Central pain processing in “drug-naïve” pain-free patients with Parkinson's disease
Abstract
Background
Despite its clinical relevance, the pathophysiology of pain in Parkinson's disease (PD) is still largely unknown, and both central and peripheral mechanisms have been invoked.
Objectives
To investigate whether central pain processing is altered in “drug-naive” pain-free PD (dnPD) patients.
Methods
Using event-related functional MRI (fMRI), functional response to forearm heat stimulation (FHS) at two different intensities (41°C and 53°C) was investigated in 20 pain-free dnPD patients, compared with 18 healthy controls (HCs). Secondary analyses were performed to evaluate associations between BOLD signal changes and PD clinical features and behavioral responses.
Results
During low-innocuous FHS (41°C), no activation differences were found between dnPD patients and HCs. During high-noxious FHS (53°C) a significantly increased activation in the left somatosensory cortex, left cerebellum, and right low pons was observed in dnPD patients compared to HCs. In the latter experimental condition, fMRI BOLD signal changes in the right low pons (p < .0001; R = −0.8) and in the cerebellum (p = .004; R = −0.7) were negatively correlated with pain intensity ratings only in dnPD patients. No statistically significant difference in experimental pain perception was detected between dnPD patients and HCs.
Conclusions
Our findings suggest that a functional remodulation of pain processing pathways occurs even in the absence of clinically overt pain symptoms in dnPD patients. These mechanisms may eventually become dysfunctional over time, contributing to the emergence of pain symptoms in more advanced PD stages. The comprehension of pain-related mechanisms may improve the clinical approach and therapeutic management of this disabling nonmotor symptom.
1 INTRODUCTION
Pain symptoms are relatively frequent (Barone et al., 2009; Defazio et al., 2008; Tinazzi et al., 2006) in Parkinson's disease (PD) and may arise during both premotor and motor phases with a disabling effect on patients’ quality of life (Defazio et al., 2013; Tinazzi et al., 2006). Notably, these symptoms are known to be clinically heterogeneous, as they may encompass different causes, such as musculoskeletal, dystonic, radicular, neuropathic and central pain (Ford, 2010; Wasner and Deuschl, 2012). Despite its clinical relevance, the pathophysiology of pain in PD is still largely unknown, and both central and peripheral origins as well as neuropathic and nociceptive mechanisms have been invoked (Scherder et al., 2005; Tinazzi et al., 2008; Wasner and Deuschl, 2012; Zambito-Marsala et al., 2017, 2011).
Over the last years, compelling evidence (Scherder et al., 2005) has supported the idea that neurodegenerative mechanisms in PD may involve brain areas directly or indirectly contributing to the so-called “pain matrix” (Davis, 2000; Garcia-Larrea and Peyron, 2013). This complex network, first described by Melzack (1999), encompasses several brain areas implicated in the supraspinal pain processing (Wasner and Deuschl, 2012). As clarified in a meta-analysis of human data from positron emission tomography (PET), functional magnetic resonance imaging (fMRI), electroencephalography, and magnetoencephalography studies (Apkarian et al., 2005), pain-induced activations are commonly found within primary and secondary somatosensory, insular, anterior cingulate, and prefrontal cortices and thalamus. Moreover, other regions such as basal ganglia, brainstem, cerebellum, amygdala, hippocampus, and areas within the parietal and temporal cortices can also be involved, with a degree of inconsistency between studies mainly related to the presence of several factors influencing pain perception (i.e., cognition, mood, and context). Thus, it has been proposed (Tracey and Mantyh, 2007) that the pain matrix is to be considered not as a single entity but rather as a substrate that is actively modulated by a variety of brain regions. The interplay between these areas determines, in large part, the pain experience (Tracey and Mantyh, 2007).
