Structural insights into aberrant cortical morphometry and network organization in psychogenic erectile dysfunction
Conflict of Interests: All the authors declare no biomedical financial interests or potential conflicts of interest regarding the publication of this article.
Abstract
Functional neuroimaging studies have revealed abnormal brain dynamics of male sexual arousal (SA) in psychogenic erectile dysfunction (pED). However, the neuroanatomical correlates of pED are still unclear. In this work, we obtained cortical thickness (CTh) measurements from structural magnetic resonance images of 40 pED patients and 39 healthy control subjects. Abnormalities in CTh related to pED were explored using a scale space search based brain morphometric analysis. Organizations of brain structural covariance networks were analyzed as well. Compared with healthy men, pED patients showed significantly decreased CTh in widespread cortical regions, most of which were previously reported to show abnormal dynamics of male SA in pED, such as the medial prefrontal, orbitofrontal, cingulate, inferotemporal, and insular cortices. CTh reductions in these areas were found to be significantly correlated with male sexual functioning degradation. Moreover, pED patients showed decreased interregional CTh correlations from the right lateral orbitofrontal cortex to the right supramarginal gyrus and the left angular cortex, implying disassociations between the cognitive, motivational, and inhibitory networks of male SA in pED. This work provides structural insights on the complex phenomenon of psychogenic sexual dysfunction in men, and suggests a specific vulnerability factor, possibly as an extra “organic” factor, that may play an important role in pED. Hum Brain Mapp 36:4469–4482, 2015. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Psychogenic erectile dysfunction (pED) is defined as the persistent inability to achieve and maintain erection satisfactorily for sexual performance owing predominantly or exclusively to psychological or interpersonal factors, such as anxiety, depression, loss of self-esteem, and other psychosocial stresses [Lizza and Rosen, 1999; Rosen, 2001]. Epidemiologic studies have found exclusively psychogenic etiology in about 40% of men with erectile dysfunction (ED) [Aydin et al., 2001; Caspari et al., 1999; Feldman et al., 1994]; particularly, this rate reaches up to about 70% in the affected men under 40 years [Caskurlu et al., 2004; Karadeniz et al., 2004; Mellinger, 1992]. As a common male sexual disorder, pED can have profoundly adverse effects on the quality of life and psychological well-being of both sufferers and their partners [Guest and Das Gupta, 2002; Latini et al., 2006].
It has been known that the brain plays a central role in the control of male sexual function [Argiolas and Melis, 2005; McKenna, 1999]. In the past 15 years, numerous functional neuroimaging studies [see Stoleru et al., 2012 for a review] investigated the brain dynamics involved in normal male sexual arousal (SA) induced by visual [Karama et al., 2002; Mouras et al., 2003; Stoleru et al., 1999], auditory [Ethofer et al., 2007; Miyagawa et al., 2007; Rauch et al., 1999], olfactory [Bocher et al., 2001; Savic et al., 2001] and tactile [Georgiadis et al., 2009, 2010] sexual stimuli. These studies have revealed an involvement of widespread brain regions in male SA, such as the lateral occipitotemporal cortex, the inferior temporal cortex (ITC), the parietal cortex, the orbitofrontal cortex (OFC), the medial prefrontal cortex (PFC), the insula (INS), the cingulate cortex, and the ventral premotor area (vPM) as well as, for subcortical regions, the amygdalas, the claustrum, the hypothalamus, the caudate nucleus, the thalamus, the cerebellum, and the substantia nigra [Stoleru et al., 2012]. The activations of these circuits compose a neurobehavioral model of brain processes in male SA, consisting of cognitive, motivational, emotional, and autonomic components. The cognitive component comprises a process of appraisal through which stimuli are evaluated as sexual incentives. The emotional component refers to the specific hedonic quality of rising arousal. The motivational component relates to the processes that direct behavior to a sexual goal. The autonomic component regards the autonomic and endocrinological responses (e.g., cardiovascular, respiratory, and genital) associated with SA (for more detailed discussion of this model) [see Redoute, et al., 2000; Stoleru, et al., 1999, 2012]. Furthermore, these components are controlled by inhibitory processes [Stoleru, et al., 2003]. It has been demonstrated that in the absence of sexual stimuli, brain regions, such as the medial OFC, the left lateral OFC, the lateral temporal cortex (LTC), the angular gyri (ANG), and the posterior cingulate cortex (PCC), may exert continuous inhibitory controls on SA; and these regions deactivate during the development of SA to release such inhibition [Poeppl et al., 2014; Stoleru et al., 2012].
For male sexual dysfunction, a few functional neuroimaging studies have found abnormalities in the central mechanisms controlling male sexual functions [Cera et al., 2012b; Hagemann et al., 2003; Montorsi et al., 2003; Redoute et al., 2005; Stoleru et al., 2003]. Particularly, pED patients during sexual stimuli showed abnormally increased or extended activations in the regions of medial PFC, OFC, and cingulate cortex known to mediate the inhibitory control of unfolding male SA [Cera et al., 2012b; Hagemann et al., 2003; Montorsi et al., 2003], and decreased activations in ITC mediating the cognitive processes of male SA [Hagemann et al., 2003], and in INS and ACC involved in modulating the autonomic functions of male SA [Cera et al., 2012b]. Since pED is primarily considered as a functional disorder, little attention has been paid to its neuroanatomical correlates. However, a recent study that used structural magnetic resonance imaging (MRI) and focused on subcortical structures observed gray matter (GM) atrophy of nucleus accumbens and hypothalamus in pED [Cera et al., 2012a]. A more recent study [Zhang et al., 2014] using diffusion-based imaging (DTI) revealed white matter (WM) microstructural changes in multiple WM tracts in pED patients. These results illustrated that, in addition to the functional impairments, brain structural alterations may be involved in pED.
