Time-dependent differences in cortical measures and their associations with behavioral measures following mild traumatic brain injury
Funding information: U.S. Army Medical Research and Materiel Command, Grant/Award Numbers: W81XWH-11-1-0056, W81XWH-12-1-0109, W81XWH-12-1-0386
Abstract
There is currently a critical need to establish an improved understanding of time-dependent differences in brain structure following mild traumatic brain injury (mTBI). We compared differences in brain structure, specifically cortical thickness (CT), cortical volume (CV), and cortical surface area (CSA) in 54 individuals who sustained a recent mTBI and 33 healthy controls (HCs). Individuals with mTBI were split into three groups, depending on their time since injury. By comparing structural measures between mTBI and HC groups, differences in CT reflected cortical thickening within several areas following 0–3 (time-point, TP1) and 3–6 months (TP2) post-mTBI. Compared with the HC group, the mTBI group at TP2 showed lower CSA within several areas. Compared with the mTBI group at TP2, the mTBI group during the most chronic stage (TP3: 6–18 months post-mTBI) showed significantly higher CSA in several areas. All the above reported differences in CT and CSA were significant at a cluster-forming p < .01 (corrected for multiple comparisons). We also found that in the mTBI group at TP2, CT within two clusters (i.e., the left rostral middle frontal gyrus (L. RMFG) and the right postcentral gyrus (R. PostCG)) was negatively correlated with basic attention abilities (L. RMFG: r = −.41, p = .05 and R. PostCG: r = −.44, p = .03). Our findings suggest that alterations in CT and associated neuropsychological assessments may be more prominent during the early stages of mTBI. However, alterations in CSA may reflect compensatory structural recovery during the chronic stages of mTBI.
Abbreviations
-
- CSA
-
- cortical surface area
-
- CT
-
- cortical thickness
-
- CV
-
- cortical volume
-
- ESS
-
- Epworth Sleepiness Scale
-
- HCs
-
- healthy controls
-
- MTBI
-
- mild traumatic brain injury
-
- TBI
-
- traumatic brain injury
-
- TP
-
- time-point
-
- TPs
-
- time-points
1 INTRODUCTION
Traumatic brain injury (TBI) is a highly prevalent condition, affecting an estimated 1.7 million annually in the United States (Faul, Xu, Wald, & Coronado, 2010). Of these, it is estimated that ∼75% of injuries can be classified as mild traumatic brain injury (mTBI) (Centers for Disease Control and Prevention (CDC), 2003), often described as “concussion.” Most mTBIs resolve quickly and without complications (McCrea et al., 2003). However, a significant proportion of individuals who sustain an mTBI continue to experience chronic postconcussive symptoms, which may include deficits in attention, concentration, and memory, and chronic complaints of fatigue, headaches, mood lability, and sleep difficulties (Bigler, 2008; Haboubi, Long, Koshy, & Ward, 2001; Packard, 2008; Pare, Rabin, Fogel, & Pepin, 2009). Notably, ∼50% of patients with an mTBI will experience chronic sleep disruption in the months and years after their injury (Orff, Ayalon, & Drummond, 2009), including poor sleep quality, delayed sleep phase, daytime hypersomnia, and/or impaired daytime vigilance (Baumann, Werth, Stocker, Ludwig, & Bassetti, 2007; Castriotta et al., 2007; Makley et al., 2008; Parcell, Ponsford, Redman, & Rajaratnam, 2008; Rao et al., 2008; Verma, Anand, & Verma, 2007; Williams, Lazic, & Ogilvie, 2008). Moreover, the presence of a sleep problem following an mTBI is problematic, as it is typically associated with poorer recovery and exacerbation of neuropsychiatric complications (Gilbert, Kark, Gehrman, & Bogdanova, 2015). Finally, recent evidence suggests that sleep may play a critical role in brain repair and recovery processes by enhancing neurotoxin clearance (Xie et al., 2013) and increasing the proliferation of oligodendrocyte precursor cells, which are necessary for myelin repair and regrowth (Bellesi et al., 2013). Sleep is essential to recovery but patients with mTBI often obtain insufficient quantity and quality of sleep to optimize recovery.
Although the effects of mTBI on specific brain areas and its long-term effect on brain and behavior have been previously investigated (Dean et al., 2013; Dean and Sterr, 2013; McInnes, Friesen, MacKenzie, Westwood, & Boe, 2017), the natural progression of recovery from mTBI has not been clearly documented using multiple structural imaging techniques. For instance, it would be useful to know how cerebral gray and white matter volumes or their morphology differ over the natural course of recovery so that departures from normal can be identified and appropriate interventions initiated as soon as possible. At present, our understanding of the recovery process has been hindered by the inconsistency of injury time frames studied across various investigations. For example, previous studies have explored functional, structural, and symptomatic complaints within 1 month post-mTBI (Ling, Klimaj, Toulouse, & Mayer, 2013; Paniak et al., 2002), 3 months post-mTBI (Laborey et al., 2014; Ling et al., 2013; Wang et al., 2015), and 6 months or more post-mTBI (De Kruijk et al., 2002; Novack, Alderson, Bush, Meythaler, & Canupp, 2000; Zhou et al., 2013). Studies on mTBI, conducted at a given time-point postinjury, provide valuable information about postconcussive symptoms and functional and structural recovery. However, when injury groups are studied in isolation, it is difficult to visualize the larger picture of brain recovery. Therefore, a better understanding of the complex brain mechanisms that unfold in the months following mTBI is needed, which might lead to more reliable and cost-effective rehabilitation techniques for those suffering from mTBI. Keeping that in mind, in our study, we subcategorized mTBI individuals into three groups depending on their time since injury (0–3 months, 3–6 months, and 6–18 months).