Previous PET studies (Brefel-Courbon et al., 2005, 2013) have demonstrated abnormal pain-related responses in PD patients under chronic dopaminergic treatment, with or without persistent pain. Thus, these studies have provided evidence that several cortical areas involved in pain perception were recruited during pain stimulation, but no information have been supplied about how the painful inputs have been centrally processed.
fMRI techniques have allowed the in vivo evaluation of the role and function of each brain region and their potential interrelationship, by looking at the blood oxygen level dependent (BOLD) signal fluctuations. As it has been demonstrated that the BOLD signal reflects the firing of neural populations with a strong correlation between its amplitude and local field potential data (Logothetis & Wandell, 2004), it has been suggested that BOLD signal is more related to synaptic activity rather than neural activity per se, providing information about the processing of neuronal information converging at the synaptic level. To date, only a few fMRI studies (Polli et al., 2016, Petshow et al., 2016) with different paradigms and designs (i.e., resting-state, task-related block-design), have been performed in PD patients aiming to study pain processing and perception, ending to controversial findings.
Some of the discrepancies arising from both PET and fMRI studies may stem from potential confounding effects. First, the aforementioned heterogeneous clinical presentation of pain symptoms, which may weaken the definition of inclusion/exclusion research criteria; second, dopamine replacement therapies may influence pain perception (Gerdelat-Mas et al., 2007) and modulate the functional response of pain-related networks (Brefel-Courbon et al., 2005, 2013); third, the presence of chronic pain may itself induce a plastic reorganization of the pain network which may bias interpretation of pain-related responses; finally, fMRI block-designed studies may trigger expectations and cognitive sets, which may potentially affect pain perception and processing (Ploner et al., 2017).
To overcome these issues, we sought to explore central nociceptive processing in early, “drug-naive” PD patients (dnPD) not experiencing pain symptoms, by using an event-related fMRI design. Based on previous neurophysiological evidence (Zambito-Marsala 2017, 2011; Tinazzi et al., 2008), we hypothesize that a functional reorganization of central nociceptive pathways may occur early in the course of the disease even in the absence of clinically overt pain complaints, reflecting specific neuropathological PD-related changes. Indeed, several evidence from neuropathological studies (Braak et al., 2004; Hornykiewicz, 1998) have shown that both noradrenergic and serotonergic pathways are severely affected by neurodegenerative processes during the prodromal stage of PD, prior to the degeneration of the substantia nigra (Braak et al., 2004). Therefore, we hypothesize that these two monoamine systems may determine aberrant central pain-induced responses, even in the absence of pain symptoms.
2 MATERIALS AND METHODS
2.1 Study populations
From November 2015 to November 2016, we screened 62 consecutive dnPD patients attending the Movement disorders unit of the First Division of Neurology at the University of Campania “Luigi Vanvitelli” (Naples, Italy) according to the UK Parkinson's Disease Society Brain Bank Diagnostic Criteria (Gibb and Lees, 1988). Disease severity was assessed using the Hoehn and Yahr (H&Y) stages (Hoehn and Yahr, 1967) and the Unified Parkinson's Disease Rating Scale part III (UPDRS III) (Fahn and Elton, 1987). Global cognitive functions and clinically significant depression were screened by Mini-Mental State Examination (MMSE) (Folstein et al., 1975), Frontal Assessment Battery (Apollonio et al., 2005) and Beck Depression Inventory (BDI) (Beck et al., 1961) respectively. Patients were screened for the presence of pain symptoms by means of a clinical interview and the King's Parkinson's disease pain scale (KPP) (Chaudhuri et al., 2015). Inclusion criteria were (a) PD onset after the age of 40 years to exclude early onset parkinsonism and (b) H&Y stage ranging from 1 to 2. Exclusion criteria were (a) pain related to PD or any disease causing potential acute or chronic pain (KPP score > 0); (b) any previous or actual treatment with dopaminergic or anticholinergic agents, antidepressants or other centrally acting drugs and current use of analgesic drugs; (c) clinically relevant tremor [see Table 1 for tremor subscore (Romenets et al., 2012)]; (d) major depression according to DSM-IV criteria and BDI cut-off score (>9); (e) clinical and neurophysiological (i.e., sensory and motor nerve conduction velocities) evidence of peripheral neuropathy or of any disease potentially causing sensory impairment; (f) cognitive impairment (MMSE < 26); and (g) any other neurological disorder or clinically significant or unstable medical condition.
