Relations between cortical thickness, serotonin 1A receptor binding, and structural connectivity: A multimodal imaging study
Funding information: National Institute of Mental Health, Grant/Award Numbers: F30MH109412, K01MH091354, P50MH062185, R01MH074813, R01MH090276, R01MH40695; Columbia University; American Foundation for Suicide Prevention; National Alliance for Research on Schizophrenia and Depression (NARSAD)
Data Acquisition was performed at Columbia University. Data processing and analysis was performed at Stony Brook University.
Abstract
Serotonin 1A (5-HT1A) receptors play a direct role in neuronal development, cell proliferation, and dendritic branching. We hypothesized that variability in 5-HT1A binding can affect cortical thickness, and may account for a subtype of major depressive disorder (MDD) in which both are altered. To evaluate this, we measured cortical thickness from structural magnetic resonance imaging (MRI) and 5-HT1A binding by positron emission tomography (PET) in an exploratory study. To examine a range of 5-HT1A binding and cortical thickness values, we recruited 25 healthy controls and 19 patients with MDD. We hypothesized increased 5-HT1A binding in the raphe nucleus (RN) would be negatively associated with cortical thickness due to reduced serotonergic transmission. Contrary to our hypothesis, raphe 5-HT1A binding was positively correlated with cortical thickness in right posterior cingulate cortex (PCC), a region implicated in the default mode network. Cortical thickness was also positively correlated with 5-HT1A in each cortical region. We further hypothesized that the strength of 5-HT1A-cortical thickness correlation depends on the number of axons between the raphe nucleus and each region. To explore this we related 5-HT1A–cortical thickness correlation coefficients to the number of tracts connecting that region and the raphe, as measured by diffusion tensor imaging (DTI) in an independent sample. The 5-HT1A–cortical thickness association correlated significantly with the number of tracts to each region, supporting our hypothesis. We posit a defect in the raphe may affect the PCC within the default mode network in MDD through serotonergic fibers, resulting in increased ruminative processing.
1 INTRODUCTION
Major depressive disorder (MDD) is predicted to be the leading cause of disability in the world by 2030 (Mathers and Loncar, 2006). In the United States, the lifetime prevalence of MDD is 16.2% (Kessler et al., 2003), and estimated to cost over 200 billion healthcare dollars in 2010. Despite this, an understanding of its pathophysiology has been elusive. Imaging modalities such as magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), and positron emission tomography (PET) can be used to identify structural and functional brain abnormalities associated with MDD, providing information about its underlying pathophysiology. Here we evaluate the relationship of serotonin 1A (5-HT1A) receptor binding in the raphe nucleus as measured by PET binding potential (BPF), a pathophysiological occurrence that can potentially be pharmacologically modulated, to cortical gray matter thickness measured by structural MRI, a structural mechanism that we cannot yet modulate directly. By studying the association between these two phenomena, we can shed light on a biological mechanism that could be disrupted in MDD, and potentially pave the way for additional medical interventions.
1.1 Serotonin 1A receptor function
In the brain, serotonin is synthesized in the raphe nuclei (RN), located in the midbrain, pons, and medulla (Hornung, 2003). The RN of the midbrain is primarily responsible for efferent serotonergic tracts that project to most of the brain. Serotonin released by neurons of the RN activates both 5-HT1A heteroreceptors on the dendrites of post-synaptic target neurons and pre-synaptic 5-HT1A autoreceptors on the dendrites and cell bodies of raphe neurons (Banerjee, Mehta, & Kanjilal, 2007). Somato-dendritic 5-HT1A autoreceptors hyper-polarize serotonin neurons in RN, providing negative feedback control of RN firing rate, and thus of synaptic 5-HT release. Postsynaptic terminal field and heteroreceptors are highly expressed in cortical and other target areas involved in many brain functions including reward processing and behavioral/emotional control (Barnes and Sharp, 1999). Elevated 5-HT1A autoreceptor and heteroreceptor densities have been found in antidepressant naive (AN) MDD patients when compared with healthy controls, using the 5-HT1A antagonist radioligand, [11C]-WAY100635 (Hesselgrave and Parsey, 2013; Parsey et al., 2006; Sargent et al., 2000). 5-HT1A autoreceptor upregulation in MDD may lead to a deficiency in synaptic serotonin, consistent with the monoamine-deficiency hypothesis (Belmaker and Agam, 2008; Kaufman, DeLorenzo, Choudhury, & Parsey, 2016). However, the extent of 5-HT1A autoreceptor elevation in MDD varies widely across patients (Kishi et al., 2009).
1.2 Cortical thickness
In vivo MRI and postmortem studies have reported reduced cortical thickness in MDD patients, with bilateral cortical thinning in the medial orbitofrontal cortex (OFC), fusiform gyrus, insula, rostral anterior and posterior cingulate cortex, and unilateral thinning in the left middle temporal gyrus, right inferior temporal gyrus, and right caudal ACC (Du et al., 2012; Hoexter et al., 2013; Lai, 2013; Schmaal et al., 2016; Singh et al., 2013). However, such results have not been consistent. Our recent study of 222 subjects found no differences in cortical thickness when comparing patients with MDD and those without (Perlman et al., 2017). Moreover, although a meta-analysis in the Enhancing Neuro Imaging Genetics through Meta Analysis (ENIGMA) study found reduced cortical thickness in several brain regions in MDD, these effect sizes were very small (−0.5% to −1.3%) (Schmaal et al., 2016). Indeed, some studies have found increased thickness (Qiu et al., 2014; Reynolds et al., 2014). One study found that cortical thickness in the posterior cingulate cortex (PCC), a key area in the default mode network (DMN), correlated positively with depression severity (Truong et al., 2013).
