Dynamic functional connectivity of neurocognitive networks in children
The authors declare no conflict of interest.
Abstract
The human brain is highly dynamic, supporting a remarkable range of cognitive abilities that emerge over the course of development. While flexible and dynamic coordination between neural systems is firmly established for children, our understanding of brain functional organization in early life has been built largely on the implicit assumption that functional connectivity (FC) is static. Understanding the nature of dynamic neural interactions during development is a critical issue for cognitive neuroscience, with implications for neurodevelopmental pathologies that involve anomalies in brain connectivity. In this work, FC dynamics of neurocognitive networks in a sample of 146 youth from varied sociodemographic backgrounds were delineated. Independent component analysis, sliding time window correlation, and k-means clustering were applied to resting-state fMRI data. Results revealed six dynamic FC states that re-occur over time and that complement, but significantly extend, measures of static FC. Moreover, the occurrence and amount of time spent in specific FC states are related to the content of self-generated thought during the scan. Additionally, some connections are more variable over time than are others, including those between inferior parietal lobe and precuneus. These regions contribute to multiple networks and likely play a role in adaptive processes in childhood. Age-related increases in temporal variability of FC among neurocognitive networks were also found. Taken together, these findings lay the groundwork for understanding how variation in the developing chronnectome is related to risk for neurodevelopmental disorders. Understanding how brain systems reconfigure with development should provide insight into the ontogeny of complex, flexible cognitive processes. Hum Brain Mapp 38:97–108, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The human brain is intrinsically organized into a complex set of functional networks. These networks engender and constrain a broad range of complex cognitive functions, including language, reasoning, and cognitive control. The structure of cognition is undergirded by ability of brain functional networks to dynamically express various functional configurations. Until recently, however, our understanding of intrinsic connectivity networks (ICNs) has relied on static measures of functional neural connectivity (FC) that assume spatial temporal stationarity throughout the measurement period. Observations that FC within and between ICNs is dynamic, or fluctuating over time [Allen et al., 2014; Chang and Glover, 2010; Hutchison et al., 2013; Sakoglu et al., 2010], underscores the need for detailed examination of FC across discrete time windows. These discoveries of the human chronnectome, or time-varying functional coupling in ICNs, are increasing our understanding of fundamental properties of functional brain networks, and of how neural systems flexibly coordinate to support cognitive and behavioral dynamics [Calhoun et al., 2014].
High-level attentional and cognitive control processes develop over the course of childhood and adolescence [Bunge and Wright, 2007; Luna et al., 2010]. It is increasingly clear that the emergence of these complex processes is supported by reconfiguration and maturation of functional brain networks. Studies of conventional static FC demonstrate that although ICNs are established in early life, even during the fetal period [Thomason et al., 2015], they undergo continued refinement over the first two decades of life by a protracted trajectory of brain development and experiential learning [Fair et al., 2007; Fransson et al., 2011]. Research shows that ICNs become more segregated with age, with age-related increases in within-network FC [Dosenbach et al., 2010; Fair et al., 2007; Sherman et al., 2014; Thomason et al., 2008; Uddin et al., 2011]. There are also age-related changes in between-network integration, with children displaying weaker between-network FC compared with adults [Uddin et al., 2011].
While static FC approaches have provided us with critical information about the topological organization of functional brain networks during development, they say little about the dynamic interplay within and between functional brain networks at the time scale in which cognitive processes occur. Recent advances in the field of dynamic FC have made this all the more accessible [see reviews by Calhoun et al., 2014; Hutchison et al., 2013]. Dynamic FC studies have demonstrated reoccurring patterns of brain FC, or FC “states,” that are reproducible over time and across individuals [Allen et al., 2014; Chang and Glover, 2010]. FC dynamics have shown promise for predicting mental and vigilance states [Shirer et al., 2012] and for characterizing disease [Damaraju et al., 2014a; Rashid et al., 2014]. Because dynamic FC approaches more closely approximate the unfolding of cognitive processes in real time, they may also help us to better understand developmental gains in cognitive and behavioral flexibility. Static and dynamic FC approaches are complementary, and both are needed to understand functional neural organization during early life, and how cognitive functions emerge and evolve over the course of maturation.