In recent years, a number of structural brain measures, such as cortical thickness (CT), cortical volume (CV), and cortical surface area (CSA) have been proposed to be of importance in evaluating changes in brain structure following mTBI (Dall'Acqua et al., 2016; Govindarajan et al., 2016; Zhou et al., 2013). Although these cortical metrics of brain structure tend to covary together to some extent, following an mTBI, they each reflect different facets of morphology that contribute uniquely to overall brain function. Cortical measures also play a potentially important role in evaluating attention abilities and sleep quality (Altena, Vrenken, Van Der Werf, van den Heuvel, & Van Someren, 2010; Spira et al., 2016; Stoffers et al., 2012; Westlye, Grydeland, Walhovd, & Fjell, 2011). For instance, in mTBI patients, significant cortical thinning in the right precuneus and anterior cingulate gyrus was associated with poor performance on memory and attention tasks (Zhou et al., 2013). In patients with persistent insomnia, cortical thinning was reported in the anterior cingulate cortex, precentral cortex, and the lateral prefrontal cortex (Suh, Kim, Dang-Vu, Joo, & Shin, 2016). Reduced CV within the superior frontal cortex was also reported to be associated with poor sleep quality (Chao, Mohlenhoff, Weiner, & Neylan, 2014; Sexton, Storsve, Walhovd, Johansen-Berg, & Fjell, 2014). Reduced gray matter volume within the bilateral lateral orbitofrontal cortices and bilateral inferior frontal gyri pars orbitalis was also associated with sleep interruptions due to repeated awakenings (Lim et al., 2016). Nonetheless, it is unclear the extent to which different structural measures of the brain and their associated capacities pertaining to better attention abilities and sleep vary independently of one another or whether the dynamics of one structural measure depends on the dynamics of another following mTBI. Previously, Mota and Herculano-Houzel (2015) showed the interdependent nature of structural measures, such as cortical folding, CSA, and CT, reporting that the changes in cortical folding depended not only on CSA but also on CT. Taken together, such studies interpret the dependence of brain performance on the integrated impact of surface area and cortical thickness in a healthy brain, but this possibility has not been extended to TBI. While prior structural neuroimaging has not been able to reliably identify consistent morphological changes associated with mTBI, it is conceivable that these metrics, when applied in conjunction with one another, may prove more sensitive to subtle changes during the recovery process.
In this study, our primary goal was to explore differences in multiple brain structural measures, such as CT, CV, and CSA at different stages post-mTBI. Our second goal was to examine the association between the three brain morphology metrics, attentional processes, and sleep-related outcomes for all the regions of interest which showed differences in structural measures at the various time points in the year following injury. We hypothesized that the differences in each of the three brain morphological measures would (i) display unique and significant structural differences across different stages post-mTBI and (ii) show that differences in CT, CV, and CSA would correlate with differences in attention and sleep measures.
2 MATERIALS AND METHODS
2.1 Participants
A total of 87 adults, recruited from the general population within the greater metropolitan area of Boston, MA and New England, participated in this study. Thirty-three participants were included as healthy controls (HCs, mean age = 24.52 ± 3.0 years, 19 female) and 54 participants with a recent mTBI were included in the mTBI group (mean age = 22.40 ± 4.6 years, 33 female, time since injury between 0 and 18 months, mean = 5.73 ± 3.9 months). Any participant from the HC group or mTBI group with any history of drug or alcohol abuse or current use of illicit substances was excluded. Current alcohol use was required to be lower than the Center for Disease Control criteria for excessive alcohol use (www.cic.gov/alcohol). All HCs were recruited as part of a separate study (although no data from these subjects regarding cortical thickness, volume, or surface area have been published previously) but with the same scanning parameters and on the same scanner as the mTBI group. Neuropsychological testing was completed at the Social Cognitive and Affective Neuroscience laboratory located at McLean Hospital. All participants underwent high-resolution anatomical brain imaging using a Siemens Tim Trio 3T scanner (Erlangen, Germany) located at the McLean Hospital Imaging Center.
2.1.1 Inclusion/exclusion criteria for HCs
All the HCs were screened via a comprehensive telephone interview and were excluded if there was any history of psychiatric or neurological disorder, significant medical problems—including head injury, sleep disorders—or current use of psychotropic medications that could affect neuroimaging. Additionally, the inclusion eligibility of all the HCs was determined using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV Axis I Disorders) (SCID) (First, Spitzer, Gibbon, & Williams, 2002). All the HCs met inclusion criteria and none of them met diagnostic criteria for any current/lifetime Axis I disorder.
