compared with controls over several brain regions (Figure 2a). Power increases were particularly evident at frequencies below ∼15 Hz and were strong between 8 and 10 Hz. These power increases in chronic pain subjects occurred in areas of the medial prefrontal cortex (EEG electrodes FP1 and FP2), and the dorsolateral prefrontal cortex (F8), primary sensorimotor and temporal cortices (FC5 and T3) and parietal association cortex (P3, PZ, P4, and CP6). Plots of power differences separately within the theta, alpha and beta ranges mapped onto a surface representation of the head again showed that although power differences occurred primarily in the alpha range, differences also occurred in both the theta and beta ranges (Figure 2b). Within the alpha range, significant power increases in chronic pain subjects occurred in the frontal (FP2, F4, and F8), primary sensorimotor (FC5), temporal (T3), and parietal (CP1, P3, PZ, and P4) cortices. A similar pattern of differences between groups, although less widespread, also occurred in the theta and beta ranges (theta: F7, F8, FC5, T3, T5; beta: F8, FC5, T3, CP2, T5, P3, PZ, O2). Importantly, in theta, alpha and beta ranges, at no EEG electrode was power significantly greater in controls compared to chronic pain subjects.
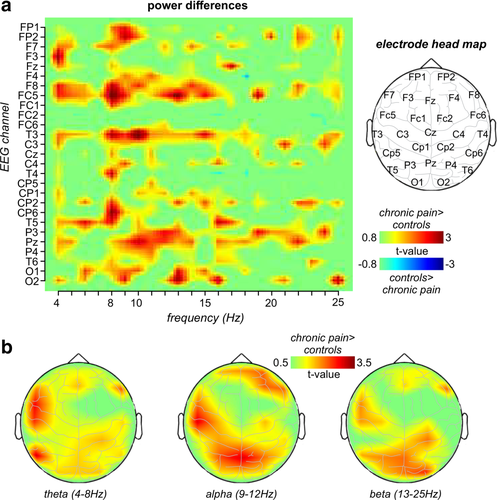
Spatial distribution of power differences: (a) Plots of t values representing power differences between controls and chronic pain subjects at each of the 27 EEG channels, across the 4–25 Hz frequency range. Note that power is greater in chronic pain subjects than in controls. To the right is an outline showing the approximate location of each of the 27 EEG electrodes. (b) Plotted on a surface representation of the head, t values representing power differences between controls and chronic pain subjects separately within the theta, alpha, and beta bands
While there were significant power differences between controls and chronic pain subjects over multiple areas of the cerebral cortex, in only a few regions was power significantly linearly correlated to either pain intensity (Figure 3a) or duration (Figure 3b) in the chronic pain subjects. In the theta range no region displayed a significant relationship between power and pain intensity. In the alpha range, only at T4 (r = .60, p = .007) was there a significant positive correlation. Within the beta range, significant positive relationships occurred between power and pain intensity in the sensorimotor (FC6: r = .53, p = .02), parietal (CP6: r = .57, p = .01) (Figure 3c) and occipital (O1: r = .57, p = .01) cortices. With respect to pain duration, there were no significant relationships between theta or beta power at any EEG electrode and pain duration. However, there were significant positive relationships between alpha power and pain duration in the frontal (FP2: r = .52, p = .02) and parietal cortices (CP5: r = .50, p = .02, P4: r = .66, p = .002) (Figure 3c).
