Effect of rs1063843 in the CAMKK2 gene on the dorsolateral prefrontal cortex
The authors declare no potential conflicts of interest for this manuscript.
Abstract
Recently, a single nucleotide polymorphism (SNP) in the CAMKK2 gene (rs1063843) was found to be associated with lower expression of the gene in the dorsolateral prefrontal cortex (DLPFC) and with schizophrenia (SCZ) and deficits in working memory and executive function. However, the brain mechanism underlying this association is poorly understood. A functional magnetic resonance imaging (fMRI) study (N = 84 healthy volunteers) involving multiple cognitive tasks, including a Stroop task (to measure attentional executive control), an N-back task (to measure working memory), and a delay discounting task (to measure decision making) to identify the brain regions affected by rs1063843 was performed. Across all three tasks, it was found that carriers of the risk allele consistently exhibited increased activation of the left DLPFC. In addition, the risk allele carriers also exhibited increased activation of the right DLPFC and the left cerebellum during the Stroop task and of the left caudate nucleus during the N-back task. These findings helped to elucidate the role of CAMKK2 in cognitive functions and in the etiology of SCZ. Hum Brain Mapp 37:2398–2406, 2016. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Schizophrenia (SCZ) is a severe heritable mental disorder with an estimated heritability of 81% [Sullivan et al., 2003]. Since 2008, genome-wide association studies (GWAS) have suggested many candidate SCZ risk genes, including ZNF804A, TCF4, CACNA1C, and MIR137 [Ferreira et al., 2008; O'Donovan et al., 2008; Ripke et al., 2011; Stefansson et al., 2009]. Almost all of these candidate genes are thought to be associated with an impaired dorsolateral prefrontal cortex (DLPFC), suggesting that this brain region is important in the etiology of SCZ [Bigos et al., 2010; Thurin et al., 2013; van Erp et al., 2014]. A recent genome-wide expression study (eGWAS) performed on DLPFC brain tissue suggested that one of the most promising SCZ candidate genes is calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) [Luo et al., 2014]. This gene is downregulated in the DLPFC of SCZ patients [Luo et al., 2014]. Luo et al. [2014] further searched for genetic variants within the gene and in its putative regulatory regions (50 kb upstream and downstream of the gene) that may regulate its expression and found that three single nucleotide polymorphisms (SNPs) were significantly associated with expression levels of CAMKK2 in human DLPFC. However, only a SNP in intron 17 of CAMKK2 (rs1063843, C/T) was independently verified. This SNP, rs1063843, was also found to be significantly associated with SCZ (sample size: 17,154 patients and 113,469 controls) with the T allele that was associated with relatively low expression of CAMKK2 mRNA was more common among SCZ patients. This association has been replicated by a recent, independent study [Atakhorrami et al., 2016].
The gene encoding CAMKK2 is located at 12q24.2 and includes 18 exons [Hsu et al., 2001]. The most well-characterized substrates of CAMKK2 include calcium/calmodulin-dependent protein kinase 1 (CAMK1), calcium/calmodulin-dependent protein kinase 4 (CAMK4), and AMP-activated protein kinase (AMPK) [Anderson et al., 1998; Hurley et al., 2005]. CAMKK2 can phosphorylate these substrates in a calcium/calmodulin-dependent manner [Hsu et al., 2001]. CAMKK2 in the brain acts primarily through CAMK1 and CAMK4. In animal studies, loss of CAMKK2, CAMK1, or CAMK4 impaired neuronal differentiation and migration, neurite outgrowth, and synapse formation [Michaelsen and Lohmann 2010; Peters et al., 2003; Saneyoshi et al., 2008; Takemoto-Kimura et al., 2010; Wayman et al., 2008], all of which play important roles in brain and cognitive functions. Human studies have reported an association between rs1063843 and cognitive functions including executive function and working memory, as well as intrinsic and extrinsic motivation [Luo et al., 2014]. However, evidence for a neural mechanism underlying this association is lacking.
