Virtual water maze learning in human increases functional connectivity between posterior hippocampus and dorsal caudate
Conflict of Interest: The authors declare no conflict of interest.
Abstract
Recent work has demonstrated that functional connectivity between remote brain regions can be modulated by task learning or the performance of an already well-learned task. Here, we investigated the extent to which initial learning and stable performance of a spatial navigation task modulates functional connectivity between subregions of hippocampus and striatum. Subjects actively navigated through a virtual water maze environment and used visual cues to learn the position of a fixed spatial location. Resting-state functional magnetic resonance imaging scans were collected before and after virtual water maze navigation in two scan sessions conducted 1 week apart, with a behavior-only training session in between. There was a large significant reduction in the time taken to intercept the target location during scan session 1 and a small significant reduction during the behavior-only training session. No further reduction was observed during scan session 2. This indicates that scan session 1 represented initial learning and scan session 2 represented stable performance. We observed an increase in functional connectivity between left posterior hippocampus and left dorsal caudate that was specific to scan session 1. Importantly, the magnitude of the increase in functional connectivity was correlated with offline gains in task performance. Our findings suggest cooperative interaction occurs between posterior hippocampus and dorsal caudate during awake rest following the initial phase of spatial navigation learning. Furthermore, we speculate that the increase in functional connectivity observed during awake rest after initial learning might reflect consolidation-related processing. Hum Brain Mapp 36:1265–1277, 2015. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
To date, much of the neuroimaging work on spatial navigation in human has focused on blood-oxygen-level-dependent (BOLD) activation during encoding and retrieval processes. It is well established that the hippocampus plays a central role in spatial navigation, with previous studies often highlighting its functional importance to place-based navigation strategies [Doeller et al., 2008; Hartley et al., 2003; Iaria et al., 2003; Marchette et al., 2011; Spiers and Maguire, 2006]. The contribution of dorsal striatum to spatial navigation is also of significant interest, with this region most often associated with nonspatial navigation strategies such as landmark-based navigation or route following [Doeller et al., 2008; Hartley et al., 2003; Hirshhorn et al., 2012; Iaria et al., 2003]. However, it is worth noting that work in rodents has demonstrated an important functional dissociation between medial (caudate) and lateral (putamen) subdivisions of dorsal striatum. The caudate appears to play a generalized role during the initial learning phase of goal-directed tasks, whereas the putamen is likely involved in a shift to habitual behavior during the late learning phase [Khamassi and Humphries, 2012; Yin and Knowlton, 2004, 2006]. Here, we investigated how spatial navigation learning and stable performance modulates connectivity between hippocampus and striatum in human using resting state functional magnetic resonance imaging (rs-fMRI).
rs-fMRI measures the extent to which fluctuations in BOLD signal in spatially remote brain areas are temporally correlated at rest and is thought to reflect functional connectivity [Biswal et al., 1995; Fox et al., 2005]. Previous studies have demonstrated that functional connectivity can be modulated by task performance [Barnes et al., 2009; Stevens et al., 2010; Tung et al., 2013; Waites et al., 2005] or task learning [Albert et al., 2009; Lewis et al., 2009; Tambini et al., 2010; Vilberg and Davachi, 2013]. Studies have typically used a rest-task-rest design to identify changes in functional connectivity between pretask and post-task performance or learning. For example, performance of an n-back working memory task modulated functional connectivity in both task active and negative networks for several minutes post-task performance [Barnes et al., 2009]. Motor learning increased functional connectivity within a fronto-parietal resting-state network when compared to the same motor task without learning [Albert et al., 2009].
Task modulated functional connectivity has also been found to correlate with changes in behavioral performance, suggesting a link between postlearning functional connectivity and consolidation-related processing. Visual perceptual learning modulated functional connectivity between task-related network nodes, and the change in functional connectivity was correlated with behavioral performance [Lewis et al., 2009]. Associative learning modulated hippocampal-neocortical functional connectivity, which was also correlated with behavioral performance [Tambini et al., 2010; Vilberg and Davachi, 2013]. In sum, these studies confirm that rs-fMRI provides a sufficiently sensitive method to detect anatomically specific changes in functional connectivity, and that the observed changes in functional connectivity can correlate with subsequent behavioral performance.
Here, we used rs-fMRI to investigate how systems level functional connectivity is modulated by active spatial navigation learning. We measured rs-fMRI before and after early and late stages of virtual water maze learning, a task that is analogous to the commonly used paradigm in rodent [D'Hooge and De Deyn, 2001]. Our a priori anatomical hypothesis focused on characterizing functional connectivity between subregions of hippocampus and striatum. The hippocampus was selected as the starting point for this analysis due to the overwhelming evidence in humans and rodents that this structure is involved in spatial learning [for reviews, see, Martin and Clark, 2007; Spiers and Maguire, 2007]. We specifically targeted functional connectivity between hippocampus and striatum as we previously observed extensive activation in dorsal caudate and putamen during early and late phases of virtual water maze learning [Woolley et al., 2013]. Furthermore, others have suggested that interaction between these structures is critical during spatial navigation [Brown and Stern, 2014; Brown et al., 2012]. Therefore, we sought to characterize functional connectivity between hippocampus and striatum, and determine if it is modulated by virtual water maze learning.