Emerging data demonstrate the utility of dynamic FC approaches for understanding functional neurodevelopment. In a recent study of 51 individuals (ages 9–32 years), Hutchison and Morton [2015] found that although the structure and topology of FC states is stable across age, there are age-related changes in the frequency and dwell time (how long an individual spends in a given state) of certain FC states. Additionally, older individuals showed a higher frequency of state transitions during a cognitive control task, but not during rest. Other work demonstrates that connections among ICNs are more variable with increasing age [Allen et al., 2011; Qin et al., 2015]. These findings are in agreement with seminal research by McIntosh et al. [2008] in children (ages 8–15) and young adults (ages 20–33) showing that electrophysiological variability increases with age, which corresponds with reduced behavioral variability and more accurate performance. Together, these data have led to prevailing theory that (1) higher brain variability reflects greater complexity and a greater capacity for information processing, and (2) developmental gains in cognitive functioning are undergirded by increased neural temporal dynamics [Hutchison and Morton, 2016; McIntosh et al., 2008]. Importantly, knowledge of FC dynamics and their possible associations with mental activity during developmental years, when higher-order cognitive functions are emerging, is limited.
In the present study we aimed to characterize dynamic FC, or variation in FC over time, in a sample of 146 youth, ages 7–16 years. To increase the generalizability of our findings, we combined two youth cohorts from varied socioeconomic and demographic backgrounds. We focused on interactions within and between subsystems of core neurocognitive networks thought to be critical for developmental gains in cognitive functioning [see reviews by Menon, 2011, 2013]. These include (i) the frontoparietal central executive network (CEN) anchored in dorsolateral prefrontal cortex and posterior parietal cortex; (ii) the default mode network (DMN) anchored in posterior cingulate cortex, medial prefrontal cortex, and medial temporal lobe, and (iii) the salience network (SN) anchored in anterior insula and anterior cingulate cortex. For completeness, secondary analyses examining whole brain ICNs were also conducted. ICNs were identified using group independent component analysis (ICA), and time-varying FC within and between ICNs was estimated using a series of sliding windows. To identify connectivity states that are quasi-stable, that is, that reoccur over time and are reproducible across subjects, we applied k-means clustering to windowed FC correlation matrices. We leveraged the unconstrained nature of the resting-state, as dynamics are thought to be more prominent during rest periods when individuals freely engage in several forms of mental activity [Delamillieure et al., 2010] that are posited to differentially affect brain functional organization. We also aimed to examine the functional significance of observed FC states by evaluating their correspondence with measures of thought content during the scan, and tested for age-related change in variability in FC between networks, and in frequency of FC states.
The present study extends prior work in at least three ways. First, the prior study by Hutchison and Morton [2015] in 51 individuals (ages 9–32 years) provided critical insight into the functional relevance of particular FC states by evaluating changes in their frequency during a cognitive control task versus rest. The present study complements this work by evaluating, for the first time, the relevance of dynamic FC states for self-generated mental activity in children during the resting-state, which is particularly conducive to mind-wandering [Filler and Giambra, 1973]. We also extend this work by testing a large sample of children from varied demographic backgrounds, consistent with calls for larger, more representative neuroimaging studies [Falk et al., 2013]. Finally, we apply nearly identical analytic strategies as our prior study in adults [Allen et al., 2014], facilitating comparison between studies.
MATERIALS AND METHODS
Participants
Resting-state scans from 146 children and adolescents (youth) were used in this study (mean age: 12.28 years, range 7–16, 57% female). An additional 11 participants were scanned but excluded from the present study because their movement exceeded 2 mm, as described below. Half of the participants (n = 73) were scanned at Stanford University's (SU) Richard M. Lucas Center for Imaging (Stanford, CA), and the other half (n = 73) at Wayne State University's (WSU) Magnetic Resonance Research Facility (Detroit, MI). These samples overlapped in age, t(144) = 1.01, P = 0.311, and data were collected using similar imaging parameters. Importantly, however, the two samples differed in their ethnic and socioeconomic distribution. As shown in Table 1, the SU sample was predominantly Caucasian with annual household incomes of $100,000 or more. In contrast, the majority of WSU participants were African American with household incomes less than $40,000 per year. There were more females in the WSU than in the SU sample, χ2(1) = 6.28, P = 0.009. These samples were combined to achieve a larger, more diverse youth cohort (see Supporting Information Fig. S1 for age and gender distribution, by site).