The aim of this work is to explore structural changes of the cerebral cortex and alterations in the cortical structural network organization related to pED. In the brain morphometry analyses, we applied a scale space search analysis method [Zhao et al., 2013] that can produce a multi-scale description of brain structural changes to address the Gaussian kernel smoothing issue, i.e. in exploratory studies, in general, no prior information is available to determine the optimal smoothing filter size, and a single-filter analysis could not completely detect all signals with different sizes contained in the image data [Han et al., 2006; Jones et al., 2005; Lerch and Evans, 2005]. The brain connectivity networks were assessed with structural covariance network (SCN) analysis [Evans, 2013]. As it has been discussed beforehand, pED patients have been reported to show abnormal dynamics in multiple brain regions associated with the development or inhibition of male SA. Here, we hypothesize that pED patients would show neuroanatomical alterations in these regions. Moreover, it has been known that the complex brain structural network supports the dynamic emergence of coherent physiological activities that span distinct brain regions making up a functional network [Bullmore and Sporns, 2009]. The neurobehavioral components of male SA are conceived as closely interrelated and coordinated [Stoleru et al., 2012]. The reported WM microstructural alterations in pED [Zhang et al., 2014] might indicate neuroanatomical connectivity abnormalities. Therefore, we predict that the pathology of pED may be also correlated with abnormal cortical structural network architecture.
MATERIALS AND METHODS
Subjects
Male patients who visited the outpatient clinic at the Henan Provincial People's Hospital and complained of male sexual dysfunction were recruited from August 2012 to July 2013. The diagnosis of pED (generalized type) was conducted following current guidelines [Wespes et al., 2006], that is, absence of organic (vasculogenic, neurogenic, hormonal, anatomical, and drug-induced) ED. Physical examinations (penile duplex Doppler ultrasonography for vascular impairment, RigiScan test for Nocturnal Penile Tumescence, electrocardiogram exam) and laboratory tests (thyroid-stimulating hormone level, prostate-specific antigen, and serum sexual hormone status) specifically for genitourinary, endocrine, vascular, and neurological systems were conducted for all patients. Detailed records of medical, sexual, and relevant drug history and surgical disorders were acquired from each patient as well.
This study included 40 right-handed heterosexual patients (mean age = 28.5 ± 6.4 years) who were diagnosed as pED, had experienced ED for more than 6 months, and were in a stable heterosexual relationship for at least 1 year; 23 patients were excluded from this study because some of them had experience of ED <6 months, or were diagnosed having premature ejaculation, or had hormonal defects, vascular or genital impairments, or had been using medication that might affect sexual function or that was designed to enhance sexual performance during the previous 14 days before the scanning.
Thirty-nine healthy right-handed heterosexual men (mean age = 30.0 ± 3.4 years), with sexual intercourse experience and free from any urosexual symptoms or signs, were enrolled for this study as normal controls. The same clinical examinations were performed on all the healthy subjects.
Psychiatric and Symptom Assessments
All the participants were assessed by an experienced psychiatrist for structured diagnosis of DSM-IV disorders using the Mini International Neuropsychiatric Interview (M.I.N.I) [Sheehan et al., 1998] and for measuring psychiatric symptoms using the Brief Psychiatric Rating Scale (BPRS) [Overall and Gorham, 1962]. All the participants did not meet the criteria of DSM-IV disorders and did not show any prominent psychiatric symptom. Furthermore, this study selected the International Index of Erectile Function (IIEF) [Rosen et al., 1997] to assess the sexual functioning for all the participants. IIEF has been demonstrated to have high sensitivity and specificity for discriminating functional and dysfunctional samples and detecting treatment-related changes in ED; and concurrent validation against other comparable measures has been shown [DeRogatis, 2008]. Therefore, IIEF has been frequently recommended as a primary end point in clinical trials of ED, and has become a standard in that regard [Kandeel, 2007; Rosen et al., 2002]. In addition, questionnaires of Self-Rating Anxiety Scale (SAS) [Jegede, 1977] and Self-Rating Depression Scale (SDS) [Zung, 1965] were implemented to evaluate the levels of anxiety and depression for all the participants.
Ethics Statement
The study was approved by the ethics committee of Henan Provincial People's Hospital and conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all the subjects.
MRI Image Acquisition
Brain MRI was acquired on a 3T GE Discovery 750 MRI scanner (General Electric, Milwaukee, WI), using a dedicated 8-channel head coil. Three-dimensonal (3D) brain structural images were obtained via a high resolution T1-weighted inversion recovery fast spoiled gradient recalled (IR FSPGR) sequence with the following parameters: TR = 8.3 ms, TE = 2.26 ms, flip angle = 12°, matrix resolution = 256 × 256, 160 slices, FOV = 256 × 256 mm2, and an isotropic resolution of 1 × 1 × 1 mm3.