METHODS
We first performed a hierarchical clustering analysis to parcellated hippocamapus into functional subdivisions. We then identified which subregions of hippocampus and striatum are functionally connected either before or after learning, and tested whether the observed functional connectivity was mediated by additional cortical nodes. Finally, we determined the extent to which hippocampus-striatum functional connectivity changed over the course of learning, and whether learning-related changes correlated with future behavioral performance.
Subjects
Eighteen human female subjects (aged 20–28, mean age 23.1) participated in this study. The data reported here are a subset from a study that performed direct comparisons between human and rodent subjects [Woolley et al., 2013]. Only female rodents were tested in that study, and to maximize similarity for interspecies comparisons, only female humans were tested. All subjects were right handed with no history of neurological disease. Prior to testing, subjects were required to provide written informed consent to the procedures, which were approved by the Ethics Committee of KU Leuven in accordance with the Declaration of Helsinki.
Task
A custom virtual environment analogous to the water maze was constructed in Blender (www.blender.org) and rendered in MATLAB (2007b, The Mathworks). The environment consisted of a circular pool (diameter = 16 virtual reality units (vru), height = 0.5 vru) situated 0.5 vru above ground level in the center of a square room (length = 20 vru, height = 8 vru). Within the pool was a hidden platform 1.6 vru in diameter. There was only one distinguishing feature in the environment, a black cross located on a wall approximately half way between the floor and the ceiling in the opposite corner of the room to the quadrant in which the hidden platform was located. Subjects viewed the room from a first-person perspective, and moved around by pressing buttons on an MRI compatible button box (Current Designs). Movement was restricted to either forward displacement or orienting (i.e., rotating left and right). A single button press resulted in a forward movement of 0.1 vru or rotation of 1.5°. Data were recorded at 25 Hz.
Trial Procedures
Over the course of the experiment, subjects performed “search,” “prediction,” and “control” trials. All trials began from one of four starting zones (separated by 90°) located at the perimeter of the pool, with the exact position within a given starting zone varying by ±10° from trial to trial. Subjects always faced the center of the pool at the beginning of the trial.
The goal of search trials was to navigate to the hidden platform as quickly and directly as possible. When the goal location was successfully intercepted, the walls of the room turned green for 1 s, after which the subject remained at the same location for a further 3 s. During this 4 s period, forward movement and orienting were not possible. The maximum time limit for search trials was 45 s. If a trial reached the maximum time limit, the walls of the room turned red for 1 s, after which the subject remained at their final unsuccessful location for a further 3 s.
Prediction trials required the subject navigate to where they thought the hidden platform was located and indicate this position via a button press. If the goal location was correctly identified, the walls of the room turned green for 1 s, after which the subject remained at the same location for a further 3 s. If the goal location was not correctly identified, the walls of the room turned orange, movement was again possible, and two additional predictions were allowed. After three incorrect predictions or 45 s (whichever came first), the walls of the room turned red for 1 s, after which the subject remained at their final unsuccessful location for a further 3 s.
During control trials, subjects moved freely within the pool. No distinguishing features were present on the walls, preventing any goal-directed navigation. Control trials were matched to the average duration of search trials (between 10 and 20 s) and finished in a similar manner, with the only difference being that the color of the walls always turned blue (which did not relate to feedback provided during other trials).
Experimental Protocol
Four testing sessions were completed, each on a separate day. The first session familiarized subjects with the experimental procedures and trial order prior to scanning. During this session, a limited number of trials were performed in a different environment to that used in the main experiment.
One or two days later, subjects returned for the first scan session. From this session onward, the environment and the location of the hidden platform was unchanged. Subjects performed six runs of trials, with each run lasting at least 8 min. The order of presentation of search, prediction, and control trials was determined as follows: each run always started with a search trial. An unsuccessful search trial was repeated until the hidden target zone was successfully intercepted. Once a successful search trial was completed, a prediction and control trial followed with a randomized order of presentation. This sequence was then repeated. The current trial type was always displayed in small text at the top of the screen. Subjects rested for 5–10 s between trials and were required to fixate on a white cross in the center of a black screen. A second identical scan session was performed 7 days after the first. Subjects performed an average of 5.6 trials per run in scan session 1 (range: 5–9) and 5.9 trials per run in scan session 2 (range: 5–7).
Between scan sessions, subjects performed a training session during which only behavioral data were acquired. The behavioral training session was performed 3 days after scan session 1 and also consisted of six runs of trials each lasting at least 8 min. Trials were performed in a similar order to the scan sessions, except that control trials were not included and the rest periods were reduced to between 2 and 4 s.
Resting-State Protocol
During the scan sessions, subjects were scanned for 7 min in a resting state before and after task performance (i.e., two scans in each session, four scans in total). Subjects were required to fixate on a white cross in the center of a black screen, and were instructed to relax and think of nothing in particular.
Behavioral Analysis
The time taken to find the hidden platform on search trials was used to quantify performance (trial time). To test for learning within each session, we conducted a one-way repeated measures ANOVA (Runs 1–6). Statistical analyses were performed in Statistica 9 (StatSoft). The α-level was set to 0.05. Part of this data was previously reported in Woolley et al. [2013].