| SU (n = 73) | WSU (n = 73) | P-value | |
|---|---|---|---|
| Age, m (SD) | 12.47 (1.88) | 12.09 (2.54) | 0.311 |
| Sex, females (%) | 34 (46.57) | 49 (67.12) | 0.009 |
| Pubertal maturation, n (%) | 0.313 | ||
| Pre/early pubertal (Tanner stages 1–2) | 25 (34.25) | 30 (41.1) | |
| Mid/late pubertal (Tanner stages 3–5) | 26 (35.61) | 40 (54.79) | |
| Not reported | 22 (30.14) | 3 (4.11) | |
| Race/Ethnicity, n (%) | <0.001 | ||
| Caucasian | 44 (60.27) | 23 (31.51) | |
| African American | 0 (0) | 32 (43.83) | |
| Latino | 3 (4.11) | 3 (4.11) | |
| Asian | 3 (4.11) | 0 (0) | |
| Mixed race | 19 (26.03) | 7 (9.59) | |
| Not reported | 4 (5.48) | 8 (10.96) | |
| SU Household Income, n (%) | |||
| Less than $50,000 | 9 (12.33) | ||
| $50,000–$75,000 | 11 (15.07) | ||
| $75,000–$100,000 | 10 (13.69) | ||
| $100,000 or more | 25 (34.25) | ||
| Not reported | 18 (24.66) | ||
| WSU Household Income, n (%) | |||
| Less than $40,000 | 41 (56.16) | ||
| $40,000–$60,000 | 14 (19.18) | ||
| $60,000–$80,000 | 5 (6.85) | ||
| $80,000–$100,000 | 2 (2.74) | ||
| $100,000 or more | 8 (10.96) | ||
| Not reported | 3 (4.11) |
- Abbreviations: Stanford University, SU; Wayne State University, WSU; number, n; mean, m. P-values derived from two-sample t-tests for age, and chi-square for sex, pubertal maturation, and race. Income comparison between sites are not available as they were measured by using different ordinal scales.
All participants were fluent in English and had no reported history of brain injury. Pubertal status was assessed for all participants using the self-report Tanner stages questionnaire [Marshall and Tanner, 1968]. Twenty-five participants did not report their Tanner stage (see Table 1). There was a trend for those participants to be younger than those who did report their Tanner stage, t(144) = 1.85, P = 0.066. Parents and youth gave informed consent and assent, respectively, as approved by the local Institutional Review Board.
Post-Scan Questionnaire
Immediately following the MRI scan, participants completed a brief, semi-structured questionnaire that inquired about their internal experiences during the scan (provided in Supporting Information). Children indicated the percent of time they spent thinking about past, present (during scan), or future, and their self versus others. Using a 0–6 point scale (0 = strongly disagree; 6 = strongly agree), participants also quantitatively rated whether they thought about happy or sad things during the scan. Self-report data were available for 66% of the study sample (n = 97 participants), with more data available in the WSU than in the SU sample, χ2(1) = 54.77, P < 0.001. Self-report data were used to ascribe initial functional relevance of dynamic connectivity states, as described below.
fMRI Data Acquisition
Imaging was performed with either a 3 T GE system (SU) or 3 T Siemens Verio scanner (WSU). Imaging protocol and parameters were similar at the two sites. All participants completed a 6-minute resting-state scan, during which they were asked to lie quietly in the scanner with their eyes closed. A total of 180 T2*-weighted blood-oxygenation-level-dependent (BOLD) images were acquired for each participant. At SU, a custom built single-channel quadrature head coil coupled with a T2*-sensitive gradient echo spiral in/out pulse sequence was used (Glover and Law, 2001] with the following parameters: repetition time [TR] = 2,000 ms; echo time [TE] = 30 ms; flip angle = 77°; FOV [field of view] = 220 mm, 29 slices; slice thickness = 4.0 mm. At WSU, T2*-weighted BOLD images were acquired with a 12-channel head coil using echo-planar imaging with the following parameters: TR = 2,000 ms; TE = 25 ms; flip angle = 90°; FOV = 220 mm, 29 slices; slice thickness = 4.0 mm.
fMRI Data Preprocessing
Preprocessing was conducted using the SPM8 software package (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/). The first three frames were removed to allow for signal stabilization. Images were then slice-time corrected, realigned, spatially normalized to the Montreal Neurological Institute (MNI) template, and smoothed using an 8 mm full-width at half-maximum Gaussian kernel. Given our interest in temporal modulation, we normalized voxel time series (using z-score transformation) to minimize possible bias in subsequent variance-based data reduction steps [Allen et al., 2014].