In this study, we explored the neural mechanism of the association between rs1063843 and cognitive function using functional magnetic resonance imaging (fMRI) data obtained from 84 subjects during three tasks: a Stroop task, an N-back task, and a delay discounting task (DDT). These tasks measure attentional executive control, working memory, and decision making, respectively, all of which have been reported to activate the DLPFC in previous fMRI studies [Boettiger et al., 2007; Callicott et al., 2003; Zhang et al., 2015]. We hypothesized that the rs1063843 risk allele would affect the activation of the DLPFC during all tasks.
MATERIALS AND METHODS
Subjects
A sample of 84 healthy controls was included in this fMRI study. Most of these subjects had been involved in one of our previous studies [Zhang et al., 2015]. All of the subjects completed the Stroop task and the N-back task during fMRI scanning. In addition, most subjects (N = 58) performed the DDT. Several subjects were excluded from task-specific data analyses due to excessive head motion (>3 mm or 3°): six for the N-back task, four for the Stroop task, and four for the DDT. All subjects were recruited by an advertisement and were screened for any personal or family history of psychiatric disorders by experienced psychiatrists during an unstructured interview. All had normal or corrected-to-normal vision and were right-handed, as assessed by the Edinburgh handedness inventory. Detailed demographic factors are presented in Table 1.
| Mean ± SD | F or χ2 | P | ||
|---|---|---|---|---|
| TT/TC | CC | |||
| N-back task | ||||
| N of subjects | 32 | 46 | ||
| Gender (male/female) | 23/9 | 37/9 | 0.78 | 0.377 |
| Age (years) | 27.50 ± 7.61 | 26.39 ± 4.92 | 0.61 | 0.437 |
| Education (years) | 12.66 ± 3.15 | 13.41 ± 2.90 | 1.20 | 0.277 |
| IQa | 114.06 ± 9.40 | 116.02 ± 11.32 | 0.65 | 0.424 |
| Accuracy: 2-back | 0.86 ± 0.15 | 0.86 ± 0.16 | 0.01 | 0.925 |
| Reaction time: 2-back (seconds) | 0.36 ± 0.11 | 0.35 ± 0.92 | 0.42 | 0.517 |
| Stroop task | ||||
| N of subjects | 32 | 48 | ||
| Gender (male/female) | 23/9 | 38/10 | 0.56 | 0.453 |
| Age (years) | 27.78 ± 7.49 | 25.96 ± 4.93 | 1.73 | 0.193 |
| Education (years) | 12.50 ± 3.25 | 13.44 ± 3.04 | 1.73 | 0.193 |
| IQa | 115.25 ± 9.01 | 115.04 ± 12.25 | 0.007 | 0.935 |
| Incongruent accuracy | 0.92 ± 0.05 | 0.92 ± 0.06 | 0.093 | 0.762 |
| Congruent accuracy | 0.99 ± 0.02 | 0.98 ± 0.03 | 0.180 | 0.672 |
| Conflict effect (seconds)b | 0.24 ± 0.11 | 0.25 ± 0.12 | 0.017 | 0.898 |
| DDT task | ||||
| N of subjects | 21 | 33 | ||
| Gender (male/female) | 12/9 | 27/6 | 3.895 | 0.048* |
| Age (years) | 27.76 ± 7.38 | 25.76 ± 5.23 | 1.365 | 0.248 |
| Education (years) | 13.10 ± 3.46 | 13.15 ± 3.04 | 0.004 | 0.950 |
| IQa | 117.85 ± 8.03 | 118.67 ± 8.58 | 0.093 | 0.761 |
| Discounted option % | 0.798 ± 0.049 | 0.678 ± 0.039 | 1.815 | 0.065 |
- a Full scale IQ as measured by Wechsler Adult Intelligence Scale.
- b In reaction time, only for the correct trials.
- *P < 0.05.