Image Acquisition
A Siemens 3 T Magnetom Trio MRI scanner with 12 channel head coil was used for image acquisition. For all subjects, a T1-weighted structural image was acquired using a magnetization prepared rapid gradient echo sequence (repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, 1 × 1 × 1.1 mm voxels, field of view: 240 × 256, 160 sagittal slices). rs-fMRI data were acquired with a descending gradient echo planar imaging (EPI) pulse sequence for T2*-weighted images (TR = 3000 ms, TE = 30 ms, flip angle = 90°, 50 oblique axial slices each 2.8 mm thick, interslice gap 0.028 mm, in-plane resolution 2.5 × 2.5 mm, 80 × 80 matrix).
Image Preprocessing
Image preprocessing was conducted using SPM8 (Wellcome Department of Imaging Neuroscience, University College London). rs-fMRI images were spatially realigned, slice time corrected to the middle slice (reference slice = 25), normalized to the standard EPI template of the Montreal Neurological Institute (MNI), and resampled into 3 mm isotropic voxels.
To improve alignment across subjects in hippocampus and striatum, a region of interest alignment procedure established by the Stark Lab was applied [Stark and Okado, 2003; Yassa and Stark, 2009]. FAST v4.1 (implemented in FSL v4.1.6) was used to create bilateral hippocampus and striatum segmentations based on each individual subject's structural image [Smith et al., 2004; Zhang et al., 2001]. Automatic segmentation results were manually checked for accuracy and adjusted where necessary. High-dimensionality diffeomorphic techniques (ROI-Demons) were then used to map the transformation between an individual's ROI segmentation and a model ROI (the model ROI for each region was a modal ROI that represented the central tendency of all subjects). ROI-demons generates a smooth 3D vector that is used to transform images between subject space and model space. After each subject's structural image was aligned to the model, the resulting transformation matrices were applied to the normalized rs-fMRI images. This technique has previously been used to improve alignment across subjects in the hippocampus and striatum [Mattfeld and Stark, 2011].
Linear regression was used to account for confounds in the measured BOLD response. Regressors of no interest included white matter and ventricle signals and their first derivatives [Fox et al., 2005], and the BOLD time course of a posterior cingulate cortex seed to ensure functional connectivity measures were independent of the default mode network. Global signal regression was not applied.
Recently, there has been considerable discussion over the impact of head motion on resting-state functional connectivity analyses. In addition to regressing out the three-dimensional motion parameters and their first derivatives, we also included regressors to deweight scans with a framewise displacement greater than 0.5 mm. A separate regressor was included for each outlier scan, with a one at the outlier time point and a zero at all other time points. Framewise displacement was calculated as the sum of the absolute scan to scan difference of the six translational and rotational realignment parameters [Power et al., 2012]. Only 1.2% of all scans exceeded this threshold, and there was no significant difference in mean framewise displacement between the four resting-state runs (one-way ANOVA: F3, 68 = 0.2, P = 0.89).
The BOLD time course in each voxel was temporally band-pass filtered between 0.009 and 0.08 Hz. Finally, rs-fMRI images were spatially smoothed with an isotropic 5 mm full-width-at-half-maximum Gaussian kernel.
Statistical Analysis of rs-fMRI Data
A hierarchical cluster analysis was performed on Run 1 data from scan session 1 (collected prior to subjects engaging in any task), with temporal correlation as a similarity metric and an average linkage function used to compartmentalize hippocampus into functional subdivisions characterized by distinct resting-state activity [Everitt et al., 2001; Mantini et al., 2011]. This analysis revealed bilateral clusters in anterior, mid, and posterior hippocampus. While the hierarchical clustering of resting-state data takes advantage of interhemispheric connectivity, functional lateralization in hippocampus is an important consideration, particularly in the context of navigation and memory [Burgess, 2002]. For this reason, unilateral hippocampal seeds were used in subsequent analyses.
Hippocampus-striatum connectivity maps were created for individual participants by calculating correlations between the average time course of all voxels in each of the six hippocampal ROIs and the time courses of each voxel in striatum [Fox et al., 2005]. After applying Fisher's r-to-z transformation to correlation maps, a random effects analysis was performed to reveal a pattern of functional connectivity that was consistent across subjects [Fox et al., 2006]. These procedures are commonly referred to as a seed-based analysis. Statistical significance was assessed separately for the prelearning and postlearning sessions at the voxel level by means of one sample t-tests with a statistical threshold of P < 0.05. One tailed tests were applied as we had an priori hypothesis that consolidation-related functional connectivity would manifest as significant correlations greater than zero [Albert et al., 2009; Tambini et al., 2010; Vilberg and Davachi, 2013]. We corrected for multiple comparisons across all seed regions by controlling False Discovery Rate (FDR) [Genovese et al., 2002]. This analysis sought to identify subregions in hippocampus and striatum that were positively correlation during the learning session, that is, either before and/or after subjects acquired the virtual water maze task. No significant hippocampus-striatum pairs were identified prelearning. Seven significantly correlated hippocampus-striatum pairs were identified postlearning.