| Parameters | dnPD (n = 20) mean ± SD | HCs (n = 18) mean ± SD | p value |
|---|---|---|---|
| Age, y | 60 ± 8.9 | 55.9 ± 5.2 | 0.1 |
| Gender (M/F) | 11/9 | 10/8 | 1 |
| Disease duration, y | 1.2 ± 0.5 | - | - |
| H&Y stage | 1.4 ± 0.5 | - | - |
| UPDRS III | 10.1 ± 7 | - | - |
| - Tremor subscores | 2.2 ± 2.9 | - | - |
| Dominant side (R/L) | 10/10 | - | 1 |
| Education, y | 9.8 ± 5 | 10.3 ± 3.7 | 0.1 |
| BDI | 6.8 ± 2.7 | 5.7 ± 2.9 | 0.2 |
| MMSE | 27.7 ± 1.2 | 28.1 ± 0.5 | 0.1 |
| FAB | 9 ± 3 | - | - |
| KPP | 0 | - | - |
- Note. dnPD = drug-naïve Parkinson's disease; HCs = healthy controls; H&Y stage = Hoehn & Yahr stage; UPDRS = Unified Parkinson's Disease Rating Scale; R = right; L = left; MMSE = Mini Mental State Examination; BDI = Beck Depression Inventory; FAB = Frontal Assessment Battery; KPP = King's Parkinson's disease pain scale; BPI = Brief Pain Inventory.
Following these criteria, 20 dnPD patients (11 male, 9 female, mean age ± SD: 60 ± 8.9) were enrolled in the study. All patients were right-handed. We also enrolled 18 age- and sex-matched, right-handed subjects as healthy controls (HCs). HCs were screened for the presence of pain by means of a clinical interview and the Brief Pain Inventory (Cleeland and Ryan, 1994). Exclusion criteria for HCs were (a) painful conditions or any disease causing potential acute or chronic pain; (b) major depression according to DSM-IV criteria and BDI scores (>9) (Beck et al., 1961); (c) any previous or actual treatment with centrally acting drugs and current use of analgesic drugs; and (d) cognitive impairment (MMSE < 26).
2.2 Standard protocol approvals, registrations, and patient consents
The experiments conform to the principles of the Declaration of Helsinki and have been approved by the ethics committee of the University of Campania “Luigi Vanvitelli”, Naples. All participants provided informed, written consent after the experimental procedure had been explained.
2.3 Stimuli
As previously described (Russo et al., 2012; Moulton et al., 2008), contact heat stimulation was performed using the contact heat evoked potential stimulator (CHEPS) (Medoc Ltd, Ramat Yishai, Israel). The CHEPS has a probe with (at one end) a thermode area of 572.5 mm2 and a heating thermo-foil (Minco Products, Inc., Minneapolis, MN), covered with a 25-lm layer of thermo conductive plastic (Kapton®, thermal conductivity at 23°C of 0.1–0.35 W/m/K), characterized by a rapid rising time at high temperature (up to 70°C/s) developed to study pain activation related to thermal and nociceptive pathways. Previous fMRI studies mainly stimulated different body areas such as trigeminal field (Russo et al., 2012; Moulton et al., 2008) and the skin of cervical region, the volar forearm or the dorsum of the hand (Chen et al., 2006). Herein, we chose a selective forearm heat stimulation (FHS). Specifically, to counterbalance the effect of a possible peripheral counterpart of the PD, more prevalent on the more affected side of the body (Nolano et al., 2008), the FSH has been conducted by two predefined stimuli on the side of the body more severely (in the half of the sample population) or less severely affected (Table 1). In HCs side of stimulations were matched with regard to the side of the body tested in dnPD patients. In details, within the dnPD patients group, fourteen patients were stimulated on the right forearm and six patients were stimulated on the left one, whereas within the HCs group, twelve subjects were stimulated on the right forearm and six subjects were stimulated on the left one. A low-innocuous stimulus at 41°C and a painful heat stimulus at 53°C to provide a high-noxious stimulus were used (Russo et al., 2012; Moulton et al., 2008). All experimental stimuli were delivered in random modality during two separate fMRI sessions.