This study's protocol was reviewed and approved by the Institutional Review Board of the Institute of Cognitive Neuroscience and Learning at Beijing Normal University. All subjects were Han Chinese and gave written informed consent for this study.
Genotyping
Genomic DNA was extracted using the standard method. To genotype rs1063843, a forward primer (5'-CCACTGAATCATACACTTCC-3') and reverse primer (5'-CCTTCCTTCATACACATAGC-3') were designed using the software Primer 6.0. Polymerase chain reaction (PCR) was performed using a 9700 thermocycler (Applied Biosystems, CA) in a 20 μl amplification mixture containing 0.5 μM of each primer, 200 μM dNTPs (Fermentas, Burlington), 1 U Taq polymerase (Fermentas, Burlington), 1× Taq buffer and 30 ng template DNA. After an initial 5 min step at 96°C, the PCR proceeded with 36 cycles of 96°C for 30 s, 53°C for 30 s, and 72°C for 30 s. After a final 10 min step at 72°C, the PCR was terminated and held at 4°C. Then, 5 μl of each amplified product was digested with 10 U of the restriction enzyme NlaIII (Fermentas, Burlington) overnight, followed by electrophoresis on a 3% agarose gel. The C allele was identified by the presence of two bands (235 and 37 bp), and the T allele was identified by three bands (82, 153, and 37 bp). Each genotype was confirmed independently by two individuals. The success rate of genotyping for this SNP was 100%, and the reproducibility of the genotyping was 100%, according to repeat analysis of 20% of the samples. Because of the small number of subjects harboring the TT genotype (four subjects for the N-back and Stroop sample; two subjects for the DDT sample), our analysis combined TT and TC individuals into one risk group.
MRI Data Acquisition
Imaging data were acquired at the Brain Imaging Center of Beijing Normal University. Subjects lay supine in a Siemens Trio 3T scanner with their heads snugly fixed with straps and foam pads to restrict head movement. The echo-planar imaging (EPI) sequence was used during the fMRI scan with the following parameters: repetition time (TR) = 2,000 ms; echo time (TE) = 30 ms; flip angle = 90°; field of view (FOV) = 200 × 200 mm; matrix size = 64 × 64; 31 axial slices; and 4.0 mm slice thickness without a gap. In addition, T1 images were acquired using T1-weighted sagittal three-dimensional (3D) magnetization-prepared rapid gradient-echo sequence and the following parameters: 176 slices; 1.0 mm thickness; TR = 2,530 ms; TE = 3.45 ms; flip angle = 7°; FOV = 256 × 256 mm; and matrix size = 256 × 256.
fMRI Tasks
We have described the N-back task and the Stroop task in a previous article [Zhang et al., 2015]. Briefly, the N-back task used a block design. The stimulus was a white circle presented randomly at one of the four corners of a grey diamond. Subjects were required to make responses according to the current location of the white circle (0-back) or the location of the white circle seen two trials previously (2-back). Using a fiber-optic response box with four buttons arranged in a diamond shape, subjects responded by pressing the button corresponding to the target stimulus. The task included two runs. Each run lasted 192 s and consisted of eight blocks in which the 2-back condition alternated with the 0-back condition. A centrally placed fixation cross was presented for 16 s before each set of 4 blocks. Each block started with a 4 s on-screen instruction (either the number “0” or “2” at the center of screen indicating the type of working memory task to be performed). There were eight trials in each block. In each 2 s trial, stimuli were presented for 500 ms followed by a 1.5 s blank. The error rate of the 2-back condition was calculated as an index of working memory.
The Stroop task included three words in Chinese (red, green, and blue) displayed randomly in one of the three colors. The subjects were required to make their response according to the color of the word, ignoring the meaning of the word. This task used the same response box as the N-back task. Subjects pressed the left button for red, the top button for green, and the right button for blue. The bottom button was not used. The task consisted of 120 trials including 84 congruent trials (the meaning of the word matched its color) and 36 incongruent trials (the meaning of the word did not match its color). Each trial began with a 500 ms fixation cross followed by the stimulus presentation (colored word) for 1 s and a 2.5 s blank. The conflict effect (the difference between the mean reaction time for the correct congruent trials and that of the correct incongruent trials) was calculated as an index of attentional executive control.