MR Image Processing and Cortical Thickness (CTh) Measurements
The MR images were processed using the CIVET MRI analysis pipeline (version 1.1.12) [Ad-Dab'bagh et al., 2006] developed at the Montreal Neurological Institute to automatically extract and coregister the cortical surfaces for each subject. The main pipeline processing steps are as follows. First, intensity nonuniformity in the raw MR images was corrected using the N3 algorithm [Sled et al., 1998], and images in the native space were linearly registered into the ICBM152 space [Collins et al., 1994]; Next, each brain volume was classified into WM, GM, cerebrospinal fluid and background using the INSECT algorithm [Zijdenbos et al., 1998], and then partial volume fractions of these tissue types were computed in each brain voxel [Tohka et al., 2004]. The inner (WM/GM interface) and outer (pial) cortical surfaces were extracted from each brain volume using the CLASP algorithm [Kim et al., 2005]. The hemispheric inner and outer cortical surfaces were modeled with a deformable polygonal mesh consisting of 81,920 triangles (40,962 vertices). To obtain accurate cross-subject correspondences, the extracted hemispheric cortical surfaces were nonlinearly aligned to a hemisphere-unbiased iterative surface template [Lyttelton et al., 2007] using a depth-potential function [Boucher et al., 2009]. Finally, the aligned cortical surfaces were rescaled back to native space dimension using the inverse of the scaling parameters of the corresponding linear volumetric transformation matrix.
It has been known that neurons within the cerebral cortex are organized into ontogenetic columns that run perpendicular to the surface of the brain [Mountcastle, 1997]. CTh is a measurement that captures the columnar architecture of the cerebral cortex. In this work, CTh was measured at each cortical surface vertex throughout the cortex in the native space using the tlink metric [Lerch and Evans, 2005] of computing the Euclidean distance between linked vertices, respectively, on the inner and outer cortical surfaces.
Scale Space Search Based Brain Morphometric Analysis
In brain morphometric analyses, it is the usual practice to smooth the image data before statistical analysis so as to increase the signal-to-noise ratio and to reduce the impact of mis-registration. The matched filter theorem [Pratt, 1991] states that the optimal smoothing filter should match the size of the target signal, for example, patterns of structural changes. However, the prior information about the signal extent, in general, is unknown in exploratory studies, so that the smoothing filter was arbitrarily selected without sufficient validation in most of the existing studies. It has been demonstrated that variations in smoothing can produce very different analysis results [Han et al., 2006; Jones et al., 2005; Lerch and Evans 2005], and there is no guarantee that a single filter could be optimal for all the signals with different sizes and shapes contained in the image data [Poline and Mazoyer, 1994]. To address this issue, we used the scale space search based brain morphometric analysis algorithm [Zhao et al., 2013] to obtain a relatively complete description of the structural changes at different scale levels (different smoothing filter sizes). Compared with conventional single-filter analyses, the scale space search method can properly capture the variations in analysis results caused by variations in smoothing, and it can obviously increase the detection sensitivity (for more details, please read [Zhao et al., 2013]).
The scale space search analysis was implemented using our Scale Space Cortical Morphometry toolbox (http://www.bic.mni.mcgill.ca/users/zhao/softwares.html) and the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat/). First, CTh measurements were smoothed using surface-based diffusion [Meyer et al., 2003] with a wide range of smoothing filters with the full width half maximum (FWHM) from 16 to 256 mm. The finest scale level of 16 mm was selected for basic suppression of image noise and reduction of the impact of mis-registration; and the coarsest scale level of 256 mm corresponded to the size of the image. Since the scale space statistical field is stationary in log(FWHM) [Siegmund and Worsley, 1995], we took 25 samples in the scale interval equally spaced on the log space. At each scale level, between-group comparison with respect to CTh was conducted between the patient and control groups using one-way ANOVA controlling for age and the mean cortical thickness (MCTh) across the whole cortex. We also explored whether CTh measurements were associated with male sexual functioning, measured as IIEF scores, by computing the Pearson's correlation coefficients at each scale level removing the effects of age and global MCTh.
Rather than treating each scale as a single image, the unified p-value [Worsley et al., 1996] was applied to correct for the multiple comparisons and to detect significant results across the entire 4-dimensional (4D) scale space (3 spatial dimensions + 1 scale dimension) using a unified critical threshold Tα (here, α = 0.05).
Structural Covariance Network Analysis
To construct cortical SCNs, first, we used the automatic anatomical labeling (AAL) atlas [Tzourio-Mazoyer et al., 2002] for brain parcellation. Since our analysis is based upon a cortical surface model, we included only those 78 AAL regions that are defined for the neocortex (Supporting Information Table S1). Subcortical structures were not included in this study. For each subject, regional CTh was then computed as the average thickness of all vertices within that region. A linear regression was performed at every cortical region to remove the effects of multiple confounding variables (age and global MCTh). The residuals of this regression were used to substitute for the raw CTh values of each region. The cortical network for each of the studied groups was then constructed as an interregional correlation matrix Rij (i, j=1, 2, …, N, here N = 78) by calculating Pearson's correlation coefficients across subjects between the residual CTh measurements of every pair of regions.