Should there be a subset of patients for whom cortical thinning does occur, it would be important to know why this happens, and what other factors mediate this thinning. The serotonin system is a promising candidate, as recent evidence suggests SSRI therapy leads to increases in cortical thickness in MDD patients (Kraus et al., 2014; Marano et al., 2015), though these results have also varied by region (Smith, Chen, Baxter, Fort, & Lane, 2014). Interestingly, activity in the DMN (including aforementioned areas such as the PCC), which has been shown to increase in MDD (Dutta, McKie, & Deakin, 2014; Hamilton et al., 2011), has also been shown to be related to serotonin. This has been demonstrated by such measures as platelet serotonin (negative relationship with DMN activity) (Scharinger et al., 2014) and 5-HT1A auto receptor binding (positive relationship with DMN activity) (Hahn et al., 2012).
1.3 Relationship of 5-HT1A binding to trophic effects and cortical thickness
Serotonin has a vital role in early neuronal proliferation and regulation. The 5-HT1A receptor is involved in cell differentiation and cessation of mitosis (Azmitia, 2001). As cortical thickness is dependent in part on the number and size of neuronal cell bodies, this would suggest a negative correlation between cortical thickness and autoreceptor binding, because higher autoreceptor binding results in less serotonin release and signaling. In adults, post-synaptic 5-HT1A receptors contribute to increased dendritic branching via interactions with microtubules and microtubule-associated proteins (Azmitia, 2001; Whitaker-Azmitia, 2001). This is supported by animal studies where serotonin deficiency in adult rodents resulted in the loss of dendrites (Whitaker-Azmitia, Borella, & Raio, 1995); an effect that is reversible with a 5-HT1A antagonist (Azmitia, Rubinstein, Strafaci, Rios, & Whitaker-Azmitia, 1995). This suggests a pathway by which, in a hyposerotonergic condition such as MDD, increased 5-HT1A autoreceptors in the raphe nucleus cause a decrease of serotonin, resulting in decreased dendritic arborization and cortical thickness.
Considering this suggested pathway, an expected correlation between 5-HT1A and cortical thickness should be greatest in regions that are heavily innervated by neurons of the RN. These cortical regions should also be the most susceptible to the effects of serotonin imbalance. Though a previous study did examine 5-HT1A binding in relation to cortical thickness in healthy controls (Kraus et al., 2012), the structural connectivity between the RN and cortical regions was not addressed. To explore this relationship, we can leverage another MRI technique—diffusion tensor imaging (DTI)—to quantify the number of white matter tracts leading from the RN to cortical regions of interest (ROIs).
Here, we examined the relationship of 5-HT1A receptor binding, cortical thickness, and white matter connectivity using a multimodal design. To examine a range of cortical thickness and 5-HT1A BPF values, we recruited patients with MDD and healthy controls. We hypothesized that elevated 5-HT1A autoreceptor binding in the RN would correlate with reduced cortical thickness across all cortical regions. Moreover, we expect that the strongest correlations with raphe 5-HT1A will be seen in cortical areas that have largest numbers of tracts (greatest structural connectivity) to the raphe as measured by DTI.
2 MATERIALS AND METHODS
2.1 PET/MRI participants
The Institutional Review Boards of Columbia University Medical Center and the New York State Psychiatric Institute approved this study. All work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). 44 subjects were enrolled across two groups: 25 controls and 19 MDD patients. Twenty-one participants were male and 23 were female. Mean age of participants was 37.4 (standard deviation: 13.6 years; Table 1). MDD diagnosis was determined via the Structured Clinical Interview for the DSM (SCID), and by a Hamilton Depression Rating Scale (HDRS) score of 15 or higher (Hamilton, 1960). Ages ranged from 18 to 65, and sex was distributed evenly. Exclusion criteria for MDD participants included inability to provide consent, substance dependence in the last 6 months or abuse in the last two months, comorbidity for another axis 1 disorder, any physical illness that may affect the brain, current pregnancy or lactation, active suicidal ideation, aggressive behavior, significant past radiation exposure, and history of head injury. Exclusion criteria for controls were the same as for MDD, except that all axis 1 disorders were excluded, including MDD and a mood disorder in a first-degree relative.
| Demographic | Control | MDD | p valuea |
|---|---|---|---|
| N | 25 | 19 | |
| Age (SD) | 38.4 (14.5) | 35.5 (13.6) | .57 |
| Females (%) | 11 (44%) | 12 (63%) | .22 |
| HDRS-24 score (SD) | 1.2 (1.9) | 26.1 (6.9) | <.01 |
| GG genotypeb (%) | 3 (12%) | 3 (16%) | .71 |
- Note. Abbreviations: PET = positron emission tomography; HDRS-24 = Hamilton Depression Rating Scale, 24 item.
- a t test was used for continuous variables, chi-square for categorical.
- b Serotonin 1A receptor polymorphism (C to G).
Three participants in each group contained the GG genotype for the 5-HT1A promotor polymorphism. We included these participants to maintain power in our study, and due to the fact that we have failed to see a genotype effect on 5-HT1A binding in a large sample (Kaufman et al., 2015).