The DDT was adapted from Mitchell et al. [2005, 2007]. Briefly, subjects were presented with two reward options: a full amount to be received at a future time point or a discounted amount to be received immediately. The two options were presented at random on the left or the right side of the screen. Subjects were required to choose between the two options. They pressed the left button if they selected the option at the left side of the screen or the right button if they selected the option at the right side of the screen. Depending on the trial, the full amounts could be ¥5, ¥10, ¥25, ¥50, ¥100, or ¥500, and the time points could be 1 week, 2 weeks, 1 month, 3 months, or 6 months in the future. The discounted amount could be 70%, 85%, 90%, or 95% of the full amount. The experiment consisted of four runs, each with 30 trials. Subjects were asked to respond in one of four ways (i.e., four conditions): Want (subjects were asked to select the option they wanted), Do not Want (subjects were asked to select the option they did not want), and two control conditions (subjects were asked to select either the full amount or the discounted amount). In each run, there were 15 trials for the Want condition, 5 trials for the Do not Want condition, and 5 for each of the two control conditions. Each trial began with a 2 s fixation, followed by a 3 s cue to indicate the condition. The stimuli (the two options) were presented for 5 s, followed by a 2 s blank. We calculated the proportion of trials for which the subjects selected the discounted option under the Want condition as an index of discounting.
fMRI Data Preprocessing and Analysis
All image preprocessing and analyses were conducted using FEAT version 6.00 (FMRI Expert Analysis Tool) for FSL 5.0.7 software (fMRIB Software Library). Preprocessing included head motion correction, slice-timing correction, brain extraction, spatial smoothing with 8-mm full-width at half maximum (FWHM) of the Gaussian smoothing kernel, and high-pass temporal filtering at 128 s. After preprocessing, the functional images were first registered to the T1-weighted images and the resulting images were subsequently registered to the MNI152 standard space (voxel size: 2 × 2 × 2 mm).
Contrast images (incongruent > congruent for the Stroop task; 2-back > 0-back for the N-back task; Want > control for the DDT) for each subject were produced by first-level analysis. It needs to be mentioned that the Want > Control contrast for the DDT, as suggested by Boettiger et al. [2007], could identify brain areas that were more active during subjective decision making relative to objective decision making or more rapid responding. However, the DDT did could not be used to determination a hyperbolic discount rate (k) because the maximum discount was fixed at 70%, and consequently this task could not be used to isolate specific decision making variables such as subjective value. Higher-level analyses were carried out using FLAME (FMRIB's Local Analysis of Mixed Effects) to calculate the effect of genotype (i.e., risk allele carriers vs. non-risk homozygotes) across the whole brain for the three tasks separately. In these analyses, we controlled for age, gender, years of education, and IQ. We reported all of our results at voxelwise P < 0.005 with a cluster size >139 contiguous significant voxels, which yields a corrected threshold of P < 0.05 using the AlphaSim method.
RESULTS
Demographic and Behavioral Data
No significant difference between genotype groups was found for all demographic factors with the exception of gender in the analysis of DDT (see Table 1). Task performance was matched between genotype groups (see Table 1). No deviation from Hardy–Weinberg equilibrium for the rs1063843 allele was found (P > 0.05).
fMRI Data
The results from whole brain activation of the contrasts (incongruent > congruent for the Stroop task; 2-back > 0-back for the N-back task; Want > control for the DDT) can be found in Supporting Information Figures S1–S3.