2.4 Imaging parameters
MRI was performed on a 3-T scanner (HDxt, GE Healthcare, USA) equipped with an 8-channel parallel head coil. Each fMRI scan consisted of 300 volumes of a repeated gradient-echo echo planar imaging sequence (repetition time (TR) = 1.500 ms, echo time (TE) = 30 ms, number of axial slices = 29, matrix = 64 × 64, field of view (FOV) = 256 mm, thickness = 4 mm, interslice gap = 0 mm). Three-dimensional T1-weighted images (FSPGR BRAVO sequence, voxel size = 0.9 × 0.9 × 1.2 mm3) were acquired in the same session to have a high-resolution anatomical reference for registration and normalization of the functional images and for brain morphometric analyses. A T2-fluid-attenuated inversion recovery (T2-FLAIR) sequence (RT = 9.000 ms, ET = 1.200 ms, IT = 2.500, axial slices = 44, matrix = 224.448, FOV = 240 mm, thickness = 3 mm, interslice gap = 0 mm) was also acquired in all subjects.
2.5 Experimental protocol
dnPD patients and HCs performed two consecutive fMRI sessions for each of the two different thermal stimuli (41°C and 53°C) according to event-related experimental designs. In each of the two fMRI session, by means of the fMRI-compatible thermode, 28 short thermal stimuli (600 ms) were applied at two different intensities with a jittered inter-stimulus interval (ISI) of 14 ± 1 s (total session duration: 7 min 45 s). Prior to the fMRI experiment, outside the scanner, dnPD patients and HCs were fully informed about the characteristics of the applied heat stimulations, and were asked to focus their attention to the experimental stimulus. Furthermore, all subjects were instructed to verbalize pain ratings: after each fMRI session, there was a delay of about 30 s during which subjects, inside the scanner, verbally rated the intensity perception of the experimental stimulus by means of a numerical rating scale (NRS) ranging from 0 (‘‘no pain’’) to 10 (“worst pain imaginable”), which were then logged in by the experimenter.
2.6 Statistical analysis of demographic, clinical and behavioral data
Differences in the distribution of categorical variables among groups were assessed by means of chisquare. For the analysis of differences among groups regarding demographic, clinical, and behavioral variables we used Mann–Whitney U test to avoid biases due to the small sample size. A p value <.05 was considered statistically significant. Analyses were performed with SPSS version 13 (SPSS Inc. Chicago, IL).
2.7 fMRI preprocessing and statistical analyses
Functional image time-courses were processed using the software package BrainVoyager QX (Brain Innovation, The Netherlands). For each subject and session, the first ten echoplanar images were discarded to allow for magnetization signal full saturation. To correct for movement artefacts, all the scans were realigned to the first included volume scan using a Levenberg–Marquardt algorithm optimizing three translation and three rotation parameters on a resampled version of each image. Besides being used to correct movement artefacts in the data, the motion parameters were carefully inspected to control that no excessive residual motion (>1 functional voxel) was present and then included in the statistical analysis as confounds (see below). The resulting head motion-corrected time series were corrected for the different slice scan times using a cubic spline interpolation procedure and then filtered in the temporal domain. For temporal filtering, a high-pass filter with cutoff set to three cycles per time course (100 s) was used to reduce linear and non-linear trends in the time courses. Using the results of the image registration with three-dimensional anatomical scans, the functional image-time series were warped into Talairach space and resampled into 3-mm isotropic voxel time series. Finally, to reduce intersubject variability in the group-level analysis, the resampled volume time series were spatially filtered (smoothing) using a 6-mm full-width-at-half-maximum Gaussian kernel.