Motion Screening and Spike Removal
We took several steps to reduce the potential influence of motion-related artifact in the data. First, movement was plotted and visually inspected for each participant using ArtRepair software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html). Participants with motion exceeding 2 mm in any direction were excluded from the study (n = 11), leaving a total sample of N = 146. Notably, movement was relatively low for the remaining 146 participants, with translational and rotational mean movement 0.2 ± 0.14 mm and 0.14° ± 0.12°, respectively. Translational and rotational root mean square (RMS) motion was 0.11 ± 0.06 mm and 0.002° ± 0.001°, respectively. Next, as we describe below, we applied a high model order ICA decomposition to minimize noise artifact [Birn et al., 2008]. Additional steps were taken to address potential motion-related artifact in derived component timeseries, following group ICA (“postprocessing”). In particular, high-motion frames were replaced with the best estimate using a third-order spline fit to the clean portions of the timeseries, following prior work [Allen et al., 2014]. Outliers were detected based on the median absolute deviation, as implemented in 3DDESPIKE (http://afni.nimh.nih.gov/afni). On average, 8.28 ± 2.64 frames were removed per component timeseries, for each participant. No more than 20 average frames were replaced across components for each participant, resulting in a minimum of 5.3 min of data. We chose to replace rather than remove high-motion frames, as removing the frames would compromise the subsequent sliding window approach. This approach also ensures that participants contribute an equal number of timepoints to the analysis. Finally, the six realignment parameters and their temporal derivatives were regressed out of the component timeseries [Damaraju and Calhoun, 2014b].
Group ICA and Component Identification
To facilitate comparison with FC states observed in adults, we conducted analyses similar to those used by [Allen et al., 2014]. First, to identify brain functional networks, whole-brain data were decomposed using spatial ICA as implemented in GIFT toolbox (v.3.0a; http://mialab.mrn.org/software/gift/). C = 100 components were derived using the Infomax algorithm. Higher-order ICA yields components that correspond with known anatomical and functional segmentations, and allow investigation of ICN subsystems [cf. Allen et al., 2014]. ICA results in a set of group aggregate spatial maps that are then back reconstructed into single subject space [Calhoun et al., 2001; Erhardt et al., 2011]. Each back-reconstructed component consists of a spatial z-map reflecting the network's coherent activity across space and an associated time course reflecting network activity over time.
A combination of spatial template-matching and visual inspection was used to identify components corresponding to CEN, DMN, and SN. Templates were derived from prior ICA analyses in youth [Thomason et al., 2011; available at www.brainnexus.com/resources/resting-state-fmri-templates) and in adults [Allen et al., 2014]. Components were also evaluated based on expectations that ICNs should be localized primarily in grey matter, have timeseries dominated by low-frequency fluctuations, and low spatial overlap with known vascular, ventricular, motion, and susceptibility artifacts [Allen et al., 2011; Cordes et al., 2000]. Convergence across goodness of fit scores and visual inspection led to characterization of 52 of the 100 components as likely ICNs as opposed to physiological, movement related, or imaging artifacts. Primary analyses focused on 25 components that corresponded with CEN, DMN, and SN. Results from the full 52 components are provided in the Supporting Information.
Postprocessing
Postprocessing was applied to component timeseries to remove remaining noise sources, including scanner drift and movement-related artifact. This included the removal of low-frequency trends (high-frequency cutoff of 0.15 Hz), and linear, quadratic, and cubic detrending. Movement “spikes” were addressed with outlier detection and removal, and with nuisance regression of the six realignment parameters and their temporal derivatives, as described above. Prior work shows that these stringent postprocessing steps do not fundamentally alter dynamic FC structures [Allen et al., 2014].
FC Estimation
ICA-derived individual participant ICN maps were used to estimate dynamic FC and standard static FC between components. Static FC was estimated from the timeseries matrix, as the (C × C) sample covariance matrix, controlling for site. Dynamic FC was estimated with a sliding window, by computing between-component correlation values from tapered windowed segments. Segments were tapered by convolving a rectangle (width = 22 TRs = 44 s) with a Gaussian (σ = 3 TRs), and slid in steps of 1 TR, resulting in W = 158 windows. Covariance of ICNs was estimated, as described previously [Allen et al., 2014]. Final dynamic FC estimates for each window were concatenated to form a C × C × W array representing the changes in covariance (correlation) between networks (components) as a function of time.
Dynamic States and Clustering
To assess the frequency and structure of reoccurring FC states, we applied k-means clustering (using the L1 distance function) to windowed covariance matrices. Only covariances between the 25 ICNs corresponding with CEN, DMN, and SN were used in the clustering analysis, resulting in 25 × (25 − 1)/2 = 300 features. We determined the number of FC states (or “clusters”) to be six using the elbow criterion of the cluster validity index, which is computed as the ratio between within-cluster distances to between-cluster distance. Cluster “centrotypes” are then used as starting points to cluster all the dynamic FC data. To examine the structure of dynamic FC states across all subjects, we evaluated group-level FC states.