In whole-brain analysis of the Stroop task, risk allele carriers (TC/TT) showed significantly higher activation than non-risk allele homozygotes in both left (BA 9, cluster size = 142 voxels, Pcorrected = 0.047; peak voxel MNI coordinate: x = −22, y = 56, z = 42, Z = 3.48, Puncorrected < 0.001) and right DLPFC (cluster size = 211 voxels, Pcorrected = 0.005; peak voxel MNI coordinate: x = 34, y = 28, z = 50, Z = 3.24, Puncorrected < 0.001; see Fig. 1). Additionally, the risk allele carriers showed significantly higher activation in the left cerebellum (cluster size = 166 voxels, Pcorrected = 0.019; peak voxel MNI coordinate: x = −66, y = −12, z = −14, Z = 3.63, Puncorrected < 0.001). Several other regions showed nominal effects (Puncorrected < 0.005; higher for risk allele carriers), but these did not survive the correction for multiple comparisons [the middle occipital lobe (cluster size = 106 voxels), insula lobe (cluster size = 66 voxels) and the middle temporal lobe (cluster size = 62 voxels) in the left hemisphere and the superior temporal lobe (cluster size = 99 voxels), the medial frontal lobe (cluster size = 79 voxels), and the anterior cingulate (cluster size = 51 voxels) in the right hemisphere]. No voxel showed lower activation in risk allele carriers than in non-risk allele homozygotes (Puncorrected < 0.005).
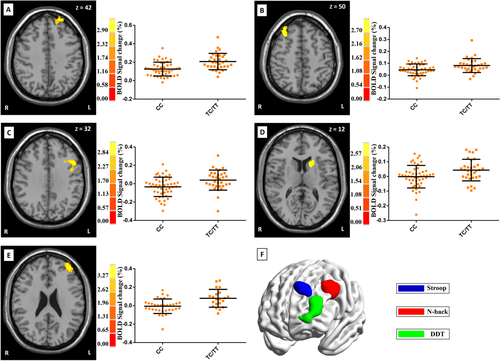
Significant associations between rs1063843 and brain activation across the whole brain during the Stroop task, the N-back task, and the DDT. For the Stroop task, risk allele carriers showed significantly higher activation than non-risk homozygotes of both left and right DLPFC (panels A and B). For the N-back task, risk allele carriers showed significantly higher activation of the left DLPFC (panel C) and the left caudate nucleus (panel D). For the DDT, risk allele carriers showed significantly higher activation of the left DLPFC (panel E). The peak voxel MNI coordinates for A–E, respectively, are as follows: −22, 56, 42; 34, 28, 50; −44, 18, 32; −20, 16, 12; −40, 48, 24. Moreover, panel F shows the surface rendering with increased activation in risk allele carriers within the left DLPFC across the three tasks. Brain graph of the panel F was visualized by using BrainNet Viewer software (https://www.nitrc.org/projects/bnv/). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For the N-back task, whole-brain analysis revealed significantly higher activation in risk allele carriers than in non-risk allele homozygotes in the left DLPFC (BA 9, cluster size = 505 voxels, Pcorrected < 0.001; peak voxel MNI coordinate: x = −44, y = 18, z = 32, Z = 3.40, Puncorrected < 0.001) but not the right DLPFC. In addition, the left striatum (mainly in the caudate nucleus) showed significantly higher activation in risk allele carriers (cluster size = 139 voxels, Pcorrected = 0.05; peak voxel MNI coordinate: x = −20, y = 16, z = 12, Z = 3.08, Puncorrected < 0.001) (see Fig. 1). Several other regions also showed higher activation in risk allele carriers than in non-risk allele homozygotes (Puncorrected < 0.005), but these effects did not survive the correction for multiple comparisons [inferior occipital lobe (cluster size = 57 voxels) of the left hemisphere, and inferior occipital lobe (cluster size = 56 voxels) of the right hemisphere and right cerebellum (cluster size = 51 voxels)]. In addition, two notably small clusters in the inferior parietal lobule (cluster size = 21 voxels) and the temporal lobe (cluster size = 24 voxels) showed lower activation in risk allele carriers than in non-risk allele homozygotes (Puncorrected < 0.005).