The variance of all image time series was estimated voxel-wise according to a random-effects convolution-based general linear model (GLM) analysis (Friston et al., 1999). For each subject, the two fMRI time series corresponding to the two separate sessions were temporally normalized to z scores (i.e., demeaned and scaled to unit standard deviation) and concatenated before entering the GLM fitting (see below). Two “event-type” GLM predictors of interest encoding the responses to the two stimulus types (41°C and 53°C) were defined using the double-gamma function as hemodynamic input function for the linear convolution (Friston et al., 1998). In total, the design matrix for the multistudy GLM included 10 predictors: 2 predictors of interest for the thermal stimuli, 6 motion confound predictors of no interest, and 1 confound predictor for the “constant” baseline. For each subject and each voxel included in the slab of imaging, the 10 “beta” weights of the 10 regressors were estimated according to a GLM fit-refit procedure which ensured a correction of residual serial correlation in the error terms according to a second-order autoregressive model. To draw population-level inferences from statistical maps, the two beta estimates for the predictors of interest at each voxel entered a second-level analysis of variance with subjects treated as random observations (random effects analysis of variance ANOVA). Before entering the ANOVA, to avoid any potential confounding effect related to the side of stimulation, age and gender, these variables were specified as covariates of no interest in the random effects analysis and therefore regressed out from the series of beta estimates at each voxel. Then, a two-way ANOVA table was calculated, with one within-subject factor for the “temperature” effect (including two levels for the two stimulus types) and one between-subject factor for the dnPD effect (including two levels for the two groups of patients and controls).
From this table, t maps of the one and two group main and differential effects (respectively from one- and two-sample t tests) for each separate stimulus type were computed and overlaid on the Talairach-normalized high-resolution “Colin-27” template. To localize the brain regions with statistically significant main effects, a threshold was applied to the t maps, which protected against false-positive voxels at 5% (corrected for multiple comparisons). To correct functional clusters for multiple comparisons, a cluster-level threshold was applied to the maps that protected against false-positive clusters at 5% (cluster-level corrected for multiple comparisons).
More specifically, starting from an (uncorrected) voxel-level threshold of p = .005, a whole-brain correction approach based on Monte Carlo simulations was used to define the minimum cluster size (Forman et al., 1995).
To test for possible correlations between BOLD responses to thermal stimuli and clinical scores, a region of interest (ROI) was functionally defined from voxels that already exhibited a statistically significant group or interaction effect in the voxel-based analysis. In this case, the individual GLM estimates for the predictors of interest were extracted for each subject and averaged across all ROI voxels, and finally correlated with individual scores.
2.8 Voxel-based morphometry analysis
Anatomical data were processed and examined using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm), where we applied the voxel-based morphometry (VBM) pipeline as implemented in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) with default parameters. This toolbox incorporates the DARTEL toolbox, which was used to obtain a high-dimensional normalization protocol (Ashburner and Friston, 2005). Within this pipeline, the three dimensional T1-weighted images were bias-corrected, tissue-classified, and registered using linear (12-parameter affine) and nonlinear transformations (warping) within a unified segmentation framework (Ashburner and Friston, 2005). Subsequently, the warped GM segments were affine-transformed to the Montreal Neurological Institute (MNI) standard space and were scaled by the Jacobian determinants of the deformations to account for the local compression and stretching that occurs as a consequence of the warping and affine transformation (modulated GM volumes). Finally, the modulated volumes were smoothed with a Gaussian kernel of 8-mm full-width at half maximum (FWHM). The GM volume maps were statistically analyzed using a GLM model and gaussian random field theory. The GLM model included the total intracranial volume (TIV), gender and age as covariates of no interest. The statistical inference was performed at the voxel level, with a family-wise error (FWE) correction for multiple comparisons (p < .05).
3 RESULTS
3.1 Demographic, clinical, and behavioral data
Demographic and clinical parameters of dnPD patients and HCs groups are summarized in Table 1. Pain intensity ratings were not significantly different between dnPD patients and HCs at any level of experimental stimuli (NRS 41°C mean ± SD: dnPD group = 3.3 ± 2.1; HCs group: = 2.8 ± 1.8; p = 0.4; NRS 53°C: dnPD group = 7.2 ± 1.8; HCs group = 6 ± 2.6; p = 0.1).