To determine whether the interregional CTh correlations was significantly distinct between the groups of pED patients and normal controls, correlation coefficients contained in the correlation matrices Rij were converted into z values that were approximately normally distributed through Fisher's r-to-z transform. A Z statistic was then used to compare these transformed z values to determine the significance of the between-group differences in correlations [Cohen, 2003]. A false discovery rate (FDR) at a q value of 0.05 was applied to correct for the multiple comparisons in the correlation analysis.
Post Hoc Analyses
Previous studies have reported that impaired sexual function in men is significantly associated with negative psychological effects, e.g. anxiety and depression [Feldman et al., 1994; Sugimori et al., 2005]. In the present sample, sexual functioning scores (IIEF) were negatively correlated with the anxiety (SAS) and depression (SDS) scores (Pearson's r = −0.32, P < 0.01 uncorrected; Pearson's r = −0.36, P < 0.005 uncorrected, respectively). In addition, anxious or depressive symptoms may have significant impacts on brain morphometry [Boes et al., 2008; Ducharme et al., 2014]. Therefore, we repeated the between-group comparisons in CTh and the SCN analysis after controlling for SAS and SDS scores, to ensure that these variables were not driving our results.
RESULTS
Demographic and Clinical Data
Demographic, psychiatric, and behavioral features for the groups of pED patients and normal controls are shown in Table 1. The two groups were not significantly different in age, intracranial volume and total BPRS scores (P > 0.1 uncorrected). All the participants (total BPRS < 35) did not show prominent psychiatric symptoms. The pED patients had lower IIEF score (P < 0.0001 uncorrected), and higher SAS and SDS scores (P < 0.005 uncorrected), than the normal controls. All the participants (SAS < 60, SDS < 70) did not reach severe anxiety and depression levels.
| pED | NC | P-value | |
|---|---|---|---|
| Age in years (M, SD) | 28.45, 6.44 | 30.02, 3.44 | 0.18a |
| ICV in cm3 (M, SD) | 1437.63, 103.96 | 1451.17, 91.64 | 0.59a |
| Total BPRS (M, SD) | 20.68, 0.31 | 20.00, 0.27 | 0.11a |
| IIEF (M, SD) | 34.82, 11.90 | 62.65, 9.45 | 1.27e-14b |
| SAS (M, SD) | 43.82, 7.52 | 37.12, 8.97 | 0.0018b |
| SDS (M, SD) | 47.97, 11.08 | 36.96, 9.15 | 8.51e-5b |
- M: mean; SD: standard deviation; ICV: intracranial volume; BPRS: Brief Psychiatric Rating Scale.
- a The P value was obtained with a two-tailed two-sample t-test.
- b The P value was obtained with a one-tailed two-sample t-test.
Scale Space Search Based Brain Morphometric Analyses
Alterations in CTh
The scale space search analysis for between-group comparison revealed significant CTh reduction in multiple cortical regions in pED patients compared with normal controls (P < 0.05, unified P-value corrected). As we predicted, these regions are well in line with the ones related to the pathological brain mechanisms of male SA in pED [Cera et al., 2012b; Hagemann et al., 2003; Montorsi, et al., 2003]. Hierarchical presentation of the patterns of significant CTh reduction in patients at selected scale levels is shown in Figure 1. At finer scales (16 ≤ FWHM ≤ 32 mm), CTh reductions were detected in relatively small and distributed areas in the superior PFC, the dorsal medial prefrontal cortex (dmPFC), the ventral medial prefrontal cortex (vmPFC), the left vPM, the right precentral gyrus, the postcentral gyri, the left inferior parietal lobule (IPL), the lateral and medial occipital cortex, the left heschl gyrus (HES), LTC, ITC, the temporal poles (TPO), the right INS, the rostral ACC, the midcingulate cortex (MCC), and PCC. With the scale increased, some of these patterns shrank and disappeared, whereas, some enlarged and emerged with surrounding patterns. Consequently, at coarser scales (90 ≤ FWHM ≤ 181 mm), CTh reductions were identified as large patterns in the right INS, the left dmPFC, the right vmPFC, the right ACC and the anterior MCC. No significant CTh increase was found in the entire scale space. When controlling for SAS and SDS scores, these results remained significant but slightly reduced in spatial extent (see Supporting Information Fig. S1), confirming the initial finding.
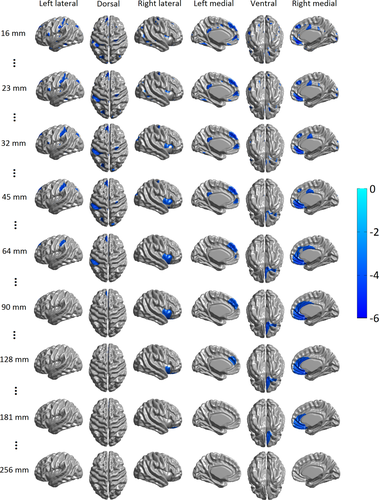
CTh reductions in pED compared with normal controls when controlling for age and global mean cortical thickness (T maps thresholded at P < 0.05, unified P-value corrected) detected using scale space search. Selected scale levels were illustrated here. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Associations between male sexual functioning and CTh
Correlation analysis was carried out to investigate the associations between CTh and male sexual functioning, measured as IIEF scores. Significant positive correlations between IIEF scores and CTh (P < 0.05, unified P-value corrected), that is, poor male sexual functioning was associated with smaller CTh, were found (see Fig. 2): at finer scales (16 ≤ FWHM ≤ 45 mm), in the inferior lateral PFC, INS, the precentral gyri, the left HES, LTC, ITC, IPL, the lateral and medial occipital cortex, vmPFC, the left and right precuneus, the left rostral ACC, the right MCC, PCC, the left medial OFC; at coarser scales (90 ≤ FWHM ≤ 128 mm), in the right medial occipital cortex. These results were highly consistent with the patterns of CTh reduction in pED patients described above, especially in the right INS, vmPFC, the left rostral ACC, the right MCC, PCC, and the right TPO.