For the DDT, only the left DLPFC (BA 10/46, cluster size = 668 voxels, Pcorrected < 0.001; peak voxel MNI coordinate: x = −40, y = 48, z =24, Z = 3.93, Puncorrected < 0.001) showed significantly different activation between genotypes following whole-brain analysis. Consistent with our findings in the other two tasks, risk allele carriers showed significantly higher activation of the left DLPFC than did non-risk allele homozygotes. In addition, the risk allele carriers showed increased activation of the inferior parietal lobule (cluster size = 99 voxels, Puncorrected < 0.005), but the differences did not survive the correction for multiple comparisons. Finally, only a small cluster in the insula (cluster size = 83 voxels) showed less activation in risk allele carriers than in non-risk allele carriers (Puncorrected < 0.005).
Finally, we extracted and correlated the mean PFC signals across the three tasks (although the areas were different for different tasks as shown in Fig. 1). A significant correlation was found between the Stroop task and the N-back task (r = 0.331, P = 0.019), but not between the Stroop task and the DDT (r = −0.183, P = 0.203) or between the N-back task and the DDT (r = −0.004, P = 0.979).
DISCUSSION
This work is the first fMRI study that provides convergent evidence from three different tasks that links CAMKK2 gene polymorphisms at rs1063843 to brain function. Our whole brain analyses of working memory (N-back task), attentional executive control (Stroop task), and decision making (DDT) consistently demonstrated significantly higher activation of the left DLPFC in risk allele carriers (TT/TC) compared with non-risk homozygotes. Given the comparable performance between genotypes (P > 0.05), we believe that this result reflects the inefficiency and complementary activity of the left DLPFC in risk allele carriers because the risk allele carriers needed more activation of the left DLPFC to achieve performance levels similar to the non-risk allele homozygotes (CC). As mentioned above, CAMKK2 is consistently downregulated in the DLPFC of SCZ patients [Luo et al., 2014], and the risk allele is associated with lower expression of CAMKK2 in the DLPFC [Luo et al., 2014]. In addition, previous research has shown that (1) the DLPFC involved in both the Stroop task and the N-back task and is impaired in SCZ patients (based on a meta-analysis of 41 fMRI studies) [Minzenberg et al., 2009]; and (2) the DLPFC (mainly the left side) is the only brain region that is activated by both the N-back task and the DDT (based on a meta-analysis of 37 studies of the DDT and 41 studies of the N-back task) [Wesley and Bickel, 2014]. Our results indicate that the left DLPFC represents a common pathway through which rs1063843 could affect multiple cognitive functions. Biologically, the rs1063843 polymorphism could affect CAMKK2 expression in the DLPFC [Luo et al., 2014], in turn affecting neuronal processes, such as differentiation and migration, neurite outgrowth, and synapse formation [Michaelsen and Lohmann, 2010; Peters et al., 2003; Saneyoshi et al., 2008; Takemoto-Kimura et al., 2010; Wayman et al., 2008], all of which are associated with both cognitive function and SCZ.
Topographically, the significant regions of the DLPFC varied somewhat across the different tasks, although they were located in close proximity to one another. This finding may be attributable to functional heterogeneity along the anterior-to-posterior and the dorsal-to-ventral axes of the DLPFC [Cieslik et al., 2013; Konishi et al., 2005; Shamosh et al., 2008; Wesley and Bickel, 2014]. First, the location of the significant cluster in the Stroop task was more anterior than that in the N-back task, which is consistent with previous findings that the posterior DLPFC is mainly related to working memory and action execution whereas the anterior DLPFC is involved in attention and action inhibition, especially in conflict resolution tasks [Cieslik et al., 2013; Konishi et al., 2005]. Second, the location of the significant cluster in DDT was more anterior and ventral than those in the N-back and Stroop tasks. Indeed, previous studies have found that the activated region in the DLPFC during the DDT was more anterior and ventral than in other tests of executive function [Shamosh et al., 2008; Wesley and Bickel 2014]. An anterior and ventral subregion of the DLPFC has been implicated in information integration, especially when the information is abstract [Green et al., 2006], which is consistent with the abstract goal and process involved in the calculation of delay discounting. This anterior and ventral subregion of the DLPFC could represent the expected reward through cooperation with other regions such as the orbital frontal cortex and/or the ventral striatum [Chen et al., 2015; Hare et al., 2009; Kahnt et al., 2011; Staudinger et al., 2011]. In fact, the significant DLPFC clusters observed during DDT in the current study were in close proximity to the orbital frontal cortex, making an effect of rs1063843 on the reward-related functions plausible. This speculation is supported by the association of rs1063843 with motivation [Luo et al., 2014].