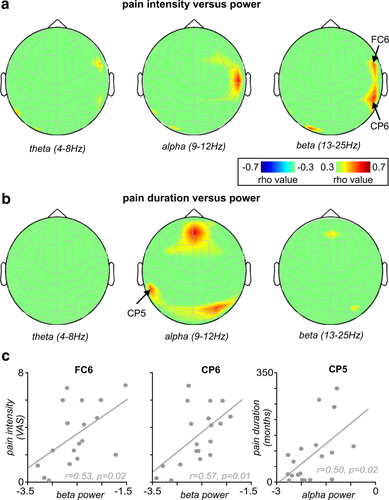
The relationship between EEG power and pain: (a) Plots of the relationship between pain intensity and EEG power in the theta, alpha and beta bands at each of the 27 channels, on a surface representation of the head. (b) Plots of the relationship between pain duration and EEG power in the theta, alpha, and beta bands at each of the 27 channels, on a surface representation of the head. (c) Plots of the correlation between pain intensity and beta power in electrodes FC6 and CP6, and the correlation between pain duration and alpha power in electrode CP5
3.3 Thalamic GABA concentration
GABA-edited MEGA-PRESS spectroscopy was not acquired in two of the chronic pain subjects (patients declined MRI), and we excluded a further two chronic pain and two control subjects due to either outlying GABA:Cr values or inadequate signal-to-noise for the spectra analysis. This resulted in data from 15 chronic pain subjects and 18 controls. In direct contrast to our previous work (Henderson et al., 2013), we found no significant thalamic GABA difference between chronic pain and control subjects (mean ± SEM GABA:Cr: chronic pain: 0.18 ± 0.01, controls: 0.17 ± 0.01, p = .27) (Figure 4a,b). Additionally, there was no significant relationship between thalamic GABA and on-going pain intensity (r = .14, p = .62) or pain duration (Spearman's rho = .05, p = .85).
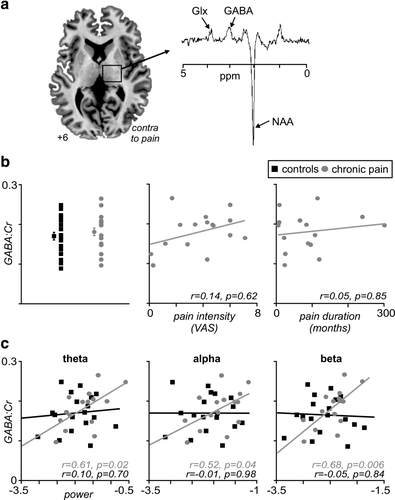
Thalamic GABA concentration: (a) Axial MR-image representing the voxel placement in the thalamus contralateral to the side of on-going chronic pain in the pain subjects, together with a diagram depicting the chemical shift axis. Note GABA concentration was measured at 3.01 parts per million (ppm). (b) Distribution of GABA:Cr ratios for chronic pain (grey circles) and control (black squares) subjects, demonstrating the lack of difference between groups. Plots of the correlations between pain intensity and pain duration with thalamic GABA concentration, demonstrating a lack of correlation with these measures. (c) Plots demonstrating the correlation between EEG power and thalamic GABA concentration in both chronic pain and control subjects in each of the theta, alpha, and beta ranges. Note the moderate to strong correlations in all bands in the pain subjects, while no such relationship exists in the control group. Glx, glutamate; NAA, N-Acetylaspartic acid
3.3.1 Thalamic GABA concentration and its association with power
Although there was no difference in thalamic GABA content between control and chronic pain subjects, we found that the relationship between thalamic GABA and cortical power was remarkably different between the two groups (Figure 4c). In controls, there was no significant relationship between thalamic GABA and overall theta power (r = .1, p = .70), alpha power (r = −.01, p = .98), or beta power (r = −.05, p = .84). In contrast, chronic pain subjects displayed significant positive relationships between thalamic GABA and overall theta power (r = .61, p = .02), alpha power (r = .52, p = .04), and beta power (r = .68, p = .006). Furthermore, the relationships between thalamic GABA and overall theta and beta power were significantly different between control and chronic pain groups (all p < .05, two-tailed, Fisher r-to-z transformation).
3.3.2 Spatial distribution of relationships between thalamic GABA and power
In addition to the overall differences between groups in the relationship between thalamic GABA and EEG power, there were striking differences in the spatial distributions of the relationships. In control subjects, at none of the EEG electrodes was there a significant relationship between thalamic GABA and theta, alpha or beta power (Figure 5a). In contrast, in chronic pain subjects, multiple electrodes showed positive correlations between thalamic GABA and theta, alpha, or beta power (Figure 5b). Within the theta range, significant correlations between power and thalamic GABA occurred in the frontal (FP1 r = .66, FP2 r = .59, F3, r = .61, FZ r = .61, F4 r = .57, F8 r = .62, FC1 r = .57), sensorimotor (FC6 r = .61, CZ r = .55), temporal (T3 r = .67), parietal (CP1 r = .78), and occipital cortices (O1 r = .72)(all p < 0.05; Figure 5c). More restricted significant correlations occurred between thalamic GABA and alpha power (F8 r = .57, T3 r = .60, CP5 r = .55, CP1 r = .73, O1 r = .54) and thalamic GABA and beta power (F7 r = .62, F3 r = .57, F8 r = .58, T3 r = .66, CZ r = .66, CP5, r = .78, CP1 r = .86, O1 r = .76). At no EEG electrode was there a significant negative relationship between thalamic GABA and power.