2.1.2 Inclusion/exclusion criteria for mTBI individuals
An mTBI was defined based on the criteria established by the American Congress on Rehabilitation Medicine (Head, 1993) and later adopted by the Department of Veterans Affairs and the Department of Defense (Management of Concussion/mTBI Working Group, 2009) as a traumatically induced event that was associated with an alteration in mental state (e.g., confusion, disorientation), consciousness (i.e., loss of consciousness <30 min; alteration of consciousness up to 24 h) and post-traumatic amnesia up to 24 h. Individuals with any history of neurological, mood, or psychotic disorder with an onset prior to the mTBI, or who suffered a loss of consciousness exceeding 30 min following an injury were excluded. Although the study was funded by the U.S. Army Medical Research and Materiel Command, none of the participants were active duty military and none of the head injuries were caused by exposure to combat.
2.1.3 Grouping of mTBI individuals
In this study, eligible individuals with mTBI were grouped into one of three subcategories based on time-since injury: <3 months, between 3 and 6 months, and between 6 and 18 months. Eighteen individuals experienced an mTBI (mean age = 24.56 ± 6.09 years, 11 female) within the preceding 3 months (TP1), 22 experienced an mTBI (mean age = 21.77 ± 3.53 years, 14 female) between 3 and 6 months prior to evaluation (TP2), and 14 experienced an mTBI (mean age = 20.61 ± 2.56 years, 8 female) between 6 and 18 months prior to the evaluation (TP3). Groups were different in “age” (F(3,86) = 4.98, p < .05, one-way ANOVA), but not “gender” (χ2(3) = 0.26, p > .05, Pearson's Chi-square). Demographic information of all the groups (HCs and three mTBI groups) is summarized in Table 1.
| Healthy controls | MTBI overall | MTBI (TP1) | MTBI (TP2) | MTBI (TP3) | Statistical | |
|---|---|---|---|---|---|---|
| Demographics | (N = 33) | (N = 54) | (N = 18) | (N = 22) | (N = 14) | significance |
| Mean age (S.D.) | 24.52 (3.0)a | 22.40 (4.6) | 24.56 (6.1)b | 21.77 (3.5) | 20.61 (2.6)a,b | F (3,86) = 4.98* |
| (in years) | ||||||
| Gender | 58 | 61 | 61 | 64 | 57 | χ2(3) = 0.26 |
| (% female) | ||||||
| Time-since-injury (TSI) in months | - | 0 < TSI ≤18 | 0 < TSI ≤ 3 | 3 < TSI ≤ 6 | 6 < TSI ≤ 18 | - |
| ATT | - | 105.02 (13.4) | 104.05 (9.1) | 108.45 (12.9) | 100.86 (17.8) | F (2,53) = 1.47 |
| ESS | - | 8.89 (3.6) | 8.39 (3.6) | 8.86 (4.0) | 9.57 (3.1) | F (2,53) = 0.41 |
| PSQI | - | 6.25 (2.7) | 5.67 (2.4) | 6.59 (2.9) | 6.50 (2.6) | F (2,53) = 0.66 |
- Note. Abbreviation: TP = time-point.
- Superscripts “a” and “b” denote the groups that significantly differ at *p < .05.
2.1.4 Consent, compensation, and IRB approval
Written consent was obtained from each participant before the experiment. Additionally, each participant was thoroughly briefed on the potential risks and benefits of the study and participants were financially compensated for their time. The experimental protocol was approved by the Institutional Review Board of McLean Hospital, Partners Health Care, and the U.S. Army Human Research Protections Office (HRPO). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2 Data acquisition
2.2.1 Magnetic resonance imaging
All participants were instructed to rest, relax, and try their best to stay motionless during scanning. Neuroanatomical data were acquired using a 3D magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence which consisted of 176 sagittal slices (voxel resolution = 1 × 1 mm, field of view (FOV) = 256 mm) with TR/TE/FA/inversion time of 2100 ms/2.30 ms/12°/1100 ms encompassing the whole brain.
2.2.2 Attention and sleep measures
The mTBI participants completed three well-validated assessments: The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, Tierney, Mohr, & Chase, 1998) for attention (ATT), a combination of digit span and coding subtests, the Epworth Sleepiness Scale (ESS) (Johns, 1991) and the Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). No such data were recorded from HCs. The RBANS ATT index is a measure of speed and accuracy of information processing, with a mean of 100 and standard deviation of 15. Here a lower RBANS ATT index score represents difficulty in basic attention processing. The use of the RBANS has been shown to be a clinically valid and reliable screening tool for patients with traumatic brain injury (McKay, Casey, Wertheimer, & Fichtenberg, 2007). ESS measures the severity of daytime sleepiness and PSQI is a measure of sleep problems, which takes into account several facets of sleep, including sleep latency, sleep duration, and sleep disturbances. ESS scores range from 0 to 24, where higher scores represent severe excessive daytime sleepiness and PSQI scores range from 0 to 21, where higher scores represent poor sleep quality. A subset of other, unrelated behavioral data from this mTBI sample have been reported elsewhere (Killgore et al., 2016).