In addition to the DLPFC, we identified an effect of rs1063843 on the activation of the left cerebellum during the Stroop task and on the activation of the left caudate nucleus (the dorsal region of the striatum) during the N-back task. Previous studies have shown that the cerebellum expresses the highest level of CAMKK2 among the entire cortex [Anderson et al., 1998; Luo et al., 2014]. Furthermore, the caudate nucleus showed the most abundant expression of CAMKK2 among all subcortical regions [Anderson et al., 1998; Luo et al., 2014]. The caudate nucleus receives direct input from the DLPFC and projects directly to the DLPFC. Together with the prefrontal cortex, the caudate nucleus constitutes the frontostriatal loop, which may form the neural basis of working memory. Lesion of the caudate nucleus in primates has been reported to impair working memory in the same manner as lesion of the prefrontal cortex [Mair et al., 2002; Voorn et al., 2004]. In human studies of SCZ, the striatum has been reported to be sensitive to antipsychotic mediation [Keedy et al., 2014; Sarpal et al., 2013]. Thus, we examined previous studies of healthy volunteers, especially those at high risk for SCZ. An fMRI study showed that the activity of the caudate—in addition to the DLPFC—was elevated during an N-back task [O'Daly et al., 2011] in healthy volunteers upon induction of symptoms by amphetamine. In structural MRI studies of the caudate nucleus, subjects with schizotypal personality disorder but no prior neuroleptic exposure showed a reduced volume [Koo et al., 2006; Levitt et al., 2002] and altered shape [Levitt et al., 2004, 2009] of the caudate nucleus, especially at its head (i.e., the dorsal region). Moreover, both the volume and shape of the caudate head showed significant correlations with working memory [Levitt et al., 2002, 2004]. Similarly, the volume of the caudate head was correlated with working memory in another study of subjects at a high risk for SCZ with no prior neuroleptic exposure [Hannan et al., 2010]. Based on these data, it seems that the rs1063843 risk allele can affect working memory via the dorsal caudate nucleus.
In conclusion, the current study used three cognitive tasks and fMRI to provide convergent evidence of a link between the rs1063843 SNP of CAMKK2 and the function of the DLPFC. In addition, this polymorphism was associated with the function of the striatum during the N-back task. These results extend previous findings regarding the association between rs1063843 and cognitive function and could help to elucidate the role played by CAMKK2 rs1063843 in the etiology of cognitive functions and SCZ.
ACKNOWLEDGMENTS
Thanks to Elsevier WebShop for editing the manuscript. Jun Li had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Jun Li, Qi Dong, and Chuansheng Chen designed the study and wrote the protocol. Xiongying Chen, Bingqian Han, Dawei Li, and Hongjie Wu managed the literature searches and analyses. Qiumei Zhang, Jinguo Zhai, Min Chen, Boqi Du, Xiaoxiang Deng, Feng Ji, Chuanyue Wang, and Yutao Xiang selected the sample and evaluated them. Ping Yu, Xiongying Chen, Wan Zhao, and Zhifang Zhang undertook the statistical analysis. Ping Yu wrote the first draft of the manuscript.