FC Variability and Zones of Instability
FC estimates between some ICNs exhibit greater temporal variability than others. We estimated temporal variability in FC by calculating the standard deviation across the sliding window correlation. Larger standard deviations indicate more variable (or less stable) functional connections between ICNs. “Zones of instability” (ZOI) are defined as connections that are more variable over time [Allen et al., 2014].
Relation of Dynamic FC to Internal Thought
To ascribe functional significance to observed resting-state dynamic FC states, we evaluated subject-specific FC data for correspondence with thought content reported in the post-scan questionnaire. In particular, we investigated (1) mean dwell time, or how long a participant is in each state; and/or (2) fraction of (total) time spent in each state. We evaluated the correspondence of the FC measures with the percent of time youth thought about: (1) self- versus others; (2) past, present, or future; and (3) had positive versus negative thoughts. We also examined the effects of age on dwell time and fraction of time spent in each state. To further control for possible contaminating effects of subject motion, we included subject-wise RMS motion as a nuisance regressor in follow-up analyses.
Age Effects
We tested for age-related change in (1) temporal variability between ICNs, (2) dwell time and fraction of time spent in each FC states, and (3) number of state transitions during the scan.
RESULTS
Core Neurocognitive Networks
To identify ICNs, we first performed group-level ICA on resting-scan scans of 146 youth. We identified 52 components as likely ICNs, and focused on 25 of those that correspond with subsystems of DMN, SN, and CEN (Fig. 1). Static and dynamic connectivity results for the full 52 components are provided in Supporting Information. Overall, derived ICNs are similar to those observed in previous high model order ICA decompositions [Allen et al., 2014; Hutchison and Morton, 2015; Smith et al., 2009]. Component information and spatial maps are provided in Supporting Information.
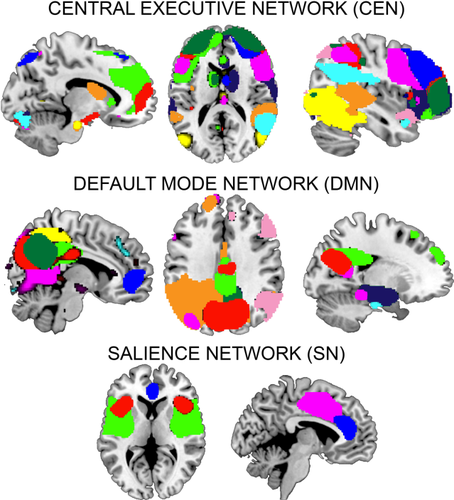
Core neurocognitive networks. Group independent component analysis was used to parcellate the brain into intrinsic connectivity networks (ICNs). See Supporting Information Table S1 for color key and more detailed information on each component ICN. [Color figure can be viewed at wileyonlinelibrary.com.]
Static FC
Figure 2 displays standard static FC, or FC computed over the entire scan length and averaged over participants, for the CEN, DMN, and SN subsystems. Patterns of FC are consistent with previous findings of positive correlation within ICNs, particularly within DMN, and negative correlation between DMN and CEN subsystems [Buckner et al., 2008; Thomason et al., 2008].
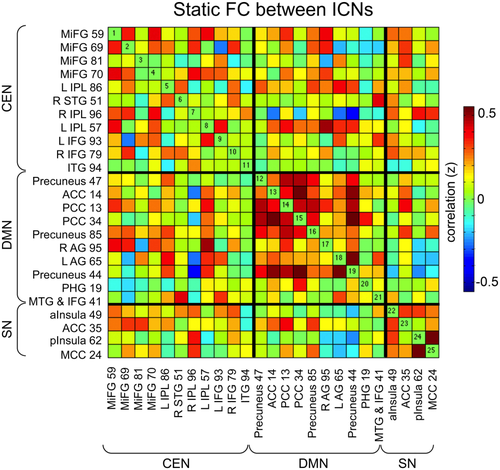
Static functional connectivity (FC) of core neurocognitive networks in children. FC was averaged for all participants for the entire length of the resting-state scan to produce the static correlation matrix. Intrinsic connectivity networks (ICNs) are labeled with their corresponding component number. See Supporting Information Table S1 for more detailed information on each component. Abbreviations: left, L; right, R; middle frontal gyrus, MiFG; inferior parietal lobe, IPL, superior temporal gyrus, STG; inferior frontal gyrus, IFG; anterior cingulate cortex, ACC; posterior cingulate cortex, PCC; angular gyrus, AG; parahippocampal gyrus, PHG; middle temporal gyrus, MTG; anterior insula, aInsula; posterior insula, pInsula; middle cingulate cortex, MCC. [Color figure can be viewed at wileyonlinelibrary.com.]