2.3 Data analysis
2.3.1 Identification of affected brain areas following mTBI
The “recon-all” pipeline in FreeSurfer (version 6.0) (https://surfer.nmr.mgh.harvard.edu/fswiki) was used to process anatomical images for all the participants (HCs and individuals with mTBI). Processing involved motion-correction, brain extraction (i.e., removal of skull, skin, neck, and eye-balls), automated transformation to the Talairach co-ordinate system, intensity correction, volumetric segmentation, and smoothing using a 15 mm full-width at half-maximum (FWHM) Gaussian kernel. For each HC and mTBI participant, we visually inspected raw T1-weighted image data to determine any possible imaging artifacts, which could affect FreeSurfer's segmentation accuracy. Accuracy of the FreeSurfer generated skull-stripped brain masks and brain surfaces (pial and white) were visually inspected for all the participants from the HC and mTBI groups. The measures of CT, CV, and CSA were calculated separately for the left and the right hemispheres for each participant. CT is defined as the mean distance from the white–grey matter interface to the nearest point on the pial surface (grey matter–CSF interface) and from that point on the pial surface back to grey/white matter interface (Fischl and Dale, 2000), CV is defined as the amount of grey matter that lies between the white–grey matter interface and pial matter (Winkler et al., 2010), and CSA is the sum of the areas of the triangles making up the surface model and is defined as the extent of the two-dimensional surface enclosed by the outer layer of the cerebral cortex (http://cna.hanyang.ac.kr/research/research02.htm) (Fischl, Sereno, & Dale, 1999) (Figure 1). For vertex-by-vertex general linear model (GLM) estimation across the left and the right cortical surface, CT, CV, and CSA were used as individual dependent variables. This method was used to generate statistical parametric maps to identify the brain areas, which showed significantly different CT, CV, or CSA in those with a mTBI (TP1, TP2, or TP3) compared to HCs and within three TPs (TP1 versus TP2, TP1 versus TP3, and TP2 versus TP3). These statistical maps display the distribution of p values. Effects of “age” (demeaned) and “gender” were regressed out when performing group analyses. As the group-wise sample size in our study is small and the differences in brain structure between HCs and mTBI groups and within the mTBI groups are not expected to be localized finely, we selected a moderately larger smoothing kernel size of 15 mm. Moreover, unlike volume-based analysis, larger smoothing kernel size in surface-based analysis never extends into bone/air/white matter. Furthermore, we used a cluster forming threshold (CFT) of p < 0.01. Multiple comparisons were corrected at a clusterwise statistical threshold (CWP) of p < 0.05 using Monte-Carlo simulations.
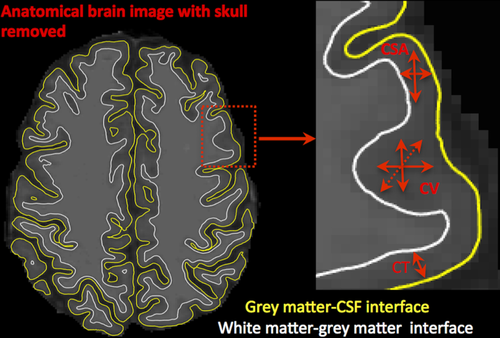
Cortical thickness (CT), cortical volume (CV), and cortical surface area (CSA). Representation of cortical measures (CT, CV, and CSA) within original anatomical brain image [Color figure can be viewed at wileyonlinelibrary.com]
2.3.2 Association between structural measures, ATT, and sleep measures
The method described above was used to generate statistical parametric maps to identify the brain areas, which showed significant differences in CT, CV, or CSA when compared across each of the three time-points and when compared to HC group. Multiple brain regions identified over the whole brain, which showed significant differences in CT, CV, or CSA between mTBI groups and HC group or across time-points (i.e., from TP1 to TP2, and/or from TP1 to TP3 and/or from TP2 to TP3), were selected as regions of interest (ROIs). Subject-wise CT, CV, and CSA of corresponding ROIs were calculated by performing a whole brain parcellation into 34 brain areas using the “Desikan–Killiany” atlas (Desikan et al., 2006). In this atlas, the automated method used to subdivide the human cerebral cortex into 34 cortical ROIs is both anatomically valid and reliable with average intraclass correlation coefficients of 0.835 across all of the ROIs (Desikan et al., 2006). Partial correlation analyses were performed between structural measures (CT, CV, and CSA), attention (RBANS ATT), and sleep measures (ESS and PSQI) for all ROIs identified during the initial analysis, after considering the effects of “age” (demeaned), “gender,” and corresponding whole-brain structural measures. The correlation analysis was performed only for the ROIs; therefore, partial correlations were not corrected for multiple comparisons.
3 RESULTS
3.1 Structural measures for HCs versus three mTBI groups
CT: Compared to HCs, 2 clusters—the right insula and the right superior temporal gyrus (STG)—in the mTBI group at TP1 showed significantly greater CT (Figure 2a–d). Compared to HCs, 4 clusters—the left rostral middle frontal gyrus (RMFG), the left supramarginal gyrus (SMG), the right lateral orbitofrontal cortex (LOFC), and the right postcentral gyrus (PostCG)—in the mTBI group at TP2 showed significantly greater CT (Figure 2e–h). These findings are summarized in Table 2. There were no significant difference in CT between HCs and mTBI group at TP3. Within the three TPs also, we did not find significant differences in CT, that is, for TP1 versus TP2, for TP2 versus TP3, or for TP1 versus TP3.