3.2 Imaging data
During the low-innocuous FHS (41°C), no differences in BOLD response were observed between dnPD patients and HCs. Instead, during high-noxious stimulation (53°C) a significantly increased activation in the left somatosensory cortex (SSC), left anterior cerebellar vermis (i.e., lingula) and right low pons have been observed (p < .05 cluster-level corrected) in dnPD patients compared to HCs (Figure 1). Talairach coordinates of significant cluster differences between groups are reported in Table 2. Mean values of percent BOLD signal changes extracted from the brainstem, cerebellum, and parietal cortex are reported in the figures.

Significant different activations between dnPD and HCs during high-noxious stimulation (53°C). (a–c) Activations clusters and (d–f) bar-graphs of percentage BOLD signal change extracted from significant clusters. dnPD = drug-naïve Parkinson's disease; HCs = healthy controls; SSC = somato-sensory cortex; R = right; L = left [Color figure can be viewed at wileyonlinelibrary.com]
| Stimulus | Anatomical label | BA | Talairach coordinates x, y, z | Cluster size (mm3) | t value |
|---|---|---|---|---|---|
|
High-noxious FHS (53°C) dnPD > HCs |
L Somato-sensory cortex | 40 | −41, −31, 55 | 770 | 4.3 |
| L Cerebellum (lingula) | −6, −48, −15 | 595 | 3.6 | ||
| R low pons | 5, −27, −30 | 1054 | 4.1 |
- Note. R = right; L = left; dnPD = drug-naïve Parkinson's disease; HCs = healthy controls; p < .05 cluster level corrected.
There were no local regional GM differences in both the whole-brain and ROI-based analyses between dnPD patients and HCs, neither at a statistical threshold corrected for multiple comparisons (p < .05 FWE) nor at an uncorrected threshold (p < .001; cluster size: 100).
3.3 Correlation analyses
During high-noxious FHS (53°C), a significant negative correlation was found between BOLD signal change in the right low pons (p < .0001; R = −0.8) and left cerebellum (p = .004; R = −0.7) and NRS scores of experimental pain stimulus in dnPD patients (Figure 2). No correlation was found between BOLD response in the SSC and NRS score. No correlation was found between any other clinical, demographic, and behavioral measures and imaging data.
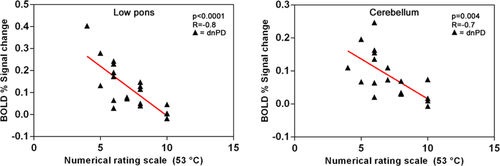
Correlation analysis between pain ratings and percentage BOLD signal change in cerebellum and brainstem clusters. dnPD = drug-naïve Parkinson's disease [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
In this study, we demonstrated that a functional reorganization of the pain-processing network occurs in pain-free, untreated PD patients in an early stage of the disease. A greater activation of the left SSC, left cerebellum and right low pons during high-noxious stimulations is present in dnPD patients when compared to HCs. In dnPD patients, brainstem and cerebellar activations were correlated with pain intensity ratings, which, interestingly, were not significantly different between the two groups at any level of heat stimulation.
Over the last years, compelling evidence (Scherder et al., 2005) has supported the idea that neurodegenerative mechanisms in PD may involve brain areas directly or indirectly contributing to the so-called “pain matrix” (Garcia-Larrea and Peyron, 2013; Davis, 2000). This complex network encompasses cortical and subcortical structures, cerebellum and brainstem, implicated in the supraspinal pain processing (Wasner and Deuschl, 2012). Among these, a prominent role in sensory-discriminative functions of pain is attributed to the SSC (Apkarian et al., 2005). In a previous imaging study the SSC showed a lower pain-induced activation in PD patients with pain symptoms compared to pain-free patients (Brefel-Courbon et al., 2013). These data suggest that an impaired pain-induced response within this area may rely on the development of pain symptoms in PD patients. Therefore, higher order pain processing regions may exert a modulatory function in PD, adjudicating a sensory peripheral input as painful or not.