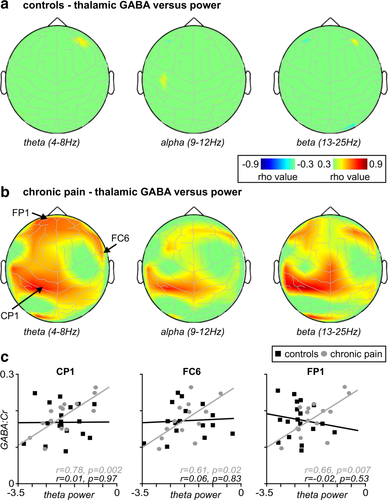
Spatial distribution of thalamic GABA versus power: (a) Plots of the relationship between thalamic GABA and EEG power in control subjects, and (b) chronic pain subjects, mapped onto a surface representation of the head, in theta, alpha, and beta bands. Note the lack of relationship in control subjects in all bands, and the relationship between GABA and power in pain subjects in all three frequency bands. (c) Plots of the correlation between thalamic GABA and theta power in EEG electrodes CP1, FC6, and FP1; note the striking difference in this relationship between groups
4 DISCUSSION
Consistent with our hypothesis, we found that chronic orofacial neuropathic pain was associated with significantly increased cortical power, particularly in theta and alpha frequency bands, compared to healthy controls. Furthermore, the region encompassing the sensorimotor cortex displayed a strong positive correlation between power and on-going pain intensity. Although we found no significant difference in thalamic GABA concentration between the two groups, which was in contrast to our previous findings and our hypothesis, there was a striking difference in the relationship between thalamic GABA and cortical power between pain subjects and controls. In the chronic pain group, significant positive linear relationships occurred between thalamic GABA and power, particularly in regions of the sensorimotor, frontal, and parietal association cortices. That is, the greater the thalamic GABA concentration, the greater the resting cortical power. In striking contrast, we detected no significant relationships between thalamic GABA and theta, alpha, or beta power in any region in the control group.
Consistent with much of the literature, the thalamocortical dysrhythmia that occurred here in individuals with orofacial neuropathic pain was characterized by a significant enhancement of activity in the theta and alpha ranges and a significant increase in power at the dominant spectral power peak. The enhanced power in pain subjects relative to controls was most marked between ∼8 and 10 Hz. These changes are consistent with many previous studies demonstrating thalamocortical dysrhythmia in chronic neuropathic pain (Sarnthein et al., 2006; Schulman et al., 2005; Stern et al., 2006; Walton & Llinás, 2010). Some studies demonstrated reduced or absent power in the alpha band (Jensen et al., 2013; Llinás et al., 1999) and enhanced power in the beta band in pain subjects (Stern et al., 2006), however these samples differed from ours and none comprised exclusively orofacial neuropathic pain. That we found a distinct increase in power at ∼8 Hz is similar to that found in neuropathic pain in the past (Sarnthein et al., 2006; Schulman et al., 2005). Interestingly though, in their neuropathic pain investigation, Schulman et al. (2005) termed this 7–9 Hz range the “high theta range.” There are indeed differences across the literature with regard to the grouping of frequencies, and different definitions of frequency bands would go some of the way to explaining some of the apparent, slight discrepancies across the EEG literature.