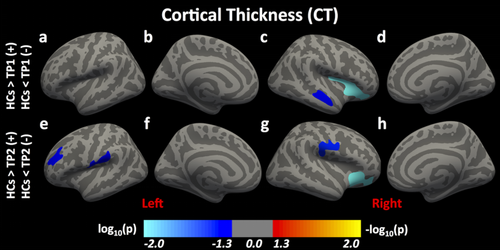
Differences in cortical thickness (CT) following mTBI. Here, we report significant differences in CT between HCs and individuals with mTBI at time-points (TPs) 1 and 2 [Color figure can be viewed at wileyonlinelibrary.com]
| MNIX, MNIY, MNIZ | Annotation | Cluster size | (+) HCs > TP 1/2/3 | |
|---|---|---|---|---|
| Cluster number | (Peak) | (Peak) | (Voxels) | (−) HCs < TP 1/2/3 |
| Cortical thickness (CT): HCs versus mTBI (TP1) | ||||
| Left hemisphere (LH) | ||||
| None | ||||
| Right hemisphere (RH) | ||||
| 1 | 31.5, 21.2, −0.1 | Insula | 3901 | (−) |
| 2 | 48.8, −19.8, −6.3 | Superior temporal gyrus | 1297 | (−) |
| Cortical thickness (CT): HCs versus mTBI (TP2) | ||||
| Left hemisphere (LH) | ||||
| 1 | −32.6, 41.1, 19.3 | Rostral middle frontal gyrus | 1453 | (−) |
| 2 | −56.5, −23.9, 21.4 | Supramarginal gyrus | 1859 | (−) |
| Right hemisphere (RH) | ||||
| 1 | 29.5, 24.9, −8.4 | Lateral orbitofrontal cortex | 2387 | (−) |
| 2 | 62.9, −12.1, 23.7 | Postcentral gyrus | 2280 | (−) |
| Cortical thickness (CT): HCs versus mTBI (TP3) | ||||
| Left hemisphere (LH)/right hemisphere (RH) | ||||
| None | ||||
- Note. Abbreviations: HCs = healthy controls; mTBI = mild traumatic brain injury; TP = time-point.
CV: We did not find significant differences in CV when compared between HCs and any of the three mTBI groups and within three mTBI groups.
CSA: Compared to HCs, 3 clusters—the right PostCG, the right inferior temporal cortex, and the right superior frontal cortex—in the mTBI group at TP2 showed significantly lower CSA (Figure 3a–d). There were no significant difference in CSA between HCs and mTBI groups at TP1 or TP3. These findings are summarized in Table 3. Within the three-mTBI groups, 3 clusters—the left STC, the left PostCG, and the right isthmus of cingulate gyrus—in the mTBI group at TP3 showed significantly higher CSA compared to TP2 (Figure 3e–h). These findings are summarized in Table 4.
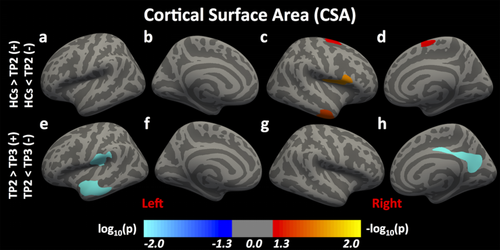
Differences in cortical surface area (CSA) following mTBI. Here, we report significant differences in CSA between HCs and individuals with mTBI at time-point (TP) 2 and between mTBI groups at TPs 2 and 3 [Color figure can be viewed at wileyonlinelibrary.com]
| MNIX, MNIY, MNIZ | Annotation | Cluster size | (+) HCs > TP 1/2/3 | |
|---|---|---|---|---|
| Cluster number | (Peak) | (Peak) | (Voxels) | (−) HCs < TP 1/2/3 |
| Cortical surface area (CSA): HCs versus MTBI (TP1) | ||||
| Left hemisphere (LH)/right hemisphere (RH) | ||||
| None | ||||
| Cortical surface area (CSA): HCs versus MTBI (TP2) | ||||
| Left hemisphere (LH) | ||||
| None | ||||
| Right hemisphere (RH) | ||||
| 1 | 40.9, −3.3, 18.0 | Postcentral gyrus | 2488 | (+) |
| 2 | 46.4, −5.7, −38.7 | Inferior temporal cortex | 1835 | (+) |
| 3 | 23.1, 2.2, 60.9 | Superior frontal cortex | 2047 | (+) |
| Cortical surface area (CSA): HCs versus MTBI (TP3) | ||||
| Left hemisphere (LH)/right hemisphere (RH) | ||||
| None | ||||
- Note. Abbreviations: HCs = healthy controls; TP = time-point.