To determine if the correlation between the hippocampus-striatum pairs identified in the previous step was direct or mediated by a third node, a partial correlation analysis was performed. For each hippocampus-striatum pair, additional nodes were defined as regions which had overlapping connectivity with both hippocampal and striatal clusters. Whole brain connectivity maps for each cluster were created using a seed-based analysis. Each of the resulting connectivity maps in a hippocampus-striatum pair was thresholded at P < 0.05 (FDR corrected). Two tailed tests were applied to ensure the inclusion of anticorrelated nodes if present. A conjunction analysis was performed to identify voxels that were significantly correlated with both hippocampal and striatal clusters [Nichols et al., 2005]. For each set of clusters (hippocampal cluster, striatal cluster, overlapping cluster), partial correlations were calculated via regularized inverse covariance estimation [Smith et al., 2011], using NetSim code (implemented in MATLAB) made available by the FMRIB Analysis Group, Oxford (www.fmrib.ox.ac.uk/analysis/netsim). This method was used to calculate partial correlations at the individual subject level, which were then converted to z scores with Fisher's r-to-z transformation. Group level statistical significance was assessed with a one sample t-test. If a partial correlation remained significant, direct functional connectivity between the hippocampus-striatum clusters was considered likely (in addition to hippocampal and striatal connectivity to the overlapping cluster).
Learning-related changes in functional connectivity between each hippocampus-striatum pair was assessed by entering Fisher's r-to-z scores from across the four resting-state runs into a repeated measures ANOVA (2 sessions × 2 phases). Statistical analyses were performed in Statistica 9. The α-level was set to 0.05. Interactions and main effects were Bonferroni corrected for the number of hippocampus-striatum pairs tested (corrected P values reported). Post hoc tests were also Bonferroni corrected. Brain-behavior correlations were then calculated to determine the extent to which learning-related changes in functional connectivity corresponded with the subsequent offline gain in behavioral performance, which was quantified as the difference between the average trial time of the last block of trials in the first scan session and the first block of trials in the additional training session.
Finally, control analyses were performed to identify the extent to which the observed learning-related increase in functional connectivity reflected activation during task performance. Some of the control data were previously published in Woolley et al. [2013], which used task-related fMRI to examine brain activity during water maze learning (whereas the present data set focuses on the exploration of changes in functional connectivity pretask and post-task learning using rs-fMRI). Cases where control data were also reported in Woolley et al. [2013] are explicitly indicated.
RESULTS
Subjects actively navigated through a virtual water maze environment and used visual cues to learn the position of a hidden platform, similar to the rodent version of the task (Fig. 1A). rs-fMRI scans were acquired before and after task performance in two sessions approximately 1 week apart. In between scan sessions, subjects performed additional task training in a behavior-only session.
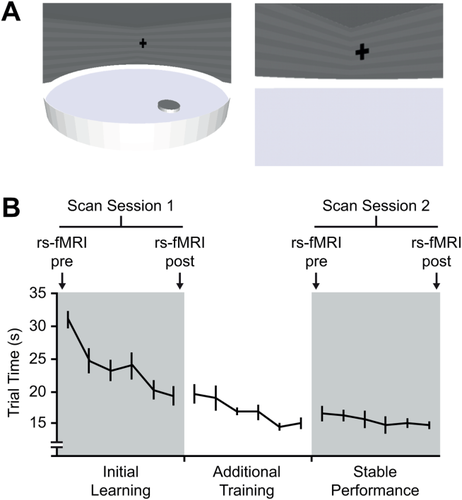
(A) Virtual water maze. The left image shows an overview of the virtual water maze environment. The right image shows the water maze when viewed from the first-person perspective. (B) The significant reduction in trial time over the course of scan session 1 was indicative of initial learning. Additional training was undertaken in a behavior-only session 3 days later. Performance was stable during scan session 2, conducted 7 days after scan session 1. rs-fMRI was measured pretask and post-task performance in both scan sessions. Error bars represent SEM. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Task Performance
The behavioral performance of subjects in scan session 1 was characterized by a goal directed but variable search pattern. Over the course of this session, the time taken to intercept the target location decreased significantly (F5, 85 = 15.6, P < 0.001; Fig. 1B). During the behavior-only training session, a further reduction in trial time was found (F5, 85 = 4.6, P < 0.001; Fig. 1B), indicating learning had not plateaued in the first scan session. The search pattern during scan session 2 was highly focused on the hidden platform location and a further reduction in trial time was not observed (F5, 85 = 0.73, P = 0.60). Figure 1B shows that behavioral performance in scan session 2 reached a plateau. A direct comparison of trial time between scan sessions revealed that performance was significantly better during the second scan session compared to the first (F1, 17 = 35.5, P < 0.001). These results confirm that behavioral performance was representative of initial task learning during scan session 1 and stable task performance during scan session 2.
Hippocampus-Striatum Functional Connectivity
Hierarchical clustering within left and right hippocampus on the first resting-state run acquired prior to learning revealed three highly correlated bilateral clusters organized along the anterior-posterior axis (labeled anterior, mid and posterior hippocampus; Fig. 2). Hippocampal clusters were divided by hemisphere and entered into a seed-based analysis to identify clusters in striatum with high functional connectivity either prelearning or postlearning. Seven hippocampus-striatum pairs were found to have significantly correlated BOLD time courses postlearning (Table 1). In general, clusters in posterior and mid hippocampus were functionally connected to clusters in ventral putamen, ventral caudate and dorsal caudate, with the majority of the pairs identified in the left hemisphere.