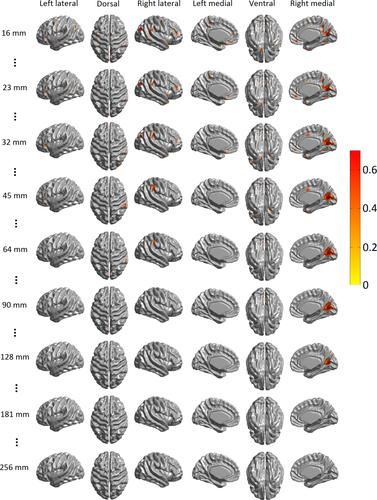
Correlations of CTh and IIEF score when controlling for age and global mean cortical thickness (Pearson's correlation coefficient r maps thresholded at P < 0.05, unified P-value corrected) detected using scale space search. Selected scale levels were illustrated here. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cortical Structural Covariance Network Organization
The between-group differences in interregional structural covariance were assessed by determining the distinctions in correlation coefficients (Table 2 and Fig. 3). In normal controls, the right orbital part of inferior frontal gyrus (ORBinf) was significantly connected (P < 0.05, FDR corrected) with the left angular gyrus (ANG) and the right supramarginal gyrus (SMG); whereas, these connectivities decreased into insignificant negative correlations (P > 0.05, FDR corrected) in pED patients. In addition, patients also showed abnormal significant negative correlations (P < 0.05, FDR corrected) between the IPL, the left opercular part of inferior frontal gyrus (IFGoperc), the right medial orbital part of the superior frontal gyrus (ORBsupmed), the left Inferior occipital gyrus (IOG), the right HES, and the right parahippocampal gyrus (PHG). Furthermore, we also found, in pED patients, increased positive correlation (P < 0.05, FDR corrected) between the right IFGoperc and the right INS, and decreased negative correlation (P < 0.05, FDR corrected) between the left supplementary motor area (SMA) and the left HES. Adding SAS and SDS scores as control variables, when computing the residual CTh measurements, did not change these results.
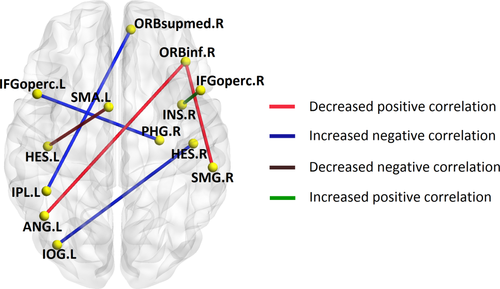
3D rendering map of significant interregional connectivity alterations in patients with pED compared with normal controls. IPL: inferior parietal, but supramarginal and angular gyri; ANG: angular gyrus; ORBinf: inferior frontal gyrus, orbital part; IOG: inferior occipital gyrus; ORBsupmed: superior frontal gyrus, medial orbital; SMG: supramarginal gyrus; IFGoperc: inferior frontal gyrus, opercular part; PHG: parahippocampal gyrus; SMA: supplementary motor area; HES: heschl gyrus; INS: insula; L: left; R: right. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Regions | Regions | Correlation coefficients (r) | Z score | |
|---|---|---|---|---|
| pED | NC | |||
| Decreased positive correlations in pED | ||||
| ANG.L | ORBinf.R | −0.42 | 0.62 | −5.01 |
| ORBinf.R | SMG.R | −0.29 | 0.57 | −4.04 |
| Increased negative correlations in pED | ||||
| IPL.L | ORBsupmed.R | −0.58 | 0.22 | −3.79 |
| IOG.L | HES.R | −0.46 | 0.46 | −4.25 |
| IFGoperc.L | PHG.R | −0.46 | 0.43 | −4.02 |
| Decreased negative correlations in pED | ||||
| SMA.L | HES.L | 0.14 | −0.74 | 4.62 |
| Increased positive correlations in pED | ||||
| IFGoperc.R | INS.R | 0.71 | −0.007 | 3.83 |
- The r values in bold indicate significant interregional correlation in CTh within group (P < 0.05, FDR corrected). All Z scores are significant (P < 0.05, FDR corrected).
- IPL: inferior parietal, but supramarginal and angular gyri; ANG: angular gyrus; ORBinf: inferior frontal gyrus, orbital part; IOG: inferior occipital gyrus; ORBsupmed: superior frontal gyrus, medial orbital; SMG: supramarginal gyrus; IFGoperc: inferior frontal gyrus, opercular part; PHG: parahippocampal gyrus; SMA: supplementary motor area; HES: heschl gyrus; INS: insula; L: left; R: right.