2.2 DTI participants
To our knowledge, no human study to date has published findings relating binding potential/cortical thickness correlations to the number of tracts projecting from the RN. However, as many of the current cohort did not undergo DTI, tractography was performed on a large separate sample from our database. The mean age of this sample was 36.6 (standard deviation: 12.9) years, and 65% of participants were female. This sample included scans from both healthy controls (n = 39) and patients diagnosed with early onset, chronic, or recurrent MDD (n = 156) using the SCID and Quick Inventory of Depressive Symptomatology (QIDS-SR). Exclusion criteria included (a) previous failure to respond to antidepressant treatment; (b) current pregnancy, fertile but not using contraception, or breastfeeding; (c) history of substance dependence in the last six months or substance abuse in the last two months; (d) lifetime history of psychosis or bipolar disorder; (e) unstable medical conditions that may warrant hospitalization; (f) significant laboratory abnormalities; (g) history of seizure or anticonvulsant use; (h) treatment by other methods including repetitive transcranial magnetic stimulation, vagal nerve stimulation, electroconvulsive therapy, antipsychotics, mood stabilizers, and psychotherapy; and (i) considered to have a high risk of suicide (Trivedi et al., 2016). The study comprised 309 participants: 195 DTI scans passed our quality control measures (as outlined previously (Delorenzo et al., 2013; Olvet et al., 2015)) and were used in the analysis (Table 2).
| Demographic | Control | MDD | p valuea |
|---|---|---|---|
| N | 39 | 156 | |
| Age (SD) | 39.2 (14.3) | 35.9 (12.5) | .21 |
| Females (%) | 21 (54%) | 106 (68%) | .10 |
| HDRS-17 score (SD) | 1.9 (0.4) | 19 (4.4) | <.01 |
- Note. Abbreviation: DTI = diffusion tensor imaging.
- a t test was used for continuous variables, chi-square for categorical.
2.3 MRI acquisition and analysis
Seventeen and 27 participants were respectively scanned using a 1.5 T Signa Advantage or 3 T Signa HDx system (General Electric, Milwaukee, WI) as described previously (Parsey et al., 2010). Previous literature has shown that within-subject variation in cortical thickness between these two field strengths is 0.17 mm (Han et al., 2006). The following parameters were employed. For the 1.5 T MRI: three-dimensional spoiled gradient recalled acquisition in the steady state; repetition time 34 ms; echo time 5 ms; slice thickness 1.5 mm and zero gap; flip angle 45°; 123 slices; field of view 22 × 16 cm; 256 × 192 matrix, reformatted to 256 × 256, resulting in a voxel size of 1.5 × 0.9 × 0.9 mm; and 11 minute acquisition time. For the 3.0 T MRI: three-dimensional spoiled gradient recalled acquisition in the steady state; repetition time 7.3 ms; echo time 3.0 ms; slice thickness 1 mm with 1 mm gap; flip angle 9°; 168 slices; field of view 260 × 260 × 168 mm; 256 × 256 matrix, with a voxel size of 1.0 × 1.0 × 1.0 mm. MRIs were processed in Freesurfer 5.3 (http://surfer.nmr.mgh.harvard.edu/) using techniques described in prior publications (Dale, Fischl, & Sereno, 1999; Dale and Sereno, 1993; Fischl and Dale, 2000; Fischl, Liu, & Dale, 2001; Fischl, Sereno, & Dale, 1999a; Fischl, Sereno, Tootell, & Dale, 1999b; Fischl et al., 2004a, 2004b; Han et al., 2006; Jovicich et al., 2006; Reuter, Rosas, & Fischl, 2010; Reuter, Schmansky, Rosas, & Fischl, 2012; Segonne et al., 2004). The segmented and skull-stripped MRI images were then examined by trained technicians and the quality of tissue segmentation and ROI designation was evaluated—14 images with poor gray/white matter separation were excluded from analysis, which has been shown to increase statistical power in group comparisons (Iscan et al., 2015); these participants were healthy controls scanned on the 1.5 T Signa Advantage scanner. ROIs were based on the Desikan-Killiany atlas, which comprises 34 defined regions per hemisphere (Desikan et al., 2006). Cortical thickness was measured as the average distance between the gray–white matter border and the pial surface of the brain for a given ROI. In addition, bilateral hippocampus and amygdala were included, making a total of 72 regions.
2.4 PET acquisition and analysis
[11C]-WAY100635 radiochemistry and input function measurement was performed as previously described (Parsey et al., 2010). Briefly, WAY100635 ([O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide trihydrochloride) was reacted with radioactive [11C]-CO2 to produce the final radiotracer. Mean injected dose was 6.49 ± 0.4 mCi. During the scan, arterial blood was sampled every 5 s automatically for the first two minutes, and manually at longer intervals after (6, 12, 20, 40, and 60 min). These samples were centrifuged for 10 min and radioactivity in the separated plasma was counted using a gamma counter (Wallac 1480 Wizard 3M Automatic Gamma Counter). To measure metabolite levels, samples collected at 2, 6, 12, 20, 40, and 60 min were processed by protein precipitation with acetonitrile followed by purification via high-pressure liquid chromatography (HPLC). The fraction of unmetabolized 11C-WAY100635 was calculated in these samples, and these fractions were fit to the Hill function, with the value at time zero constrained to 100%. The input function was the product of the interpolated unmetabolized fraction and total counts. The calculated input function values were fit to a sum of three exponentials from the time of the peak to the final data point, whereas the early rising part of the curve was fit to a straight line. These fitted values were then used as input to the kinetic analysis. Plasma-free fraction (fP) was obtained by centrifuging 200 μL aliquots of plasma mixed with tracer; fP was calculated as the ratio of ultrafiltrate to total plasma activity concentrations.
PET images were acquired on an ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN) as described in Parsey et al. (2010). Emission data were collected for 110 minutes at 20 frames of increasing duration. Image processing was performed through MATLAB (The Mathworks, Natick, Massachusetts), with extension to Functional Magnetic Resonance Imaging of the Brain's (FMRIB's) Linear Image Registration Tool (FLIRT v.5.2 (Jenkinson and Smith, 2001)) for coregistration, Brain Extraction Tool v1.2 (Smith, 2002) for skull-stripping, Statistical Parametric Mapping (SPM5) segmentation routines (Ashburner and Friston, 2005) to segment gray matter, white matter, and CSF, and the Advanced Normalization Toolbox (ANTs (Avants, Epstein, Grossman, & Gee, 2008; Avants et al., 2011)) for normalization. Frame-by-frame rigid body registration was performed to a reference frame to correct for subject motion.