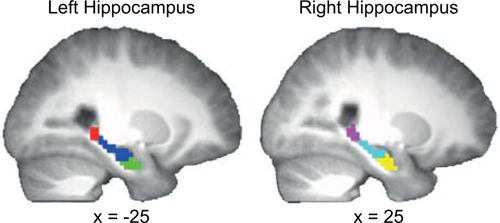
Hierarchical clustering resulted in three bilateral hippocampal subdivisions. The posterior subdivision is displayed in red (left hippocampus) and violet (right hippocampus), the mid subdivision in blue (left hippocampus) and cyan (right hippocampus), and the anterior subdivision in green (left hippocampus) and yellow (right hippocampus). Coordinates are in MNI space. The template image is the average T1-weighted structural image of 18 subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Hippocampal node | Striatal node | Peak coordinate (MNI) | k | Peak T value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left mid hippocampus | Left ventral caudate + Putamen | −9 | 11 | 1 | 36 | 4.65 |
| Left ventral putamen | −27 | −4 | −8 | 18 | 4.72 | |
| Left posterior hippocampus | Left dorsal caudate | −12 | 11 | 13 | 18 | 5.39 |
| Left ventral putamen | −27 | −7 | −5 | 12 | 5.29 | |
| Right ventral putamen | 30 | −10 | −11 | 16 | 4.84 | |
| Right posterior hippocampus | Left ventral putamen | −24 | 5 | −11 | 21 | 5.37 |
| Right ventral putamen | 30 | −10 | −11 | 22 | 6.82 | |
- k represents the number of voxels in the striatal node.
To investigate the extent to which the correlation in BOLD time courses between hippocampus-striatum pairs represented direct connectivity we performed a partial correlation analysis. First, we identified potential mediating nodes by performing a conjunction analysis between whole brain connectivity maps for each hippocampus-striatum pair. Additional nodes were defined as clusters with significant overlapping connectivity (see detailed anatomy in Table 2). We then tested if the partial correlation between hippocampus and striatum remained significant when regressing out the influence of each additional node. In all cases, the partial correlations remained significant suggesting functional connectivity between the hippocampal-striatal pairs was not fully mediated by the additional nodes that shared overlapping connectivity (Table 3).
| Hippocampal node | Striatal node | Additional node | Peak coordinate (MNI) | k | Peak T value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left mid hippocampus | Left ventral caudate + putamen | Left orbitofrontal cortex | −7 | 52 | −8 | 7 | 4.71 |
| Left ventral putamen | Left superior temporal gyrus | −39 | 12 | −19 | 4 | 5.83 | |
| Right superior temporal gyrus | 35 | 15 | −25 | 7 | 5.45 | ||
| Left posterior hippocampus | Left dorsal caudate | None | |||||
| Left ventral putamen | Left subtantia nigra | −13 | −15 | −11 | 3 | 6.16 | |
| Left ventral prefrontal cortex | −21 | 33 | −10 | 2 | 7.13 | ||
| Right ventral putamen | Left ventral prefrontal cortex | −23 | 33 | −10 | 2 | 7.65 | |
| Right posterior hippocampus | Left ventral putamen | None | |||||
| Right ventral putamen | Left ventral prefrontal cortex | −25 | 35 | −10 | 2 | 5.69 | |
- k represents the number of voxels in the additional node.
| Hippocampal node | Striatal node | Full correlation | Additional node | Partial correlation | ||
|---|---|---|---|---|---|---|
| Z | P | Z | P | |||
| Left mid hippocampus | Left ventral caudate + putamen | 4.37 (0.69) | <0.001 | Left orbitofrontal cortex | 2.75 (0.69) | <0.001 |
| Left ventral putamen | 4.47 (0.72) | <0.001 | Left superior temporal gyrus | 2.68 (0.69) | 0.0012 | |
| Right superior temporal gyrus | 2.51 (0.50) | 0.0012 | ||||
| Left posterior hippocampus | Left dorsal caudate | 3.15 (0.54) | <0.001 | None | ||
| Left ventral putamen | 4.93 (0.90) | <0.001 | Left subtantia nigra | 3.32 (0.74) | <0.001 | |
| Left ventral prefrontal cortex | 2.81 (0.49) | <0.001 | ||||
| Right ventral putamen | 3.97 (0.76) | <0.001 | Left ventral prefrontal cortex | 3.3 (0.53) | < 0.001 | |
| Right posterior hippocampus | Left ventral putamen | 3.98 (0.62) | <0.001 | None | ||
| Right ventral putamen | 5.24 (0.77) | <0.001 | Left ventral prefrontal cortex | 4.46 (0.65) | <0.001 | |
- SEM is shown in brackets. t-tests were used to determine whether the average z score differed significantly from zero.