| mTBI: time-point 2 (TP2) versus time-point 3 (TP3) | ||||
|---|---|---|---|---|
| MNIX, MNIY, MNIZ | Annotation | Cluster Size | (+) TP 2 > TP 3 | |
| Cluster number | (Peak) | (Peak) | (Voxels) | (−) TP 2 < TP 3 |
| Cortical surface area (CSA) | ||||
| Left hemisphere (LH) | ||||
| 1 | −52.2, 7.4, −14.6 | Superior temporal cortex | 3587 | (−) |
| 2 | −56.0, −17.5, 16.2 | Postcentral gyrus | 2892 | (−) |
| Right hemisphere (RH) | ||||
| 1 | 5.4, −47.2, 30.1 | Isthmus cingulate | 3480 | (−) |
3.2 Correlation analysis between structural measures, RBANS ATT, and sleep measures
For two ROIs—the right postcentral gyrus (R. PostCG) and the left rostral middle frontal gyrus (L. RMFG)—there were negative correlations between CT and the RBANS ATT index within TP2 (R. PostCG: r = −.44, p = .03 and L. RMFG: r = −.41, p = .05) (Figure 4a,b). However, the mTBI groups were not significantly different on RBANS ATT (F(2,53) = 1.47, p = .24, one-way ANOVA), ESS (F(2,53) = 0.41, = 0.66, one-way ANOVA) or PSQI (F(2,53) = 0.66, p = .52, one-way ANOVA) (Table 1).
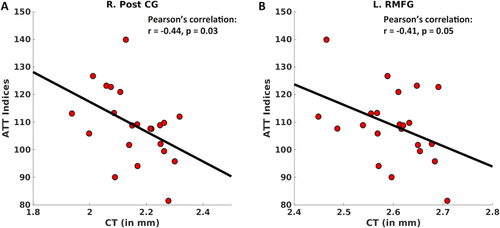
Significant partial correlations between RBANS ATT and cortical thickness (CT). After regressing out the effects of age, gender, and whole-brain CT, here we plot significant correlations found between RBANS ATT and CT for both the ROIs (a) the right postcentral gyrus (R. PostCG) (r = −.44, p = .03) and (b) the left rostral middle frontal gyrus (R. RMFG) (r = −.41, p = .05) [Color figure can be viewed at wileyonlinelibrary.com]
3.2.1 Impressions
Bilateral cortical thickening was observed during the acute stages of mTBI (i.e., within 0–3 and 3–6 months post-mTBI) compared to HCs. During the less acute stage of mTBI (i.e., 3–6 months post-mTBI), CSA was lower as compared to HCs. During the chronic stage of mTBI (i.e., 6–18 months post-mTBI), CSA was higher in comparison to acute stages of mTBI (i.e., 3–6 months post-mTBI). Moreover, in mTBI individuals, higher CT of the left PostCG and the right RMFG within TP2 was associated with lower attention scores.
4 DISCUSSION
In this study, we document time-dependent differences across several measures of brain structure following an mTBI. Our findings suggest that cortical alterations in thickness and their associated behavioral outcomes may occur at early stage of mTBI. However, cortical alterations in surface area are suggestive of trends of potential partial physical recovery with greater time since injury.
4.1 Time-dependent cortical differences following mTBI
CT: In general, previous studies on CT following an mTBI reported thinning and thickening of the cortex (Govindarajan et al., 2016; Wang et al., 2015). It has been suggested that cortical differences might depend on several factors, including the time since injury, symptom severity, regional microedema, localized microhemorrhages, and cytotoxic edema (Lewen, Fredriksson, Li, Olsson, & Hillered, 1999; Wang et al., 2015). It was also suggested that due to subsequent cortical thinning after several weeks, differences in cortical thickness were undetectable at later time points (Govindarajan et al., 2016; Lewen et al., 1999; Tate et al., 2014). However, in this study, compared to HCs, we reported thickening within the right insula and the right STG among mTBI individuals who were between 0 and 3 months of injury and within the left RMFG, left SMG, right LOFC, and right PostCG among mTBI individuals who were between 3 and 6 months of injury.
Recently, brain regions including the insula, STC, and PostCG have been shown to display greater neural activation among individuals with mTBI relative to controls (Dretsch et al., 2017). In that study, it was proposed that several psychological health symptoms such as depression and attentional bias toward negatively valenced stimuli could be responsible for the neural hyperacivation within several regions of interest in the mTBI group. However, the validity of similar mechanisms resulting in cortical thickening within these regions following an mTBI still needs to be confirmed. In a separate study, higher numbers of mTBIs were also associated with reduced CT within the bilateral insula and right middle temporal gyrus (List, Ott, Bukowski, Lindenberg, & Floel, 2015). In that study, it was hypothesized that recurrent mTBIs may induce distinct alterations, especially thinning of the cortex. Consistent with our findings, it was proposed that cortical alterations from the acute phase following an mTBI may normalize in the chronic phase. Moreover, cortical thickening within the right RMFG was reported immediately following an mTBI (Wang et al., 2015). At 3 months post-mTBI, no more cortical thinning was observed in the supramarginal gyrus (Govindarajan et al., 2016). However, we observed thickening of the supramarginal gyrus at 3 months post-mTBI. During the first year after mTBI, changes in CT indicated thickening of the prefrontal cortex, including orbitofrontal cortex in mTBI patients (Dall'Acqua et al., 2017; Wilde et al., 2012). Cortical thickening during initial scans following an mTBI and cortical thinning in later scans may reflect progressive normalization of CT, that is, physical recovery from brain lesions (Lewen et al., 1999; Wang et al., 2015). In addition, the brain areas, such as RMFG, which are more susceptible to direct impacts following a frontal–rear axis head injury, may result in the release of excitotoxins from damaged tissues causing inflammatory reactions, including microedema (Barkhoudarian, Hovda, & Giza, 2011; Lillie, Urban, Lynch, Whitlow, & Stitzel, 2013; Patterson and Holahan, 2012; Urban et al., 2012). These inflammatory reactions have been reported to elevate fractional anisotropy, thicken the cortical regions initially but cause cortical thinning over time with the reduction of microedema (Lewen et al., 1999; Ling et al., 2013).