A 5-HT1A defined raphe nucleus (RN) ROI was delineated as described previously (Delorenzo et al., 2013) (Figure 1). Briefly, an average 5-HT1A voxel-binding map was created using a previous [11C]-WAY100635 study of 52 healthy controls (Parsey et al., 2010). PET images were warped into a high resolution template space (Holmes et al., 1998) using ANTs (Avants et al., 2008, 2011) and averaged. A thresholding technique was used to extract the RN, as binding is higher in this region than in surrounding areas. The RN was then applied to individual participant's MRI by first warping their MRI to the template space, then using those parameters to inverse-warp the RN ROI into the individual participant's space.
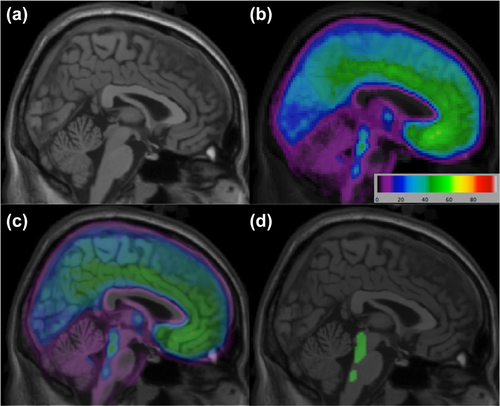
(a) Template brain. (b) Average BPF voxel image for [11C]-WAY100635 PET scans. (c) Merged PET and MRI image. (d) Raphe nucleus region of interest overlaid on template MRI [Color figure can be viewed at wileyonlinelibrary.com]
PET time activity curves obtained from the RN ROI were fit with a two-compartment model with rate constants of radiotracer between blood and tissue compartments constrained to that of the cerebellar white matter. We used these time activity curves and arterial input functions to generate a distribution volume, VT. BPF and BPND were then calculated. BPF is defined as specific regional binding (regional volume of distribution or VT minus nonspecific volume of distribution or VND) normalized to the free fraction of radiotracer in arterial blood or fp; thus BPF = (VT − VND)/fp. BPND is defined as specific binding normalized to binding in a reference region posited to have no specific binding; thus, BPND = (VT − VND)/VND. To derive VND, we used the white matter of the cerebellum as a reference region, as this region was found to be optimal for [11C]-WAY100635 imaging (Hirvonen et al., 2007; Parsey et al., 2005). Standard errors (SE) for both measures were calculated by bootstrapping both PET and plasma data (Parsey et al., 2000).
2.5 DTI weighting
To generate average number of white matter tracts between the raphe and other brain regions, DTI was performed in a separate sample at four different sites (Columbia University (GE Signa HDx), Massachusetts General Hospital (Siemens TrioTim), University of Texas Southwestern Medical Center (Philips Achieva), and University of Michigan (Philips Ingenia)), and all DTI images were transferred to and processed at Stony Brook University. Acquisition parameters were adjusted to standardize imaging prior to the data acquisition. Images were corrected for motion and gradient coil-induced distortions using the eddy current correction routine in FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). Nonbrain tissue was removed through FSL's Brain Extraction Tool. DTI images were assessed for quality by trained technicians to check for artifacts such as venetian blind, gradient-wise motion, ghost, ringing, and slice-wise intensity artifacts (Liu et al., 2010).
Probabilistic tractography was performed using the FMRIB Diffusion Toolbox (FDT) (Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007). FDT computes streamlines through each voxel by repeated sampling from each principal diffusion direction. This method yields the probability of connections from the seed (the RN ROI delineated by PET as described above) to all targets (Desikan-Kiliany-defined MRI labels) in the brain. FDT was run with 5000 samples, a maximum of 2000 steps per sample, a step length of 0.5 mm, and a tract curvature threshold of 0.2 mm. Tract number was calculated by multiplying the number of the 5000 sample fibers that met the threshold criteria by the number of voxels from the seed region. As this value is dependent on input parameters, it was fixed for all participants. All tracts connecting the RN to each region were counted.
2.6 Binding potential/cortical thickness correlation
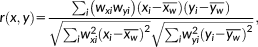
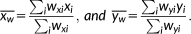
In the above formula, r(x,y) is the weighted correlation coefficient, x and y are observations (BPF/BPND and cortical thickness), and wx and wy are weights given to x and y, respectively. A greater standard error corresponded to a lower weight and vice versa. Specifically, weights were equal to the inverse of the standard errors for BPF/BPND and the inverse of the standard error of mean cortical thickness (i.e., standard deviation of cortical thickness in each region divided by the square root of the number of voxels in each region). The 95% confidence interval (CI) and 95% simultaneous Bonferroni adjusted CI (with an adjustment factor of 72 due to 72 regions being examined) for weighted correlation coefficients were calculated through bootstrapping. No weight was applied to volume measures of the amygdala and hippocampus, the only subcortical regions considered, as their standard errors could not be calculated.
2.7 Relation of tractography to binding potential/cortical thickness correlation
To test the hypothesis that correlation between RN 5-HT1A and cortical thickness would be greatest in regions with the most tracts from the RN, the strength of the weighted correlation between RN binding potential with cortical thickness in each region (r(x,y)) was examined with respect to the mean number of DTI tracts to each region. This resulted in an overall weighted correlation coefficient between tract number and cortical thickness/binding potential association. The inverse of the width of the bootstrapped 95% CI was taken as weights for the RN binding potential–cortical thickness correlation: the wider the CI, the less weight for that correlation coefficient. The inverse of the standard error of the mean (i.e., standard deviation divided by the square root of the number of DTI participants) was used as weights for the number of DTI tracts. Due to the non-normal distribution of the data, we calculated the Spearman's rank correlation coefficient between DTI tracts and the BPF-cortical thickness correlation. Due to potential site-specific differences, we corrected for site in our calculations by fitting a linear regression model to determine the differences between sites, removing calculated differences from all values, and using the adjusted values to calculate correlation coefficients.