An additional modulation of central pain processing seems to be exerted by cerebellum, due to its multiple interconnections with cortical areas (primarily frontal and parietal lobes) and sub-cortical brain structures (Borsook et al., 2013; Moulton et al., 2010; Helmchen et al., 2003). Specifically, the cerebellum is implicated in the complex sensory, emotional and neuro-cognitive aspects of pain processing in healthy subjects (Helmchen et al., 2003) as well as in patients with chronic pain conditions (Borsook et al., 2013). A meta-analysis of 47 neuroimaging studies featuring experimental pain revealed specifically localized responses within the cerebellar vermis and bilaterally in the posterior hemispheres (Moulton et al., 2010). Similarly, other studies focusing central pain-induced activations have shown that the anterior cerebellum is the commonest part to be involved in pain processing (Peyron et al., 2013; Stancak and Fallon, 2013, Farrell et al., 2005). This is in line with neuroanatomical studies showing that two ascending nociceptive pathways may rely on the cerebellum: (a) a spino-olivocerebellar pathway that conveys A-delta and C-fiber nociceptive afferent inputs to Purkinje cells in the cerebellar anterior lobe (Ekerot et al., 1991) and (b) a spino-pontocerebellar pathway conveying C-fiber nociceptive inputs to Purkinje cells in the cerebellar vermis (Wu and Chen, 1992). However, as the cerebellum is a very complex structure with a multidimensional role, and the pain perception itself is a composite subjective experience that incorporates sensory, affective, and cognitive components, further studies are required to determine the topography of the cerebellum activations and the clinical relevance in the pain processing at its different levels. Emerging imaging evidence has revealed PD-related pathological and functional compensatory changes of cerebellum even in the early stage of the disease (Wu and Hallett, 2013). Our finding of an increased cerebellar activation is in line with previous task-related and resting-state fMRI evidence of an hyperactivation of bilateral cerebellum with an increased cerebello-prefrontal connectivity during a passive tactile stimulation (Cao et al., 2011) and an increased connectivity of cerebellum (Polli et al., 2016) in PD patients with persistent pain. Taken together, these results support the hypothesis that cerebellum may play a role, either pathological or compensatory, in the development of non-motor symptoms, including sensory deficits (Wu and Hallett, 2013).
Furthermore, we have also observed a pain-induced increased activation in the right low pons, in an area encompassing the nucleus rafe magnus (NRM) and the gigantocellular/paragigantocellular (Gi/PGi) nuclei, in PD patients. The contralateral low pons activation, respect to left SSC and cerebellum, is not surprising. Indeed, many brainstem nuclei involved in pain processing are characterized by contralateral projections to modulate descending anti-nociceptive pathways (Keltner et al., 2006). The brainstem has a crucial role in pain processing. In particular, the diffuse noxious inhibitory controls (DNIC), a pain modulatory network of descending pathways projecting from the brainstem, is an efficient inhibitory control of nociceptive inputs arising from the spinal and medullary dorsal horn to higher centers (Le Bars, 2002). In this context, the NRM and nucleus Gi/PGi complex seem to exert a very important activity (de Oliveira et al., 2006), mediated by noradrenergic and serotonergic transmission (Basbaum and Fields, 1984). Several evidence from neuropathological studies (Braak et al., 2004; Hornykiewicz, 1998) have shown that both noradrenergic and serotonergic pathways are severely affected by neurodegenerative processes during the prodromal stage of PD, prior to the degeneration of the substantia nigra (Braak et al., 2004). Therefore, these two monoamine systems may be disrupted even in the earliest stages of the disease, thereby contributing to the aberrant activation here observed in dnPD patients, even in the absence of pain symptoms. Notably, noradrenergic and serotonergic transmissions seem to be involved in pain processing in PD also during the disease course. Indeed, levodopa administration has been shown to reduce pain-induced activation in several brain areas (Brefel-Courbon 2005, 2013) and modulate pain thresholds (Gerdelat-Mas et al., 2007) in PD patients with and without pain complaints, whereas apomorphine injection did not show a similar effect (Dellapina et al., 2011). This would suggest that the potential analgesic effect of levodopa may be likely related not only to a dopaminergic stimulation but also to its effect on noradrenergic and serotonergic synapses (Carta et al., 2007; Dolphin et al., 1976). In line with this hypothesis, a clinical trial has shown that duloxetine, a selective serotonin and norepinephrine reuptake inhibitor, may improve pain complaints in PD patients (Djaldetti et al., 2007).