CV: CV is a composite of both CT and CSA, therefore, changes in CV could be due to changes in either CT or CSA, or both. Therefore, significant increase in CT and significant reduction in CSA or vice versa could be responsible for an unknown CV proportionality across the cortex or even the absence of differences in CV in the three mTBI groups, as observed in our study. Previously in a study on gene identification, it was reported that measures of grey matter volume are less sensitive than CT or CSA, where CT and CSA are also distinct from genetic origins (Winkler et al., 2010). In that study, there was no clear interpretation made from regional grey matter volume differences in terms of genetic influences. In the same study, it was also reported that since the variability in CSA was higher compared to CT, variability on CV might therefore be more associated with CSA as compared to CT. Our findings are partially consistent with these mechanisms as we also found more variability in CT measures as compared to CV and CSA. We acknowledge the fact that the preceding analogy is not ideal and is made between mTBI groups and a gene identification study but the geometrical relationships between these three cortical measures (CT, CV, and CSA) and relatively more dependence of CV on CSA compared to CT may partially explain the underlying mechanisms behind our findings.
CSA: We observed greater CSA at the later stages of mTBI (i.e., 6 and 18 months post-mTBI). Specifically, we found that there were many regions with significantly lower CSA at TP2 compared to HCs but greater CSA at TP3 compared to TP2. The observed differences in CSA contrast with prior findings, as decreases in CSA were previously reported to be one of the earlier existing and sensitive biomarkers for the quantification of brain damage following mTBI (Dall'Acqua et al., 2016). However, larger CSA was shown by others to be associated with complex brain interactions and better cognitive skills (Raznahan et al., 2011; Schnack et al., 2015). In humans, a larger proportion of CSA due to larger surface convolutions is attributed to an extended and dynamic network of brain projections (Hofman, 2014). This generation of an extended dynamic network may not be quick and immediate but might instead be a slow process, which could be the backbone for brain plasticity resulting in compensation of behavioral skills following an injury. Significant increases in CSA, regardless of increases in CT, have also been associated with an increase in radial column units during expansion of the neocortex in primate evolution (Rakic, 2009). Integration of these neocortical columns at higher levels of information processing sets the neural basis of multiple brain regions and their unique features to interact dynamically, which could result in greater synaptic plasticity (Budd and Kisvarday, 2012; Hofman, 2014). Moreover, at the chronic stage of mTBI (i.e., 6–18 months post-mTBI), increases in CV rather than CT and CSA individually, which can account for changes in both CT and CSA, could be an indication of an increase in the formation of dendrites resulting in modest remodeling of the cortex over time (Killgore et al., 2016). These improvements in functional and structural abnormalities could also be closely associated with beneficial neural reorganization of the affected brain hemisphere. Experience-based changes in brain structure over time, also known as experience-dependent neural plasticity, were also found to be beneficial for reducing behavioral and physical disorders (Kerr, Cheng, & Jones, 2011).
In sum, the differences in multiple structural measures following an mTBI might indicate that various brain systems change at different rates. Previous brain imaging studies on mild, moderate, and severe TBI showed that although TBI patients performed equally well as HCs, they recruited a larger number of brain areas, including the frontal and posterior cortices (da Costa et al., 2015; Turner and Levine, 2008). Larger recruitment of these areas during later stages of mTBI could be due to reduced involvement of damaged brain areas immediately after an mTBI or greater compensatory recruitment in more chronic stages. Diffusion imaging studies on TBI have also reported microstructural white-matter alterations that differ at various stages following injury, such as axonal swelling and/or an increase in glial cells (Pasternak et al., 2014), causing variations in CT, CV, or CSA across the recovery period following mTBI.
4.2 Attention and sleep measures following mTBI
We observed that between 3 and 6 months post-mTBI, abnormally higher cortical thickening within the left RMFG and right PostCG compared to HCs, was negatively associated with performance on measures of attention. Previous work has shown that both RMFG and PostCG are reliably associated with attention capacities. For instance, it was reported that the posterior region within the rostral middle frontal cortex is activated by various cognitive tasks, including the ones designed to engage in internal monitoring of action, error and attention (Amodio and Frith, 2006). A positive correlation between thickness within the left posterior middle frontal cortex and performance on a dichotic listening task (a measure of executive attention) further suggested that the left middle frontal cortex is part of an executive attention network (Andersson, Ystad, Lundervold, & Lundervold, 2009). Furthermore, the PostCG or somatosensory cortex, which is the most anterior portion of the parietal lobe, is also one of the three major major sites (intraparietal, postcentral, and precentral) of activation for attention (Corbetta, 1998). A study on a group of right hemisphere stroke patients also suggested a vital role of the PostCG/somatosensory cortex in visuospatial attention (Balslev, Odoj, & Karnath, 2013). Thus, it is clear that the RMFG and PostCG play an important role in attention.