2.8 Relation of regional 5-HT1A to cortical thickness
In an additional exploratory analysis, we examined whether postsynaptic cortical 5-HT1A receptors have any relationship with cortical thickness. For each region of the brain, we measured unweighted correlation of average 5-HT1A binding with average cortical thickness across all healthy controls and all patients with MDD. In addition to testing whether or not these two variables were significantly correlated, this method allowed us to see if there was a different pattern of correlation between healthy controls and MDD across all regions.
3 RESULTS
3.1 Raphe BPF–cortical thickness correlation
Weighted correlation coefficients ranged from −0.01 in right paracentral cortex to 0.47 in right posterior cingulate (Supporting Information, Table A.1 and Figure A.1). The vast majority of regions showed positive correlation between raphe nucleus (RN; Figure 1) 5-HT1A and cortical thickness. RN BPF showed significant correlation with regional cortical thickness in two regions after adjustment for multiple comparisons, both on the right side of the brain: inferior temporal cortex (r = 0.36, 95% simultaneous confidence interval (CI): 0.05–0.61) and posterior cingulate cortex (r = 0.47, 95% simultaneous CI: 0.01–0.76; Figure 2 and Table 3). On average, the right inferior temporal cortex received 1,567 (±125) tracts from the RN, and right posterior cingulate had 21,239 (±1327) tracts from the RN (Supporting Information, Table A.2).
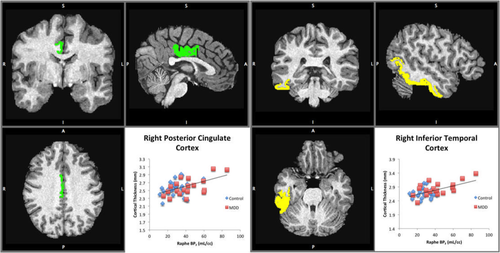
Raphe 5-HT1A BPF significantly correlates with cortical thickness in the right posterior cingulate and right inferior temporal cortex. Left: coronal, sagittal, and axial views are shown, along with the correlation with raphe BPF on the bottom right (BPND correlation not shown). Right: coronal, sagittal, and axial views of inferior temporal cortex are shown, along with the correlation on the bottom right (BPND correlation not shown) [Color figure can be viewed at wileyonlinelibrary.com]
| Region | PET measure | Weighted correlation coefficient | 95% simultaneous confidence interval (Bonferroni adjusteda) | p value (Bonferroni adjusted) |
|---|---|---|---|---|
| Right posterior cingulate cortex | BPF | 0.47 | 0.01–0.76 | .0354 |
| Right inferior temporal cortex | BPF | 0.36 | 0.05–0.61 | .0280 |
| Right posterior cingulate cortex | BPND | 0.40 | −0.06–0.69 | .1854 |
| Right inferior temporal cortex | BPND | 0.19 | −0.21–0.59 | 1.0000 |
- a Bonferroni adjustment is for 72 regions and 20000 times of resampling were used in bootstrapping.
3.2 Raphe BPND–cortical thickness correlation
When we compared raphe BPND to cortical thickness, weighted correlation coefficients ranged from −0.06 in right entorhinal cortex to 0.43 in left paracentral cortex (Supporting Information, Table A.3 and Figure A.2). As with BPF, the majority of regions showed positive correlation. A positive correlation was also found in right posterior cingulate cortex (r = 0.40, bootstrapped 95% CI: 0.16–0.58). However, this correlation did not survive the Bonferroni correction (95% simultaneous CI: −0.06 to 0.69).
3.3 Relation of tract number to 5-HT1A-cortical thickness correlation
When RN BPF to cortical thickness correlations were compared with the number of tracts from the RN to each region, the overall correlation was statistically significant (r = 0.26, CI: 0.10–0.40; Figure 3). When the same analysis was carried out with BPND, a similar positive correlation was found (r = 0.31, CI: 0.19–0.45; see Table 4 and Supporting Information, Figure A.3).
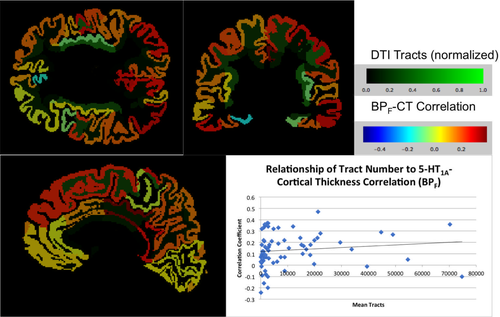
Correlation coefficients are significantly related to the number of tracts from the raphe nucleus to each region. Coronal, sagittal, and axial views of the labeled brain, along with the correlation on the bottom right. White matter tracts (normalized) are indicated by the green color bar while correlation between cortical thickness (CT) and binding potential (BPF) are indicated by the multichrome color bar [Color figure can be viewed at wileyonlinelibrary.com]
| Binding potential measure | Rank-based weighted correlation coefficient | Bootstrapped 95% confidence intervala | p value |
|---|---|---|---|
| BPF | 0.26 | 0.10–0.40 | .0027 |
| BPND | 0.31 | 0.19–0.45 | <.0001 |
- a 1000 times of resampling were used in bootstrapping.