Learning-Related Changes in Functional Connectivity
Next, we determined if the high level of functional connectivity observed between hippocampus-striatum pairs was specifically related to early water maze learning, or whether it could be accounted for by task performance alone. A significant session x phase interaction was found only for the left posterior hippocampus—left dorsal caudate pair (F1, 17 = 9.35, P < 0.05; Fig. 3A). Figure 3B displays the increase in functional connectivity from prelearning to postlearning in session 1 (P < 0.01), and no change from pretask to post-task performance in session 2 (P = 1). Significant interactions or main effects were not found in other hippocampus-striatum pairs (Fig. 4A–F).

(A) Significant postlearning functional connectivity was identified between left posterior hippocampus and left dorsal caudate (P < 0.05 FDR corrected across all seed maps). Coordinates are in MNI space. The template image is the average T1-weighted structural image of 18 subjects. (B) The increase in functional connectivity between left posterior hippocampus and left dorsal caudate was learning specific. * indicates a significant interaction (P < 0.05 Bonferroni corrected for the total number of pairs tested). Error bars represent SEM. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
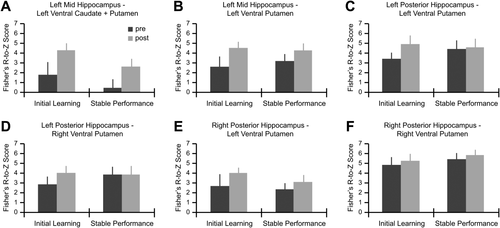
Bar plots show the change in connectivity prelearning and postlearning, and prestable and poststable performance between (A) left mid hippocampus and left ventral caudate + putamen, (B) left mid hippocampus and left ventral putamen, (C) left posterior hippocampus and left ventral putamen, (D) left posterior hippocampus and right ventral putamen, (E) right posterior hippocampus and left ventral putamen, and (F) right posterior hippocampus and right ventral putamen. No significant main effects or interactions were observed in the pairs of regions displayed here (P < 0.05 Bonferroni corrected for the total number of pairs tested). Error bars represent SEM.
We then tested if the increase in functional connectivity from prelearning to postlearning between the left posterior hippocampus and left dorsal caudate pair was correlated with behavioral performance during the first block of trials in the additional training session 3 days later. We found that larger increases in functional connectivity corresponded with greater offline gains in behavioral performance (rs = 0.47, P < 0.05; Fig. 5). Control correlations were performed to determine the specificity of this effect. First, neither prelearning (rs = 0.39, P = 0.11) nor postlearning (rs = 0.10, P = 0.69) connectivity between left posterior hippocampus and left dorsal caudate was correlated with subsequent offline gains in behavioral performance. Second, the change in connectivity from prelearning to postlearning for the other hippocampus-striatum pairs was also not correlated with subsequent offline gains in behavioral performance (rs ≤ 0.40, P ≥ 0.10).
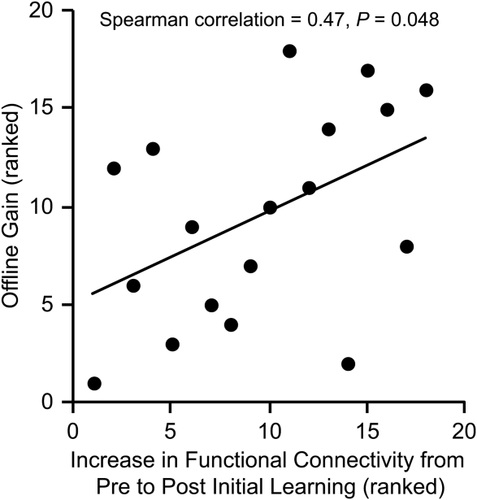
The increase in functional connectivity from prelearing to postlearning between left posterior hippocampus and left dorsal caudate was significantly correlated with offline gain in behavioral performance.
Finally, we conducted a set of control analyses to identify the extent to which the observed learning-related increase in functional connectivity reflected activation during task performance. We previously reported dorsal caudate and medial prefrontal cortex involvement during the early learning phase of the same virtual water maze task used in the present study [Woolley et al., 2013]. If an increase in functional connectivity is simply the result of ongoing task-related activity, similar increases in functional connectivity between left posterior hippocampus and medial prefrontal cortex and left dorsal caudate and medial prefrontal cortex might be expected. However, functional connectivity between these regions did not change over the course of the experiment (main effect of session: F1, 17 ≤ 1.08, P ≥ 0.31; main effect of phase: F1, 17 ≤ 0.12, P ≥ 0.73). Furthermore, task-related activity during initial learning in left posterior hippocampus and left dorsal caudate was not correlated with the increase in functional connectivity between these regions (rs ≤ 0.28, P ≥ 0.26), or with offline gain in behavioral performance (rs ≤ 0.14, P ≥ 0.58).
DISCUSSION
Our findings demonstrate that the early learning phase of a virtual Morris water maze task modulates functional connectivity between hippocampus and striatum during awake rest. Functional connectivity between left posterior hippocampus and left dorsal caudate increased from preinitial task learning to postinitial task learning (scan session 1) but not from prestable task performance to poststable task performance (scan session 2), indicating that the observed increase was specifically related to spatial navigation learning. Moreover, the magnitude of the increase in functional connectivity between left posterior hippocampus and left dorsal caudate was correlated with the offline gain in spatial navigation performance between the end of training in scan session 1 and the start of the training session 3 days later. This finding suggests that the post-task increase in functional connectivity between these areas might reflect consolidation-related processing.