DISCUSSION
Functional neuroimaging studies have revealed disruptions in the brain mechanisms for male sexual functions in pED [Cera et al., 2012b; Hagemann et al., 2003; Montorsi et al., 2003]. However, the neuroanatomical correlates of pED have remained unclear. The present study demonstrates pED-related changes in cortical morphometry and the coordination of SCN by using CTh data from MRI. Our primary findings are: (1) that pED patients showed reduced CTh than normal controls in widespread cortical regions, most of which have been reported to show abnormal dynamics in pED; (2) that CTh reductions in most of these identified regions were significantly associated with degradation of male sexual functioning; (3) that pED patients showed abnormalities in interregional morphological correlations. Overall, these findings are important, since a better understanding of the underlying neuroanatomical correlates will probably improve both diagnosis and therapeutic approaches of pED.
Abormalities in CTh in pED
The most prominent pattern of CTh reduction in pED patients compared with normal controls was in vmPFC, as it had large spatial extent and the longest lifetime in the scale space even after controlling for SAS and SDS scores (see Fig.1 and Supporting Information Fig. S1). We found that the CTh decrease in this region was significantly correlated with the degrading of male sexual functioning. vmPFC, especially the subgenual cingulate cortex, has been repeatedly reported to play a critical role in modulating negative mood states [Drevets et al., 2008; Mayberg et al., 1999]. Mayberg and colleagues [Mayberg et al., 2005] reported that stimulating this region could relieve severe, intractable depression. Such negative psychological states, e.g. depression and anxiety, have been associated with male sexual dysfunction [Feldman et al., 1994; Sugimori et al., 2005]. In the present sample, male sexual functioning (IIEF) was negatively correlated with anxiety (SAS) and depression (SDS) severities. One considerable explanation for this association is that the negative psychological states may adversely affect the male central inhibitory mechanism of SA [Bancroft, 1999]. It has been revealed that the attempted inhibition of male SA generated by visual sexual stimuli in healthy men was associated with the activation of vmPFC [Beauregard et al., 2001]. Such inhibitory activation has been conceived to be related to the involvement of vmPFC in the function of self-relevant processing [Northoff and Bermpohl, 2004], which exists also in the sexual domain [Heinzel et al., 2006]. This means that vmPFC may play an important role in the disengagement from the evaluation of the erotic character of sexually relevant stimuli by redirecting attention to self-relevant experiences [Stoleru et al., 2003]. Consistent with this concept, previous functional studies repeatedly found that pED patients showed larger activity levels in vmPFC during erotic stimuli, which were thought as increased inhibitory control of SA [Cera et al., 2012b; Hagemann et al., 2003; Montorsi et al., 2003]. All the evidence suggests that the structural abnormality of vmPFC may be in favor of an adaptive cortical plasticity related to the negative psychological effects, which might be associated with the abnormal inhibitory mechanism of male SA in pED. In addition, pED patients showed CTh reductions in PCC, LTC, and the right dmPFC, which were also proposed to contribute to inhibition of SA [Poeppl et al., 2014; Stoleru et al., 2012] and showed abnormal activations during SA in pED [Cera et al., 2012b; Hagemann et al., 2003]. PCC including the adjacent retrosplenial cortex, together with the medial PFC, has been proposed to form the cortical midline circuit that is mandatory for the constitution of the self [Northoff and Bermpohl, 2004]. Negative correlations between dmPFC activations and erectile response have been reported [Moulier et al., 2006; Mouras et al., 2008]; and dmPFC activations have been suggested to be related to higher order processing of socially relevant stimuli, which could also be of particularly high self-relevance [Walter et al., 2008]. Accordingly, PCC and dmPFC may also serve the regulation of introspective and self-reflexive processes in the context of SA. The temporal cortex may be important in the intrinsic inhibition of SA, as lesions in the temporal cortex have been associated with various disturbances in sexual appetite [Braun et al., 2003] and have been assumed to be accompanied by hypersexuality [Stein et al., 2000]. Therefore, the morphometric alterations detected in these regions further illustrate the existence of structural impairment of the sexual inhibitory control network in pED.
In a recent study focusing on subcortical structures [Cera et al., 2012a], Cera and colleagues observed GM atrophy of nucleus accumbens and hypothalamus in pED, which was associated with poor sexual functioning and was believed to affect the motivational component of male SA. Since our analysis was based on a cortical surface model, the subcortical regions were not included in this work. However, we found CTh reductions in the left SMG and the right MCC, which, together with nucleus accumbens, hypothalamus and other subcortical structures, may mediate the processes directing behavior to a sexual goal [Redoute et al., 2000; Stoleru et al., 1999, 2012]. Particularly, poor male sexual functioning was significantly associated with reduced CTh in the right MCC. These findings might support the hypothesis that the impairment of the sexual motivational network of SA is an important factor causing psychogenic sexual dysfunction [Georgiadis and Kringelbach, 2012]. Nevertheless, the impairment of sexual motivational circuits in pED patients, in contrast, might also be a consequence of ED, because ED could induce reactive negative mood effects as a consequence of unsatisfactory sexual experience [Shiri et al., 2007], which could decrease the patients’ sexual desire [Lourenco et al., 2011]. Specific studies on the causal relationship between the impairment of the sexual motivational network and pED are required.