3.4 Regional BPF–cortical thickness correlation
Unweighted correlation between regional 5-HT1A and regional cortical thickness in both groups can be seen in Figure 4. Correlation in controls (0.80) and MDD (0.73) were significant (p < .01 for both), and the slopes of the linear regressions were similar (19.96 mL cc−1/mm in controls versus 23.09 mL cc−1/mm in MDD). The offset was greater in the MDD regression relative to controls, reflecting higher BPF in this group.
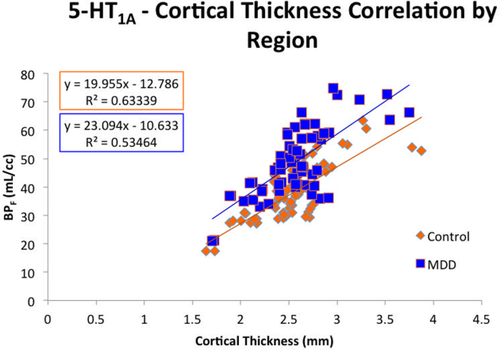
Regional 5-HT1A is significantly correlated with cortical thickness. Control and MDD correlations are plotted adjacent to each other for comparison. Correlation is significant for both groups, and a test for interaction was negative (p = .33) [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
4.1 Positive correlation of 5-HT1A with cortical thickness
In our exploratory study we found, contrary to our hypothesis, higher binding potential in the raphe nucleus (RN) was associated with greater cortical thickness in right posterior cingulate cortex (PCC) and right inferior temporal cortex. A possible explanation for this positive correlation may be found in preclinical studies. Animal studies have found that postsynaptic 5-HT1A may have a neuroprotective role in adults in preventing apoptosis and maintaining dendritic arbors (Bielenberg and Burkhardt, 1990). This is thought to be mediated by the astrocyte protein S-100β, a known inhibitor of apoptosis (Brewton, Haddad, & Azmitia, 2001) whose release is activated by the 5-HT1A receptor (Ahlemeyer, Beier, Semkova, Schaper, & Krieglstein, 2000). Thus, even though an increase in RN 5-HT1A would lead to a hyposerotonergic state, this may result in an increase in astroglial 5-HT1A receptors. Perhaps more importantly, it could result in a sensitization of these receptors and an increase in cortical thickness by this mechanism, which was not included in our initial hypotheses.
Another possible explanation is that higher 5-HT1A autoreceptor binding reflects developmental differences, that is, higher binding corresponds to more serotonergic neurons, greater numbers of serotonergic tracts, and thus more cortical targets, which results in increased cortical thickness through 5-HT1A-mediated dendritic growth and arborization or 5-HT2 mediated proliferation (Whitaker-Azmitia, 2001). The overall result of this may be thickening and subsequent overactivation of the right PCC and right inferior temporal cortex (see below). This hypothesis is supported by our finding that regional postsynaptic 5-HT1A is highly correlated with cortical thickness in both control and MDD participants (Figure 4). Local differences in correlation may be related to which serotonin receptors are more highly expressed—different regions may have different concentrations of 5-HT1A versus 5-HT2A, for example, each exerting a different effect on the cortex. However, given the significant relationship average 5-HT1A and average cortical thickness between regions, regional differences in correlation significance may have been largely driven by variance and our strict Bonferroni correction.
4.2 Effect of structural connectivity
Regardless of the direction of the 5-HT1A–cortical thickness relationship, our hypothesis about structural connectivity was supported. To our knowledge this is the first study quantifying this structural connectivity in a large representative multisite sample. Our data demonstrate that the number of tracts emanating from the RN is significantly related to the 5-HT1A/cortical thickness relationship. However, the number of tracts explains less than 10% of the variability in correlation. This percentage may be due to the anatomy of the brainstem. The RN is in close proximity to other neurotransmitter centers including the locus coeruleus (Ding et al., 2010), brainstem reticular formation (Nauta and Kuypers, 1958), and ventral tegmental area (Swanson, 1982); thus, our tractography from the region of the RN may well have included other non-serotonergic fibers, that is, adrenergic, glutamatergic, and GABAergic axons. We would therefore expect that the number of axon tracts would explain some, but not all, of the interaction between 5-HT1A and cortical thickness, due to the presence of other neurotransmitter axons.
The number of tracts connecting the raphe to various regions varied tremendously, ranging from an average of 99 ± 22 tracts projecting to the left cuneus, to an average of 74,727 ± 3771 tracts projecting to the right insula. Note that we do not expect this variability to be due to diagnosis as we have previously shown that MDD patients do not differ in their connectivity from healthy controls, on average (Olvet et al., 2015). The regions with significant 5-HT1A/cortical thickness correlation had 21,239 ± 1327 tracts, on average, from the raphe in the case of PCC, and 1,567 ± 125 tracts from the raphe in the case of right inferior temporal cortex (Supporting Information, Table A.2). Given the relatively low number of tracts connecting the raphe to inferior temporal cortex, it may be that this connection had a greater concentration of serotonergic fibers relative to other types of fibers. It should be noted that the tract number we calculated is relative, based on our input parameters. Therefore, while it may be suitable for a correlation such as we performed, it should not be taken as an absolute marker for the number of white matter tracts.