Our results are consistent with recent rs-fMRI experiments in human that were the first to show that hippocampal-cortical functional connectivity measured during awake rest following an associative encoding task is correlated with future memory performance [Tambini et al., 2010; Vilberg and Davachi, 2013]. While these studies focused on the coupling between hippocampus and task-related cortical regions we specifically targeted hippocampus-striatum functional connectivity, in part because of the established contribution of these structures to the type of spatial learning demanded by our virtual water maze task [Morris et al., 1982; Woolley et al., 2013; Yin and Knowlton, 2004]. Although our results do not exclude the involvement of other brain areas in long-term memory formation, our partial correlation analysis suggests that the high level of functional connectivity we observed between hippocampus-striatum pairs postlearning was only weakly mediated by cortical and midbrain nodes that shared connectivity with both structures.
While there is no evidence for direct monosynaptic anatomical projections between the posterior hippocampus and dorsal caudate, primate work suggests that a potential route for communication between these structures is via prefrontal cortex. For example, hippocampus projects extensively to prefrontal regions including orbitofrontal cortex, medial prefrontal cortex, and dorsolateral prefrontal cortex, all of which project to specific subdivisions of caudate and putamen [Haber and Knutson, 2010]. Although we observed overlapping functional connectivity in prefrontal cortex for several hippocampus-striatum pairs, this was not the case for the left posterior hippocampus–left dorsal caudate pair. Alternatively, there is evidence for anatomical projections to caudate from medial temporal lobe structures such as the entorhinal cortex, perirhinal cortex, and parahippocampal cortex [Suzuki, 1996]. Given the extensive anatomical connections between these structures and hippocampus, this presents another potential route for communication particularly as rs-fMRI can visualize synchronization along monosynatpic and polysynaptic pathways [Lu et al., 2011]. If functional connectivity between hippocampus and caudate is likely mediated by prefrontal cortex or a surrounding structure in the medial temporal lobe, why then did we fail to identify a mediating node? It is possible that for this specific pair of regions, the conjunction analysis was not sensitive enough to identify a potential mediating third node. Furthermore, the observed change in functional connectivity might be mediated via a more complex network than we could detect with our partial correlation approach which was limited to identifying a single mediating third node.
Interestingly, the region in dorsal caudate that displayed a learning-related increase in functional connectivity with hippocampus in the present study is similar to the caudate region we identified as being critical during early water maze learning [Woolley et al., 2013]. While this suggests that the increase in functional connectivity might reflect consolidation-related processing, it also raises the possibility that functional connectivity observed during postlearning rest might simply be a consequence of persistent activity in task-related structures. This is especially plausible as task-related activation has also been shown to predict future memory performance [Brewer et al., 1998; Wagner et al., 1998]. However, task-related fMRI activity in left posterior hippocampus and left dorsal caudate during initial learning was not correlated with the increase in rs-fMRI functional connectivity between these regions from prelearning to postlearning, or subsequent behavioral performance. Furthermore, functional connectivity between these regions and medial prefrontal cortex, also previously identified as being significantly active during early water maze learning, did not change over the course of the experiment. Together these results suggest that the change in functional connectivity between left posterior hippocampus and left dorsal caudate does not reflect a mere echo of task-related activity.
What processes might the observed modulation in functional connectivity represent? The general pattern of results found here is similar to other reports [Lewis et al., 2009; Tambini et al., 2010; Vilberg and Davachi, 2013] that have linked postlearning functional connectivity to the “consolidation through neuronal reactivation” theory that was initially developed on the basis of animal work. It was first discovered in rodent that distinct patterns of place cell activity in the hippocampus occurring during a spatial experience are replayed later during sleep [Pavlides and Winson, 1989]. It has since been reported that reactivation also occurs during awake rest [Hoffman and McNaughton, 2002; Karlsson and Frank, 2009], and in coordinated manner across distinct brain regions [Lansink et al., 2009; Qin et al., 1997]. Although it was widely speculated that hippocampal reactivation plays an important role in the consolidation of spatial information, it was only recently demonstrated that it is in fact predictive of future behavioral performance [Dupret et al., 2010]. Moreover, disrupting hippocampal replay leads to an impairment in spatial learning and memory, suggesting a causal relationship between reactivation and consolidation [Ego-Stengel and Wilson, 2010; Jadhav et al., 2012]. We speculate that the increase in functional connectivity observed here might reflect coordinated reactivation between hippocampus and caudate, and that this reactivation supports consolidation-related processing.
It has often been postulated that hippocampal function is lateralized, particularly with respect to spatial navigation processes. In a recent quantitative meta-analysis of human neuroimaging studies, it was reported that while spatial navigation retrieval is strongly lateralized to the right posterior hippocampus, spatial navigation encoding processes utilize both left and right posterior hippocampus [Kühn and Gallinat, 2014]. Given our speculation that the observed change in functional connectivity might reflect the reactivation or replay of encoding processes, a change in functional connectivity in either or both hemispheres would be predicted on the basis of prior work. Therefore, it is important to note that we do not suggest that the observed change in functional connectivity reflects a lateralized process confined to a left hemisphere network.