Dynamic FC
Using sliding windows and k-means clustering, we identified six highly structured FC states that re-occurred throughout the scan and across participants. FC states are arranged in order of emergence in Figure 3. State 1 is characterized by strong within-SN FC, anticorrelation between SN and DMN, and sparse positive FC between CEN and DMN subsystems. State 1 is unique in that connectivity within DMN is weak, including weak connectivity of the precuneus ICN with other DMN subsystems. This state is observed less frequently than are other detected states, but nonetheless represents a FC pattern that diverges substantially from the mean. State 2, which accounts for greater than 30% of windows, is characterized by weak connectivity among all ICNs. This state of diffuse integration resembles a FC state previously observed in adults, which may reflect the average of a number of additional states that are not sufficiently distinct or frequent to be separated [see Allen et al., 2014].
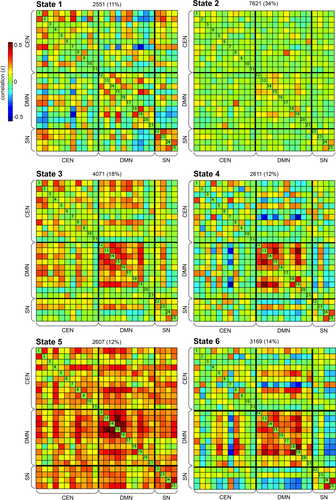
Dynamic functional connectivity (FC) states in children. Dynamic FC states are derived using k-means clustering. Total number and percentage of occurrences is listed above each state. Intrinsic connectivity networks (ICNs) are divided into central executive (CEN), default mode (DMN) and salience (SN) sub-networks. See Figure 1 and Supporting Information Table S1 for abbreviations and more detailed information on each component. [Color figure can be viewed at wileyonlinelibrary.com.]
Following State 2, State 3 appears most frequently and shows strong synchrony (i.e., positive FC) within both CEN and DMN, and between CEN and DMN. State 4 is characterized by synchrony within posterior DMN networks, which also displays strong negative correlations with select CEN components (e.g., right inferior parietal lobe). State 5 is characterized by a state of hyperconnectivity; this is the only state with positive FC between nearly all DMN and CEN subsystems. State 6 is characterized by synchrony within DMN, and strong anticorrelation between DMN and CEN subsystems. In State 6, there is strong integration of posterior insula and middle cingulate cortex ICNs of SN. Across participants, we did not observe a clear increase or decrease in the frequency of any state over the course of the scan.
Zones of Instability
We observed greater FC variability between some ICN pairs than others (see Figure 4). Connections with the greatest variability, so-called “ZOIs” [Allen et al., 2014], were observed between DMN and CEN components, and within CEN. ZOIs frequently involved the right inferior parietal lobe of CEN and the precuneus of DMN.
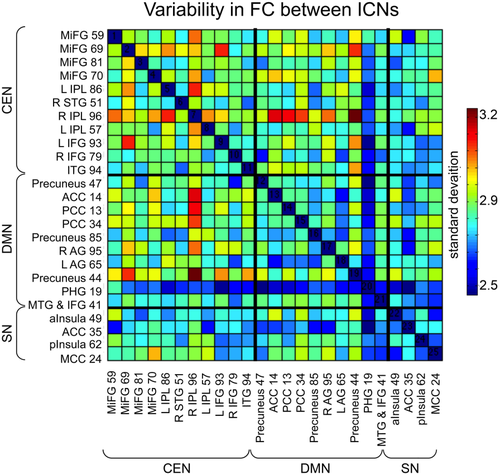
Variability in connections over time. Larger standard deviations indicate more variable, or less stable, connections over the course of the measurement period. See Figure 1 and Supporting Information Table S1 for abbreviations and more detailed information on each component. [Color figure can be viewed at wileyonlinelibrary.com.]
Relation to Post-Scan Questionnaire
To ascribe initial functional significance to observed re-occurring resting-state connectivity states, we correlated subject-specific dynamic FC values with self-reports of spontaneous thought immediately following the scan. Four out of the six observed FC states (2, 4, 5, and 6) were significantly correlated with dimensions of internal thought. Mean dwell time in State 2 was positively correlated with self-focused thought, r(97) = 0.22, P = 0.031, and negatively correlated with thoughts about others, r(95) = −0.309, P = 0.002. Participants who reported more positive thoughts spent a larger fraction of time in State 4, r(93) = 0.273, P = 0.008. Participants who reported thinking more about others spent a larger fraction of time and remained longer in State 5, r(95) = 0.235, P = 0.022, and r(95) = 0.259, P = 0.011, respectively. Finally, participants who reported thinking more about the past spent a larger fraction of time and remained longer in State 6, r(96) = 0.266, P = 0.009, and r(96) = 0.266, P = 0.009. All associations remained significant when RMS head motion was included as a nuisance regressor.