4.3 Effect of covariates
As this was a within subject design, we included a heterogeneous group in our primary analysis. However, both 5-HT1A binding and cortical thickness can differ by sex and MDD status (Kaufman et al., 2015; Parsey et al., 2002; Savic and Arver, 2014; Schmaal et al., 2016). Therefore, to remove the effects of these differences, we regressed out these factors by fitting a linear regression model to determine the effects of sex and MDD status on 5-HT1A binding and cortical thickness, removing calculated differences from all values, and using the adjusted values to calculate correlation coefficients (similar to our DTI site corrections described above). Our results were largely consistent, with adjusted right PCC cortical thickness showing significant correlation with adjusted raphe 5-HT1A BPF (r = 0.56, p < .01). The adjusted right inferior temporal cortex correlation coefficient also remains similar (r = 0.33), although the correlation is no longer significant. As stated above, without adjusting for sex and MDD status, there are no significant correlations between raphe BPND and cortical thickness. However, after correction, correlation with adjusted right superior frontal cortex cortical thickness showed significance (r = 0.41, p = .04; Supporting Information, Table A.4). This is an interesting finding given prior EEG studies finding hypoactivation of the left DLPFC and hyperactivation of right DLPFC in MDD (Henriques and Davidson, 1990; Henriques and Davidson, 1991; Saletu, Anderer, & Saletu-Zyhlarz, 2010) and functional MRI studies showing that right sided hyperactivation is associated with negative emotional judgment (Grimm et al., 2008).
In addition, while 15 of the 19 participants with MDD had either never taken antidepressants or not received medication in at least four years, 4 of them were antidepressant exposed within 4 years of the study. Our lab has previously shown that MDD patients who have had recent antidepressant exposure have significantly lower BPF, on average, than those not recently medicated (Parsey et al., 2010), so we also performed analysis excluding these participants. Compared to the analysis performed with these subjects included, correlation between raphe 5-HT1A binding and cortical thickness decreased slightly for right PCC (r = 0.44, down from 0.47) but the correlation between raphe 5-HT1A binding and right inferior temporal cortex cortical thickness was equivalent (r = 0.36). While the latter remained significant, PCC cortical thickness was no longer significantly correlated with raphe 5-HT1A. Given the change in sample size with these subjects removed and the fact that we would not expect antidepressant medication to alter the relationship between raphe 5-HT1A and cortical thickness, we hypothesize this change was due to a decrease of statistical power more than biology.
4.4 Estimation of serotonin 1A binding
Serotonin 1A binding was assessed by PET using the outcome measures BPF or BPND. BPF and BPND are based on different assumptions. However, when we performed analysis using BPF and BPND we obtained similar results. Likely due to the reduced range of the BPND estimates, the correlations with 5-HT1A exhibited reduced statistical power (Supporting information, Table A.3). While we have previously shown that BPND is inadequate for between-group comparisons (Parsey et al., 2010), our congruent findings in this study show that BPND may be suitable for within-participant comparisons, even when these comparisons are multimodal.
4.5 Possible role of the posterior cingulate and inferior temporal cortex
A robust correlation between cortical thickness in the PCC and raphe 5-HT1A binding appears to exist, as this relationship is significant both with and without adjustment for sex and MDD status. The PCC is relatively understudied in MDD, especially compared to its rostral counterpart, the anterior cingulate. However, one context in which the PCC has been studied extensively, is the default mode network (DMN) (Greicius, Krasnow, Reiss, & Menon, 2003), which comprises brain regions that show temporal synchrony when the mind is at rest. The PCC is a major part of this network, and one of this region's primary functions is internally related cognition, for example, autobiographical memory and self-related processes (Brewer, Garrison, & Whitfield-Gabrieli, 2013; Davey, Pujol, & Harrison, 2016; Leech and Sharp, 2014). One theory involving MDD is that aberrant activation of the DMN can lead to symptoms such as self-referent thinking and rumination (Hamilton, Farmer, Fogelman, & Gotlib, 2015; Nejad, Fossati, & Lemogne, 2013). Evidence for this has included increased PCC activation during an induced rumination task (Burkhouse et al., 2016), antidepressant-induced reduction in PCC activation during self-evaluation (Matthews et al., 2010), and an association of decreased DMN activation and positive response to antidepressants (Spies et al., 2017). One study also found that PCC cortical thickness correlated positively with depression severity (Truong et al., 2013).
Based on our findings, we propose a model in which a hyperactive PCC results in increased rumination, which contributes to MDD. Our finding suggests that this might be related to serotonin 1A (5-HT1A) binding. Our model is also supported by the observation that raphe 5-HT1A binding correlates positively with DMN activity in the PCC (Hahn et al., 2012). This study, combined with our findings, may provide an important link between resting-state functional connectivity and PET imaging. In addition, 5-HT1A, the DMN, and cortical thickness may all be related to the symptom domain of rumination, which could provide a useful avenue for future research (Cuthbert and Insel, 2013). Plausibility of this hypothesis is increased by the fact that patients with MDD tend to have higher 5-HT1A binding and cortical thickness in the PCC. Interestingly, a recent study has found that functional resting state connectivity between the PCC and inferior temporal cortex is also associated with rumination and depressive symptoms (Jacobs et al., 2016).
While inferior temporal cortex cortical thickness showed significant correlation with raphe 5-HT1A binding, this effect was not retained when adjusted for sex and MDD status. Nevertheless, due to its relatively high correlation coefficient in both cases, its possible role in MDD is worth considering. The role of the temporal lobe in MDD is unclear, though structural alterations in this area of the brain are thought to reflect an early neurological sign of MDD based on an imaging study of adolescents (Ramezani et al., 2014). In addition, greater temporal lobe cortical thickness has been found in MDD patients with anxiety than MDD patients without (Inkster et al., 2011). Interestingly, despite the association of increased temporal cortex-PCC connectivity with rumination mentioned previously (Jacobs et al., 2016), a separate study found decreased connectivity between these regions in MDD (Yang et al., 2016). Regardless, there is evidence that inferior temporal cortex shows connectivity with the PCC in resting state (Uddin, Kelly, Biswal, Castellanos, & Milham, 2009); therefore, we believe our finding in this region further supports an interaction between 5-HT1A and the DMN.