Wegman and Janzen [2011] previously examined changes in functional connectivity before and after object learning while navigating along a fixed route and reported an increased negative correlation between parahippocampal gyrus and caudate. While these results appear contrary to our main finding, we suggest that differences in task demands are likely to be at least partly responsible. Wegman and Janzen [2011] used a passive navigation task with a main focus on object learning. Multiple routes were presented and a total of 144 objects were viewed over the course of learning. They reported greater activation in parahippocampal gyrus for objects at decision points compared to non decision points along the route, highlighting the role of this region in distinguishing between navigationally relevant and irrelevant objects. Therefore, the prelearning to postlearning increase in negatively correlated BOLD signal between parahippocampal gyrus and caudate was related to the learning of objects and object locations important for navigation. In our task, subjects were instead required to actively navigate to and learn a single spatial location.
Noninvasive imaging experiments in human offer a very different form of evidence to that which is obtained from invasive electrophysiology experiments. rs-fMRI measures low-frequency oscillations in BOLD signal, typically less than 0.01 Hz, whereas oscillatory activity during sharp wave ripple events is in the range of 150–250 Hz. While the relationship between low-frequency oscillations that reflect the activity of millions of neurons and high-frequency oscillations measured at the level of local field potentials is not yet fully understood, simultaneous recordings in monkey suggest that local field potentials might underlie BOLD activity [Goense and Logothetis, 2008; Logothetis et al., 2001]. A further limitation of the present study and others that have sought to examine how learning modulates functional connectivity is the absence of causal evidence. In light of recent reports in rodent that a disruption of hippocampal sharp wave ripple activity leads to impaired spatial learning and memory [Ego-Stengel and Wilson, 2010; Jadhav et al., 2012], a similar form of causal evidence from human rs-fMRI studies is necessary to further strengthen the assertion that postlearning functional connectivity is a useful marker of consolidation.
Finally, we will briefly consider what type of information might be processed by posterior hippocampus and dorsal caudate during episodes of learning and consolidation. Much of the human neuroimaging literature has argued that hippocampus and caudate are part of independent memory systems [Doeller et al., 2008; Hartley et al., 2003; Hirshhorn et al., 2012; Iaria et al., 2003; Wegman et al., 2014]. The hippocampus is typically associated with place-based strategies that rely on learning the general layout of the environment, as required by the version of the water maze task used here. In contrast, the caudate is associated with response-based strategies driven by task specific cues. Within this framework it is possible that the hippocampus and caudate are not working cooperatively within the same functional network and that the activity in caudate actually reflects the inhibition of behavioral strategies that compete with hippocampus-based navigation strategies. It is the case that synaptic activity related to excitatory and inhibitory potentials both lead to a positive BOLD response [Logothetis, 2003]. However, task-related fMRI studies which have argued for the existence of independent hippocampus and striatum-dependent navigation strategies do so by providing evidence for only hippocampus or striatum activation, depending on the type of navigation performed [e.g., Doeller et al., 2008; Hartley et al., 2003; Iaria et al., 2003]. We are unaware of any study that has reported activation in both hippocampus and striatum during navigation and argued that this pattern of results is evidence for a hippocampus-based navigation strategy. Therefore, we suggest that the change in functional connectivity between posterior hippocampus and dorsal caudate observed here is more likely to reflect a cooperative interaction.
Although we observed significant postlearning functional connectivity between hippocampus and several clusters in both caudate and putamen, only functional connectivity between left posterior hippocampus and left dorsal caudate was specifically related to early water maze learning. Therefore, the results of the present study in combination with our task-related data [Woolley et al., 2013] support rodent work highlighting a functional dissociation between dorsomedial and dorsolateral striatum, which correspond to human caudate and putamen respectively. Previous studies have demonstrated that lesions confined to the dorsomedial striatum impair the initial learning of spatial navigation tasks that demand place-based strategies, whereas lesions to the dorsolateral striatum impair response-based strategies [Devan and White, 1999; Devan et al., 1999; Whishaw et al., 1987; Yin and Knowlton, 2004]. Our findings suggest that caudate (dorsomedial striatum) involvement may not only be restricted to the encoding phase of place-based learning, but that it may also play a critical role during consolidation. This is consistent with a recent report that corticosterone injections into the dorsomedial striatum of rodent immediately following the initial learning of a place-based navigation task were found to enhance future memory, while injections following a response-based version of the same task had no effect [Lozano et al., 2013]. With respect to the specific type of information processed by caudate, the consensus, within the rodent literature at least, is that it plays a generalized role in the initial learning of goal-directed behaviors and operates within the same network as hippocampus [Khamassi and Humphries, 2012; Yin and Knowlton, 2006].
In conclusion, our data provide evidence in human that spatial navigation learning modulates functional connectivity during post-task awake rest. Most strikingly, we found an increase in posterior hippocampus—dorsal caudate functional connectivity that was specific to virtual water maze learning and correlated with the subsequent offline gain in behavioral performance. The spatial learning processes examined here are similar to those typically studied in rodent, and our results provide further convergence between rodent and human models of memory.