Interestingly, in this study, we did not observe significant differences in attention or sleep measures between any of the three mTBI groups. There was no significant association observed between increased CSA and improved attention abilities or sleep quality. One possible explanation may involve the construct of “cognitive reserve,” or the ability to maintain cognitive functioning in the presence of brain damage or degenerative process (Stern, 2009, 2012). Previously, it was found that increased cognitive reserve might play a protective role against obstructive sleep apnea syndrome (OSAS)-related cognitive decline, including intelligence and attention (Alchanatis et al., 2005). Given the fact that our data did not include specific cognitive measures relevant to a range of sleep disorders, it is beyond the scope of our study to directly confirm that cognitive reserve played a role in the nonsignificant differences in attention and sleep measures across the three time-points. Regarding the sleep measures we used, another possibility is that some of the specific features of sleep biology are not well captured by self-reported measures (Lim et al., 2016). Future research in these areas is therefore needed to investigate these intriguing possibilities further.
4.3 What are the benefits of using multiple structural measures?
In this study, we report the time-line of differences in multiple cortical measures, especially CT and CSA, following mTBI. In particular, we report that when CT was significantly different following mTBI, there were no differences observed in CSA, whereas when CT did not differ across time-points, CSA appeared to be higher, which could be due to greater cortical folding. Human brain development is associated with increased cortical folding, which leads to a progressively more convoluted brain structure and gyrification along spatial and temporal scales (Armstrong, Schleicher, Omran, Curtis, & Zilles, 1995; Richman, Stewart, Hutchinson, & Caviness, 1975). Compared to other species, the folds in the human brain are unique and are associated with specific behavioral skills (Gautam, Anstey, Wen, Sachdev, & Cherbuin, 2015; Gregory et al., 2016). Approximately one-third of the brain's cortical surface is visible, whereas two-thirds of the surface is hidden from view among its folds, leading to overall greater CSA and extra space for the accommodation of additional neurons (Toro, 2012). Cortical folding also shortens the distance of cortical connections by reducing the fiber length necessary between neural regions, resulting in reduced conduction delays across axons (Buzsaki, Logothetis, & Singer, 2013; Chklovskii, Mel, & Svoboda, 2004). Mathematically, there is an interdependent relationship between cortical folding and structural cortical measures. More specifically, it is suggested that the amount of cortical folding increases as CSA increases, where CT becomes an important factor to consider (Mota and Herculano-Houzel, 2015). These relationships suggest that more brain folds lead to more CSA, and thicker cortex could be responsible for restricted brain folds, and both, that is, brain folds or CSA and CT might have unique contribution toward stronger behavioral responses. Therefore, it becomes crucial to consider multiple cortical measures to better understand the time-dependent differences in brain structure following an mTBI or in general.
5 LIMITATIONS
The present findings should be interpreted with consideration of the following, noted, limitations. First, despite having a relatively large sample size for this type of neuroimaging study, we categorized mTBI individuals into only three subcategories based on previous literature. It was, therefore, not possible to examine more fine-grained differences in associations at the acute and subacute periods postinjury. We also suggest that future studies consider employing more precise ranges of time-since-injury onsets, with particular emphasis on explicating the various periods of recovery after 6 months, which would be important for identifying the later recovery mechanisms of mTBI. Second, we did not have attention and sleep data collected from HCs, making it difficult to ascertain the extent to which individuals with mTBI experienced weaker attention abilities, higher daytime sleepiness, and worse sleep quality than the average healthy adult. Finally, the research design of our study is cross-sectional in nature. Consequently, the identified brain clusters reflect significant differences across three discrete time-points and not longitudinal changes over time within a given individual. Future work would benefit from following mTBI patients longitudinally to determine whether the differences observed here are consistent when calculated in a longitudinal design.
6 CONCLUSIONS
In summary, CT and CSA each show unique and specific patterns of differences in brain structure following mTBI. For CT, these patterns of differentiation from HCs and associated weaker attention abilities are most prominent in the first 6 months postinjury. With greater time since injury extending into the short-term and long-term chronic phases, we observe differences in CSA indicative of progressive but partial brain structural recovery, particularly characterized by increased CSA. These findings demonstrate the importance of analyzing multiple brain structural measures in order to more comprehensively understand the neural mechanisms involved following an mTBI, which may reflect brain damage during the early postacute period but compensatory physical recovery during the more chronic stages of mTBI.
ACKNOWLEDGMENTS
This research was supported by grants from the U.S. Army Medical Research and Materiel Command to WDSK (11-1-0056, and W81XWH-12-1-0386) and SLR (W81XWH-12-1-0109). The opinions, interpretations, conclusions, and recommendations in this article are solely those of the authors and are not necessarily endorsed by the Department of Defense or the U.S. Army Medical Research and Materiel Command.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare with regard to this work.