Age Effects
Increased age was associated with increased overall temporal variability between neurocognitive networks, r(146) = 0.172, P = 0.038. Age was positively associated with the fraction of time spent in State 4, r(146) = 0.198, P = 0.017, and negatively associated with the fraction of time spent in State 2, r(146) = −0.267, P = 0.001. These associations remained significant when RMS head motion was included as a nuisance regressor. Age was not significantly associated with the number of state transitions, r(146) = 0.084, P = 0.314.
DISCUSSION
Interactions within and between core neurocognitive networks, that is, CEN, DMN, SN, are considered critical for behavioral shifts and adaptive processes. Emerging data suggest that network FC is not stationary but rather, fluctuates, giving rise to highly structured patterns of FC that reoccur over time [Allen et al., 2014; Chang and Glover, 2010]. Here, we characterize dynamic FC states in a relatively large sample of youth (n = 146) from varied sociodemographic backgrounds. We found six highly structured patterns of FC that deviate, in part, from static FC averaged over the entire resting-state scan. The overall amount of time children spent or how long they remained (i.e., dwell time) in particular FC states corresponded with different types of self-reported mental activity during the scan. Although the number of state transitions did not change with age, we found age-related increases in the variability of FC among core neurocognitive networks, and differences in the time children remained in particular FC states. We discuss these results in turn, and suggest that continued research in this area will increase our understanding of how the functional architecture of the brain is established in early life, forming the backbone for the emergence of complex cognitive processes. Research in this area may also illuminate the role of dynamic FC in neurodevelopmental disorders, an idea supported by recent findings in adults with psychiatric disorders [e.g., Damaraju et al., 2014a].
Our dynamic FC analyses identified six reoccurring FC states that depart significantly from the static FC pattern. These findings, together with prior research in adult and pediatric samples [Allen et al., 2014; Hutchison and Morton, 2015; Qin et al., 2015], caution against a labeling scheme in which ICNs are treated as singular and stable entities. Rather, ICNs show significant flexibility in functional coordination with other brain systems. For instance, established “core” SN regions, such as anterior insula and anterior cingulate cortex, exhibited synchronous activity only in certain states (1, 5). Similarly, precuneus ICNs (DMN) exhibited affiliations with the DMN module in only some states (3–6), similar to prior observations in adults [Allen et al., 2014]. As Allen and colleagues point out, variable connectivity between ICNs may inform questions regarding the inclusion of the precuneus in the DMN [see Buckner et al., 2008].
We observed particularly variable connections between the CEN and DMN. These networks are highly integrated in the adult brain [Cole et al., 2010], suggesting that heterogeneous and integrative function between the CEN and DMN is established earlier in life, at least in childhood. Within these networks, connections involving the precuneus of DMN and the inferior parietal lobe of CEN were highly variable. These regions are among the most globally connected in the brain [Cole et al., 2010] and are characterized as core hubs of integration [Hagmann et al., 2008]. The inferior parietal lobe also emerged as a ZOI in adults [Allen et al., 2014], suggesting a stable, critical role for this ICN during childhood and adulthood, which may rest on its ability to flexibly associate with other ICNs. The precuneus, in contrast, did not emerge as a prominent ZOI in adults [Allen et al., 2014]. This is striking given that the precuneus is flexibly associated with other DMN subsystems in both children and adults, but a high level of integration across other neurocognitive networks was observed in children, here, but not in adults. This disparity suggests that the precuneus ICN has a privileged role in the DMN, particularly in early life [Yang et al., 2014].
Characterizing how dynamic FC evolves over the course of development should yield insight into neural factors involved in the range of increasingly complex cognitive functions that emerge across childhood and adolescence. For instance, we found age-related increases in the variability of network coupling, which is consistent with prior electrophysiological findings [McIntosh et al., 2008]. Greater functional neural variability with age may afford greater cognitive and behavioral flexibility [Hutchison and Morton, 2015; McIntosh et al., 2008]. In line with the prior dynamic FC study in 9–32 year olds [Hutchison and Morton, 2015], we found no significant relation between age and the number of FC state transitions during rest. Notably, age was positively associated with the number of states expressed during a cognitive control task [Hutchison and Morton, 2015]. Thus, what appears to be changing with age for self-generated cognition during rest is the type of FC states that are expressed, rather than the frequency of transitioning between different states. For externally directed cognition, in contrast, there does appear to be more rapid switching between FC states with age. Together, these findings suggest that developmental changes in the functional neural backbone underlying internally versus externally directed cognitive processes are distinct and may thus require several complementary measures.