Our behavioral findings demonstrate that pain intensity ratings are not different between dnPD patients and HCs. This is apparently in contrast with previous psychophysical, behavioural and neurophysiological studies (Zambito-Marsala et al., 2017, 2011; Gerdelat-Mas et al., 2007; Djaldetti et al., 2004) which have suggested a low pain threshold in both PD patients with and without pain symptoms. However, it should be underlined that most of these works have been conducted on mid-stage PD patients. Moreover, pain intensity is different from pain threshold, as described in previous pain studies (Schwedt et al., 2015).
Taken together, our imaging and behavioral findings suggest that a compensatory reorganization of pain-related brain areas, induced by early neuropathological disease-related changes, occurs in dnPD patients, not reporting pain symptoms. We hypothesize that this functional phenomenon could determine an additional recruitment of pain modulation areas to meet analgesic demands, to maintain proficiency in the early stages of PD. Within this context, the low pons, as a crucial DNIC hub, may play the prominent role, engaging higher pain processing areas, such as SSC and cerebellum, when activated by pain stimulation. These mechanisms may eventually become dysfunctional over time, affecting pain-threshold and perception and contributing to the emergence of pain symptoms in more advanced PD stages. Our hypothesis of an analgesic remodulation of central pain processing is further corroborated by the correlation analyses revealing that low pons and cerebellum activations are negatively associated with pain perception intensity in dnPD patients (i.e., the greater the activations of these areas, the lower the NRS).
It is noteworthy that the observed functional differences between dnPD patients and HCs are not related to any GM structural changes. A recent study (Polli et al., 2016) has revealed that persistent pain in PD patients under dopaminergic treatment is associated with corticometric changes in several brain areas such as prefrontal, insular, and posterior cingulate cortices. Therefore, it is conceivable that a chronic exposure to persistent pain may induce structural changes in pain-related areas that are not detectable in early PD patients not experiencing pain symptoms. However, this assumption should be taken with cautious due to the methodological differences between the two studies. Future longitudinal studies are needed to better investigate the morphometric brain changes induced by a chronic pain exposure in patients with PD.
We believe that this study has some strengths compared to previous observations. First, we have investigated drug-naive PD patients in a very early stage of disease. Second, we have used a validated pain PD scale (i.e., KPP) to rule out the presence of PD related pain in our patients. Third, we randomly stimulated patients on the side of the body more or less severely affected, to partially overcome the confounding effect of a possible PD peripheral counterpart (Donadio et al., 2016; Nolano et al., 2008) on the perception of experimental stimulations. Our patients have not been explored for the presence of cutaneous denervation as previously observed in PD patients (Donadio et al., 2016; Nolano et al., 2008). However, a peripheral deafferention due to somatic small fiber loss has not been yet accurately examined in early, drug-naive PD patients, and to our knowledge, only one study (Nolano et al., 2008) has examined the cutaneous denervation in a small number (n = 4) of patients not taking any dopaminergic treatment.
In conclusion, central pain processing may be disrupted even in early, pain-free, drug-naïve PD patients. We believe that the comprehension of pain-related mechanisms may improve the clinical approach and therapeutic management of this disabling non-motor symptom. Future longitudinal, multimodal imaging studies are needed to clarify the progression of structural and functional changes underlying pain symptoms in PD.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with the content of this article.