Normal nocturnal erections and penile hemodynamics were verified for pED patients. However, nocturnal erection and wakeful erection are, in fact, triggered by distinct systems, the former primarily depends on spinal reflexes whereas the latter is predominantly controlled by the brain [Montorsi and Oettel, 2005]. Thus, it is not surprising that pED patients also showed CTh reduction that was correlated with low sexual functioning in the regions mediating male sexual autonomic functions, such as the right INS and the left rostral ACC. INS may be related to awareness of penile erection [Arnow et al., 2002; Moulier et al., 2006], and may be also responsible for sustained penile response to erotic stimuli [Ferretti et al., 2005; Moulier et al., 2006; Mouras et al., 2003]. The rostral ACC is involved in modulating autonomic and endocrine functions such as gonadal and adrenal secretion [Devinsky et al., 1995]. Electrical stimulation to rostral ACC has been shown to bring erection in monkeys [Dua and Maclean, 1964; Robinson and Mishkin, 1968]. The structural abnormalities in these sexual autonomic regions could be related to their decreased activations during sexual stimuli in pED observed with functional neuroimaging [Cera et al., 2012b].pED patients also showed CTh loss in the left vPM and the bilateral ITC that were thought to contribute to cognitive processes of SA [Stoleru et al., 2012], and CTh measurements in these areas were significantly correlated with IIEF scores. The lateral premotor cortex including vPM has been proposed to use information from other cortical regions to select movements appropriate to the context of the action [Purves et al., 2001]. Particularly, in subjects presented with sexual relevant stimuli, the activation in vPM may reflect externally triggered preparation of movements [Stoleru et al., 2012] or the neural correlate of sexual motor imagery experienced by the subjects [Moulier et al., 2006; Mouras et al., 2003; Stoleru et al., 2003]. Functional neuroimaging studies of normal male SA indicated that the response of ITC may be related to differential perceptions of stimuli as sexual or nonsexual stimuli, and that the level of the ITC activation is correlated with the magnitude of erection [Ferretti et al., 2005; Redoute et al., 2000]. Especially, decreased activation of ITC was observed in pED patients during sexual stimuli [Hagemann et al., 2003]. These indicate that the structural abnormalities in the cognitive network of SA might also be related to the pathogenesis of pED.
It is interesting that we also observed CTh reductions in TPO and visual (occipital cortex) and auditory (HES) regions, and significant correlations between CTh and IIEF score in these regions. Activations of the visual and auditory cortices, respectively, in response to visual [Arnow et al., 2002; Beauregard et al., 2001; Mouras et al., 2003] and auditory [Ethofer et al., 2007; Miyagawa et al., 2007] sexual stimuli have been repeatedly found in healthy men. These functional study findings suggest that these sensory areas might process information related to the sexually arousing character of visual or auditory stimuli [Stoleru et al., 2012]. TPO has been proposed to bind complex, highly processed perceptual inputs to visceral emotional responses [Olson et al., 2007]. However, the reported activations in the sensory regions may be not specifically related to sexual condition [Mouras et al., 2003], and existing functional neuroimaging studies on male sexual dysfunction did not observe abnormal dynamics in these regions in patients. Thus, it needs caution to interpret these results, and specific study is needed to investigate how these structural changes are associated with pED.
Altered Structural Covariance Network Organization in pED
Male SA is a multidimensional experience involving psychosexual and physiosexual components and inhibitory processes that relay on widespread brain regions [Poeppl et al., 2014; Stoleru et al., 2012]. An understanding of how the human brain produces processes underlying male sexuality ultimately depends on the knowledge of the brain networks coordinating these brain regions. In a recent study, Zhang et al. [2014] using DTI and tract-based spatial statistics (TBSS) found that pED patients showed abnormalities in the WM microstructure. The reported reduced values of mean and axial diffusivity in the corpus callosum, corticospinal tract, internal capsule, corona radiata, external capsule and the superior longitudinal fasciculus indicate dysmyelination in these tracts, that is, impaired anatomical connectivities between the connected regions [Bar-Shir et al., 2009; Tyszka et al., 2006]. In this work, we used the interregional correlations in CTh to construct structural brain networks. Numerous existing works have demonstrated that interregional covariances in CTh are partly associated with known neuroanatomical pathways in the human brain [Gong et al., 2012; He et al., 2007; Lerch et al., 2006]. Nevertheless, CTh correlations also contain unique information. Since CTh correlations may arise from genetic, maturational and functional interaction effects [Evans, 2013], the examination of SCNs of CTh can possibly also contribute to the understanding of functional connectivities [Clos et al., 2014].