The thalamus plays a key role in the generation and maintenance of brain rhythms (Buzsaki, 2006; Hughes & Crunelli, 2005). It is thought that increases in low-frequency cortical power are due to a shift in thalamic neuron activity from a state dominated by tonic firing to one in which there is an increase in low-threshold spike burst firing (Hughes & Crunelli, 2005). This theory is supported by the finding that neuropathic pain is associated with an increase in burst firing in the ventrocaudal (Vc) thalamus and an associated increase in theta power (Lenz et al., 1989). Low-threshold calcium bursts occur when thalamocortical relay cells are in a state of hyperpolarisation; there is evidence that the TRN is capable of entraining this ‘burst firing mode’ (Golshani, Liu, & Jones, 2001), and it is argued that the TRN serves to maintain the low frequency thalamocortical oscillations (4–10 Hz) (Buzsaki, 1991; Jones, 2010). Given this, we were surprised we found no difference in thalamic GABA concentration between pain subjects and control subjects. Indeed, one would predict that neuropathic pain would be associated with increased TRN GABAergic output, which is contrary to the results of our previous studies in which we found reduced thalamic GABA content and reduced TRN blood flow in individuals with neuropathic pain (Gustin et al., 2014; Henderson et al., 2013). Although we found remarkable differences in the relationships between thalamic GABA and cortical power between subject groups, and a relationship in pain subjects suggestive of increased TRN GABAergic output, we cannot draw the conclusion that the TRN is solely responsible for the altered cortical rhythm. Indeed, we found no evidence of a difference in GABA content between groups, which raises the possibility of other mechanisms, for instance intracortical and corticothalamic projections, which include influences on both thalamic relay and possibly TRN neurons, and possibly affecting the hyperpolarization of thalamocortical relay cells.
Why thalamic GABA was reduced in our previous study but not in the present one is not known, though it is unlikely due to technical differences since similar collection and analysis techniques were used. It is possible that differences in thalamic GABA results from differences in patient characteristics and while pain duration and intensity were similar across studies, medication regimens were not (Henderson et al., 2013). In our previous investigation, 10 of the 23 pain subjects were not taking any medication compared with only 2 of 20 in this investigation. Furthermore, in our previous study, those taking medication were on relatively standard treatment regimens such as gabapentin and amitriptyline. In direct contrast, in this study, in addition to these standard medications, 8 of the 20 pain subjects were taking palmitoylethanolamide (PEA), which has been reported to target the peroxisome proliferator-activated receptor alpha as well as cannabinoid-like G-coupled receptors (Godlewski, Offertaler, Wagner, & Kunos, 2009; Lo Verme et al., 2005). While we do not have a sample large enough to explore the effects of PEA on thalamic GABA and brain activity, there is some evidence to suggest PEA may reduce neuropathic pain and thus this could have affected the results of this study (Keppel Hesselink & Kopsky, 2015). The effects of medications on thalamic GABA and resting brain activity require further detailed and directed investigations.
Although we found no reduction in thalamic GABA in this study, our finding of significant positive relationships between thalamic GABA and cortical power in individuals with neuropathic pain, but not controls, is consistent with the idea that increased GABAergic inhibition of specific thalamic nuclei such as Vc by the TRN results in increased thalamocortical power. Furthermore, this is consistent with our previous study in which we reported significant positive relationships between thalamic GABA and resting Vc-S1 connectivity strength in neuropathic pain subjects but not in controls (Henderson et al., 2013). The lack of relationship between Vc-S1 connectivity strength and thalamic GABA in controls is congruous with the lack of significant relationships between thalamic GABA and power at any of the 27 electrode locations in controls in the current study. However, this was surprising given the proposed role of the TRN and its GABAergic output in setting cortical rhythms. This, together with the lack of difference in thalamic GABA content between groups found here, leads us to question somewhat the importance of the role of the TRN in setting cortical rhythms particularly in individuals with chronic neuropathic pain.
While there are variations in the spatial distribution, or topography, of the enhanced EEG/MEG power in pain groups across the literature, most studies report enhanced low frequency power in somatosensory regions in patients relative to controls (Stern et al., 2006; Walton, Dubois, & Llinas, 2010). Our findings demonstrate the presence of more widespread activation in the patient group. Indeed, low-frequency brain rhythms (<10 Hz) show long-range cortical coherence and can span the entire cortex, whereas higher frequency oscillations (>30 Hz) tend to be associated with more restricted cortical activity (Contreras & Llinas, 2001). Furthermore, electrical stimulation immediately below the cortical surface at low frequencies activates a significantly greater cortical area than that produced by higher frequency stimulation, a difference thought to result from lateral inhibition (Llinás, Urbano, Leznik, Ramírez, & van Marle, 2005). This is consistent with our finding of increased low frequency power over a relatively large region of the cerebral cortex in individuals with neuropathic pain. Interestingly, however, only a restricted part of this power increase was correlated to on-going pain intensity, namely the sensorimotor and parietal association cortices. While we did not extend our analyses here to investigation of rhythms in the gamma band (>30 Hz), interesting questions remain as to whether the patches of lower and higher frequency cortical activity that we report on are evidence of the “edge effect,” whereby GABAergic inhibition of lateral cortical areas is diminished in disease states (Llinás et al., 2005).