Four of the six dynamic FC states covaried with retrospective self-reports of thought content during the scan. Amount of self-focused thought correlated with frequency of FC State 2, which was characterized by weak and diffuse connectivity between all ICNs. A state of weak FC was also observed in adults in the Allen et al. [2014] study, in which the authors speculate that this state signifies the average of a large number of additional states that are not sufficiently distinct or frequent to be separated. Based on our findings, it is possible that one or more of these diffuse FC states are involved in self-referential processing in children, which is known to take several forms and may recruit both separate and overlapping brain systems [Farb et al., 2007; Moran et al., 2009]. In contrast to State 2, State 6 was associated with thinking about others. This state was characterized by synchrony within DMN, and anticorrelation between DMN and CEN. This observation is consistent with the extensive apparent overlap between the DMN and regions of the “social brain” [Mars et al., 2012].
Children who reported more positive thoughts spent a larger amount of time in State 4, and children who reported more thoughts about the past spent a larger amount of time and stayed longer in State 6. States 4 and 6 were both characterized by synchrony within DMN, and anticorrelation between DMN and CEN. The DMN has been implicated in both past and positive thinking [Gorgolewski et al., 2014]. Notably, fraction of time spent in State 4 increased with increasing age, whereas State 6 did not show age-related effects. State 4 also differed from State 6 in that within-DMN correlations were more heavily concentrated among posterior DMN ICNs, and DMN-CEN negative correlations involved a more restricted number of CEN ICNs. Thus, while similar networks were involved in both positive and past-related thoughts, the pattern of FC within- and between networks differed. Investigation of dynamic FC patterns may elucidate how similar neural substrates are involved in an array of mental activities. Overall, associations with self-generated thought centered almost exclusively on DMN connections rather than SN and CEN. This is likely due to our focus on characterizing FC dynamics during the resting state, which is highly linked to DMN activity, and complements prior work comparing FC dynamics during task and rest [Hutchison and Morton, 2015].
It is important to consider several factors in interpreting our findings. The first factor involves the quantity of data available for each participant. Although we were able to achieve a relatively large and heterogeneous sample by combining data across two sites, each participant contributed a modest amount (6 min) of resting-state data. Longer scan times should improve estimates of FC variability by allowing patterns of connectivity to reoccur several more times. This should also provide greater specificity in identifying FC patterns that correspond with individual differences in reports of self-generated mental activity. Second, although we took several experimental (pretraining) and analytic steps (ICA-based denoising, motion outlier removal, motion regression) to ensure that dynamic FC patterns were driven by ICNs rather than noise, faster sampling times and concurrent physiological recordings are needed to rule out possible influences of time-varying noise. A third consideration is the use of a retrospective questionnaire to inquire about thought content during the scan. Given the unconstrained nature of resting-state, it is difficult to interrogate mental states without disrupting ongoing spontaneous processes. Innovative approaches are emerging that should offer greater precision in mapping cognitive states to neural FC states [Christoff et al., 2009; O'Callaghan et al., 2015]. Even so, there may be important individual differences that emerge in truly unconstrained states. Here, we found associations with thought content dimensions on a post-scan questionnaire, providing clues about the relevance of observed dynamic FC states for ongoing mental activity in children.
CONCLUSIONS
Using a sliding window analysis, we characterized resting-state FC dynamics among core neurocognitive networks in 146 children. We found six FC states that reoccurred over time and were consistent with and significantly expanded on standard static FC averaged across the experiment. Providing initial insight into their functional roles, some FC states corresponded with content of self-generated thought reported by children, supporting a link between brain dynamics and underlying mental experience. In addition, some connections were more variable over time, including inferior parietal lobe and precuneus. These regions exhibited membership in multiple-large scale systems, suggesting a unique role in adaptive processes in early life. With age, there were differences in the amount of time children expressed certain FC states, and age-related increases in the variability in FC among core neurocognitive networks, suggesting greater neural complexity. Continued characterization of the developing chronnectome should yield new insights into the maturation of human brain networks that support higher-level cognitive processes, and new avenues for identifying mechanisms that underlie neurodevelopmental disorders.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors thank Allison Li, Clara Zundel, Farah Sheikh, Tarek Bazzi, Nataliya Bukavyn, Sameen Jaffry, Allesandra Iadipaolo, and Farrah Elrahal at Wayne State University and Emily Dennis at Stanford University for assistance in participant recruitment and data collection, and the children and families who generously shared their time.