In this study, we observed several abnormal interregional CTh correlations in pED patients. Decreased positive correlations were found between the right ORBinf and the left ANG, and between the right ORBinf and the right SMG. The right ORBinf is located at the right lateral OFC that is thought to play a role in the assessment of the sexual relevance of stimuli [Stoleru et al., 2012]. ANG is considered to be involved in the inhibitory control of male SA [Poeppl et al., 2014; Stoleru et al., 2012]. SMG may contribute to the processes directing behavior to sexual desire [Redoute et al., 2000]. It has been postulated that the cognitive appraisal process of sexual stimuli is the first step in the development of SA, with other components depending on it [Stoleru et al., 1999]. Thus, these decreased positive correlations may indicate disassociations of the inhibitory and motivational circuits of male SA with the cognitive network of male SA, which might account for the failure of releasing inhibitory control of male SA [Cera et al., 2012b; Hagemann et al., 2003; Montorsi et al., 2003] and the impairment of sexual motivational circuits in pED patients [Cera et al., 2012a].pED patients showed increased positive correlation between the right INS and the right IFGoperc. In the above scale space search based brain morphometric analysis, the right INS showed significant CTh reduction in patients, and the right IFGoperc also showed a trend of CTh decreasing (P < 0.05, uncorrected). We could speculate that the increased correlation between the two regions may reflect a correlative cortical thinning due to shared vulnerability to an insult, as preferentially affected by the pathological process of pED.
We also noted abnormal negative correlations in pED patients. The interpretation of negative CTh correlations requires caution, as it remains unclear as to what they precisely reflect. One hypothesis is that they are related to weakened or strengthened interregional inhibitory trophic relationship between the regions [He et al., 2007; Mechelli et al., 2005]. Moreover, it has been found that negative CTh correlations are not mediated by direct fiber connections [Gong et al., 2012]. Therefore, the negative correlations observed in this work might concur with the observation of impaired WM fiber connectivities in pED [Zhang et al., 2014].
METHODOLOGICAL LIMITATIONS
Several methodological limitations in our current study need to be addressed. First, this work focused on the brain structural alterations and their correlations with the male erectile functioning measured as IIEF scores, and assessed only the two major psychological factors directly related to pED - depression and anxiety. In fact, there are several other psychological factors that may be directly associated with male sexual dysfunction, such as fear, personality, and other psychosocial stresses [Farre et al., 2004; Lizza and Rosen, 1999]. In the future work, it is desirable to include other aspects of male sexual behavior and psychological factors for a more complete understanding, using additional psychosexual or psychological tests, e.g. the Sexual Arousal Inventory (SAI) [Hoon et al., 1976], the Behavioral Inhibition/Behavioral Activation Scale (BIS/BAS) [Carver and White, 1994], the Revised NEO Personality Inventory (NEO-PI-R) [Costa and McCrae, 2000], and etc.
Second, the cross-sectional nature of this investigation prevented us from directly testing the temporal relation between cortical structural and network abnormalities and the course of pED. Future studies should include longitudinal evaluations.
Third, although possible structure-function relationships in pED derived from the present results have been discussed, multimodal approaches investigating the structure and function at the same time are expected to directly demonstrate if and how the cortical structural abnormalities are associated with functional deficits. For example, vmPFC that showed the most prominent CTh reduction and the regions that showed significantly alterations in structural connectivity can be applied as seeds to investigate the intrinsic or the male sexual behavior dependent functional connectivities in pED using resting-state or task-dependent functional MRI respectively. Moreover, sophisticated multivariate analysis and variable selection methods like support vector machines or elastic net regression may also be applicable for pooling multimodal imaging data to reveal common sites of pathology [Mueller, et al., 2012a,2012b]. In addition, the brain structural networks were obtained by calculating the interregional CTh correlations across subjects in each group. Thus, we could not explore the relation between network properties and male sexual functioning or psychiatric factors in individual subjects. Including other imaging modalities, such as resting-state functional MRI [Achard et al., 2006; Liu et al., 2008] and DTI [Gong et al., 2009; Iturria-Medina et al., 2008] data will allow measurements of spontaneous brain functional connectivity and WM integrity at the individual level.
Fourth, we used the anatomical AAL atlas for cortical parcellation to construct brain networks. Because the relationship between anatomical and functional anatomies is not precisely known, some of the regional boundaries in the atlas may be arbitrary and some functional subdivisions may not be included or separated. The use of an alternative atlas with higher resolution, which is not constrained by anatomical landmarks is desirable in the future.
Finally, subcortical regions were not included as this study was based on a cortical surface model. Although it is out of the scope of this work to replicate the pED-related subcortical structural changes reported in the previous study [Cera et al., 2012a], it will be a meaningful future work to extend the study to subcortical regions to explore their contributions to the disruptions of the brain network architecture in pED.
CONCLUSIONS
In conclusion, this work demonstrated the existence of cortical structural abnormalities in the cognitive, motivational, autonomic, and inhibitory networks for male SA in pED patients, and illustrated that such abnormalities were associated with degraded male sexual functioning. We also revealed the disassociations between the networks mediating the cognitive, motivational and inhibitory processes of male SA in pED, which could be related to the abnormal brain dynamics in pED patients. These results provide structural insights on the complex phenomenon of sexual dysfunction in men, and suggest a specific vulnerability factor, possibly as an extra ‘organic’ factor, that may play an important role in the pathogenesis of pED. Overall, the present findings are important, as a better understanding of the underlying neuroanatomical correlates of pED may assist to identify biomarkers for clinical diagnosis and could open new perspectives in therapy development for male sexual disorders.
ACKNOWLEDGMENTS
The funding organizations had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors are grateful to Dr. Shan Jiang, Department of Neurology, Henan Provincial People's Hospital, for her great collaboration and assistance on the psychiatric assessments using M.I.N.I and BPRS.