4.6 Laterality considerations
Significant correlations between 5-HT1A expression and cortical thickness were exclusively found on the right side of the brain. It should be noted that weighted correlation coefficients did not significantly differ between sides of the brain, but on average these coefficients were slightly higher on the right side (BPF: 0.16 on the right side vs 0.11 on the left side; BPND: 0.21 on the right side vs 0.17 on the left side). This accords reasonably well with a previous study that performed a similar analysis comparing cortical thickness and BPND in the raphe, where a slight majority of areas with significant correlation were on the right-hand side (Kraus et al., 2012). Interestingly, this same group also found that 5-HT1A receptor distribution differed on each side of the brain (Fink et al., 2009)—it is therefore possible that hemispheric differences play a role.
4.7 Comparison with previous studies
We did replicate the overall finding of a previous study (Kraus et al., 2012) in that 5-HT1A had a tendency towards positive correlation with cortical thickness, but we did not find significant correlations in the same brain areas. Kraus et al. did not find a positive correlation in the posterior cingulate, while we did. Conversely, we did not find significant correlation in several areas where Kraus et al. observed a correlation, including orbitofrontal cortex, precentral gyrus, and hippocampus. There is, however, overlap in that both studies found a significant positive correlation in the right inferior temporal lobe (Kraus et al., 2012). Differences in methodology between the studies included Kraus et al. including only healthy volunteers and the choice of atlas—they used the Automatic Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002), we chose Desikan-Kiliany (Desikan et al., 2006). Further, while they used a voxelwise approach, we used a regionwise approach. However, as both atlases cover the whole brain and voxel-wise measures were averages in a region, we do not expect this to account for the aforementioned differences. Simialrly, as this is a within-subjects design, we do not expect our inclusion of MDD patients to make a difference. Indeed, our results are consistent even when accounting for MDD status (see Supporting Information). We also used the more conservative Bonferroni criterion as opposed to false discovery rate; we would expect our method to yield fewer significant results due to more stringent statistical techniques.
The biggest difference between our study and that of Kraus et al. may have been our use of standard errors in weighting correlations and our use of a mixed pool of control participants and MDD patients. We believe both of these are advantageous. Our use of bootstrapped standard errors allows us to weight data with lower variance more heavily than that with high variance. We also compared the relationship of regional 5-HT1A to cortical thickness in healthy controls and MDD patients and found them to be very similar (Figure 4). Based on this, we believe that including both groups increases the variance of the data while maintaining validity.
4.8 Limitations
Our study had several limitations. First was its sample size—while more participants were enrolled, several had to be removed for quality control due to errors in gray matter/white matter segmentation by Freesurfer. These quality control measures allow us a more accurate and reliable sample, allowing increased power for analyses relative to the same sized samples in which this quality control has not been performed. In addition, we used two MRI scanners with different field strengths, which may present a potential confound. However, given that within subject variation in cortical thickness as measured by Freesurfer between the two field strengths is only 0.17 mm (Han et al., 2006), and that we were concerned with relative cortical thickness in relation to 5-HT1A binding as opposed to absolute differences in cortical thickness, this concern is mitigated. The exploratory nature of our study did reduce our statistical power substantially—we would encourage others who examine this relationship to use a priori regions that we and others (Kraus et al., 2012) have previously identified, such as the PCC. Other regions such as frontal and entorhinal cortex showed significant correlation, but did not survive multiple comparisons correction (Supporting Information, Tables A.1 and A.3). Finally, we were not able to perform DTI in the same participants as those who had PET scans, which limited our ability to explore individual variation in structural connectivity as it relates to the 5-HT1A-cortical thickness relationship.
5 CONCLUSION
There appears to be a positive correlation between serotonin 1A (5-HT1A) binding in the RN and cortical thickness in posterior cingulate cortex (PCC). This relationship is likely mediated through direct structural connectivity, as the number of white matter tracts from the RN to each region correlated significantly with the strength of this association. Our findings may point to a link between serotonergic transmission and ruminative processing, specifically involving the PCC and inferior temporal cortex. Should this be substantiated with other multimodal studies, it would add valuable insight into the pathogenesis of this poorly understood and often debilitating condition, and suggest the serotonin system as a specific treatment target for patients with MDD prone to rumination. We encourage similar analyses with other diseases that may have pertinence to our findings.
ACKNOWLEDGMENTS
We acknowledge the biostatistical computation and support provided by the Biostatistical Consulting Core at School of Medicine, Stony Brook University, as well as the scientific and data processing contributions of Dr. Laura Kunkel, Dr. Bentley Strockbine, Dr. Geoffrey Raynor, Dr. Michael Budassi, Elizabeth Bartlett, and Jonathan M. Wachtel. We also acknowledge the support in patient recruitment carried out by Dr. Greg Sullivan. The work was supported by the following grants: F30MH109412 (PI: Pillai), R01MH40695 (PI: Mann), P50MH062185 (PI: Mann), R01MH074813 (PI: Parsey), R01MH090276 (PI: Parsey), and K01MH091354 (PI: DeLorenzo) awarded by the National Institutes of Health, a Clinical and Translational Science Award (CTSA) from Columbia University, and grants from the American Foundation for the Prevention of Suicide (AFSP) and the National Alliance for Research on Schizophrenia and Depression (NARSAD).
CONFLICTS OF INTEREST
Dr Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this article. She was the recipient of a grant from Eli Lilly to support a year's salary for the Lilly Suicide Scholar, Enrique Baca- Garcia, MD, PhD. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb. Dr Mann receives royalties for commercial use of the C-SSRS from the Research Foundation for Mental Hygiene and has stock options in Qualitas Health, a start-up company making omega-3-fatty-acid products. Mr Pillai, Dr Malhotra, Ms Rupert, Mr Weschler, Mr Williams, Ms Zhang, Dr Yang, Dr Parsey, and Dr DeLorenzo report no biomedical financial interests or potential conflict of interest.