We have previously shown that orofacial neuropathic pain is associated with increased infra-slow oscillations in the ascending pain pathway, including in the spinal trigeminal nucleus, Vc, TRN, and S1, and we have provided anatomical evidence that these changes may result from chronic astrocyte activation and changes in oscillatory gliotransmission (Alshelh et al., 2016). It is possible that following nerve injury, altered Vc thalamus infra-slow oscillatory activity, in addition to altered GABAergic influences of TRN outputs to the Vc thalamus, results in increased low frequency (theta and alpha) thalamocortical rhythm in Vc-S1 recurrent loops. Increased low frequency oscillations in this relatively restricted thalamocortical network could then feasibly evoke increased oscillatory changes over a relatively wide area of the cerebral cortex, which in turn feeds back to the thalamus and so on, driving a loop of altered activity. All that said however, in this study, we were not able to demonstrate the altered GABAergic influence of the TRN—and so, while feasible, this prospect is somewhat speculative. It is also important to consider the alternative sources of thalamic GABAergic inhibition. Extrathalamic inhibitory structures, such as the zona incerta (Bartho, Freund, & Acsady, 2002), the anterior pretectal nucleus (Bokor et al., 2005), the basal ganglia (Bodor, Giber, Rovo, Ulbert, & Acsady, 2008), and the pontine reticular formation (Giber et al., 2015) also target the thalamus. Furthermore the thalamus contains a proportion of local GABAergic interneurons (Jones & Hendry, 1989; Smith, Seguela, & Parent, 1987), although the TRN contains exclusively GABAergic cells (Houser, Vaughn, Barber, & Roberts, 1980; Smith et al., 1987). It is possible that mechanisms other than the circuit with the TRN could be responsible for the findings we report on; however, we think it less likely to be these sources than the TRN. As well as our previous finding of decreased cerebral blood flow in the TRN in neuropathic pain (Henderson et al., 2013), extrathalamic inhibitory nuclei do not exhibit recurrent circuits with the thalamus (i.e., they have no thalamic inputs), nor do they directly innervate the primary somatosensory thalamus (Bartho et al., 2002; Bokor et al., 2005; Halassa & Acsady, 2016).
Several limitations influence the interpretation of the results. First, while it is common for EEG and MEG studies to discount the spectral data at and below ∼2 Hz (Llinás et al., 1999; Sarnthein et al., 2006), we omitted spectral data below 4 Hz because of high noise levels, and thus we may have excluded some interesting differences within the delta range. Furthermore, we did not collect MRS and EEG data concurrently, due to practical constraints and inherent technical difficulties. Whilst it is possible that this may have altered the relationships between thalamic GABA and cortical power, we collected MRS and EEG data on the same day, directly after one another and at a similar time of day for all participants. Last, because the voxel size in MRS is large, it is possible we may have collected chemical spectra from other thalamic nuclei as well as the TRN. However, we are confident we acquired spectra in the TRN in all participants, and the GABAergic content of the TRN is indeed well documented.
The results of this study raise important questions about mechanisms of altered resting brain rhythm in chronic neuropathic pain. Given its GABAergic output, it follows that the TRN is an integral part of thalamocortical circuitry; however, we found no direct evidence of altered thalamic GABA concentration in the pain state here, and the relationship between GABA concentration and cortical rhythms is not fully understood. While the thalamus has long been suggested to play a key role in the maintenance of pain, this too has not been fully elucidated. That we found evidence of an association between GABA concentration in the TRN and thalamocortical rhythm in pain subjects, but no such association in controls, is intriguing and offers a new avenue for investigation into the mechanisms possibly underpinning chronic neuropathic pain.
ACKNOWLEDGMENTS
The authors would like to thank many generous subjects that participated in this investigation. They would also like to thank Segar Suppiah for his assistance with all the MRI scanning. The authors declare that they have no conflicts of interest.




