The organization of intrinsic brain activity differs between genders: A resting-state fMRI study in a large cohort of young healthy subjects
Abstract
Objectives: To investigate, using resting state (RS) functional MRI (fMRI), gender-related differences of functional connectivity (FC) and functional network connectivity (FNC) of the human brain. Experimental design: One-hundred and four young healthy subjects (48/56 men/women), aged between 20 and 29 years, underwent a 10-min RS fMRI acquisition. Independent component analysis (ICA) and statistical parametric mapping were used to assess gender-related differences in RSNs, with and without correction for regional gray matter (GM) volume. The relationships among all RSNs was also assessed using a FNC method. Principal observations: For all networks, significant between-group differences of RS activity were found. Between-group comparisons of RSNs changed when adjusting for GM volume, as follows: (1) there was only marginal effect on the analysis of sensory (i.e., sensorimotor, visual, and auditory) networks; and (2) there was a significantly increased difference when cognitive networks (apart from one related to attention) were considered. Compared with women, men experienced increased FC in parietal and occipital regions in most RSNs, whereas women experienced a higher RS FC in frontal and temporal regions, and in the cerebellum. When compared to women, increased FNC was found in men between several cognitive and sensory networks, whereas women showed an increased FNC only between attention and right working-memory networks. Conclusions: The organization of intrinsic FC and FNC differ between genders. The detected differences could contribute to the understanding of the known between-gender variation in task-related recruitments, and the patterns of abnormalities detected in neurologic and psychiatric diseases with a gender prevalence. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Behavioral studies highlighted different performances between genders on various cognitive tasks, with men having a greater ability in visuo-spatial tests [Crucian and Berenbaum, 1998], and women performing better in language [Wegesin, 1998] and emotional processing [Rahman et al., 2004] tasks. Differences of brain morphology [Chen et al., 2007; Good et al., 2001; Luders et al., 2005, 2009] and function [Canli et al., 2002; Clements et al., 2006] have been considered to explain such a gender-related variability. Clearly, the definition of between-gender differences is a critical step toward a better understanding of the gender-related expression of neurologic and psychiatric disorders.
Functional magnetic resonance imaging (fMRI) studies of gender-related activity differences during active tasks provided conflicting results. Indeed, some studies demonstrated an increased parietal recruitment in men and an increased prefrontal recruitment in women during the performance of visuo-spatial and mathematical [Keller and Menon, 2009; Thomsen et al., 2000; Weiss et al., 2003], working memory [Bell et al., 2006; Goldstein et al., 2005], word generation [Bell et al., 2006], and cognitive control [Christakou et al., 2009] tasks. On the contrary, other studies showed only poor or absent gender effect during motor and cognitive tasks [Bell et al., 2006; Schlosser et al., 1998; Schmidt et al., 2009; Sommer et al., 2004; Unterrainer et al., 2005; Weiss et al., 2003]. Several factors might contribute to explain such a discrepancy, including the complexity of the active stimulations and the stimulus-related emotional contents.
An appealing unbiased strategy to assess activity differences among genders is the evaluation of resting state (RS) functional connectivity (FC). Using independent component analysis (ICA), several studies have demonstrated the occurrence of high temporal correlations between spatially distinct but functionally related brain regions, resembling specific neuroanatomical networks, which characterize the RS networks (RSNs) of the human brain [Damoiseaux et al., 2006; De Luca et al., 2006]. These networks have a high reproducibility not only across sessions [Damoiseaux et al., 2006] but also across centers and acquisition protocols, as demonstrated by a recent study performed on a very large sample of healthy subjects [Biswal et al., 2010]. The assessment of RSNs allows to examine the intrinsic functional architecture of the human brain, also called functional connectome [Biswal et al., 2010]. Recent studies explored whether RS FC differences exist between genders [Allen et al., 2011; Biswal et al., 2010; Bluhm et al., 2008; Liu et al., 2009; Tian et al., 2011; Weissman-Fogel et al., 2010; Zuo et al., 2010]. Some studies limited their analysis to a priori defined RSNs [Biswal et al., 2010; Bluhm et al., 2008; Weissman-Fogel et al., 2010] or regions of interest [Tian et al., 2011], or investigated selected characteristics of RS FC, such as asymmetry [Liu et al., 2009] or inter-hemispheric coherence [Zuo et al., 2010]. At present, only Allen et al. [2011] analyzed all possible functionally relevant brain RSNs, and detected significant influence of age and gender on RS FC. It is worth mentioning that all these studies enrolled control subjects with age spanning several decades of life, making difficult to ascertain the interaction between gender-related and age-related differences on RS FC. Additionally, they did not account for the regional distribution of gray matter (GM) volume, which is not only different among genders [Chen et al., 2007; Good et al., 2001; Luders et al., 2005, 2009], but also variable with aging according to gender [Good et al., 2001; Raz et al., 2004].
Against this background, we wished to assess gender-related differences of RS FC in a relatively large sample of young, right-handed healthy volunteers with a narrow age range (i.e., between 20 and 29 years), in which the effects of maturation and aging should be negligible. Furthermore, we sought to explore the impact on gender-related differences of RS FC of potential differences in brain morphology. This was achieved by using voxel-based morphometry (VBM) and by including in the statistical analysis the regional GM volume as a covariate. We also performed, in addition to a “classical” analysis of RS data, a functional network connectivity (FNC) analysis [Jafri et al., 2008] to investigate functional interactions among the RSNs studied.
MATERIALS AND METHODS
Subjects
We studied 104 right-handed [Oldfield, 1971] healthy controls, with no previous history of neurological, psychiatric, or cardiovascular disorders; no drug and alcohol abuse; no treatment with any psycho-active drug; and a normal neurological exam. All subjects had normal Mini-Mental State Examination scores [Folstein et al., 1975], after correction for age and education. There were 48 men (mean age = 23.5 years, range = 20–29 years) and 56 women (mean age = 22.8 years, range = 20–29 years). The mean age of the two study groups was not different at a two-sample t test (P = 0.16).
Local Ethical Committee approval and written informed consent from all individuals were obtained before study initiation.
Image Acquisition
Using a 3.0 Tesla Philips Intera scanner, the following sequences of the brain were acquired from all subjects: (a) dual-echo turbo spin echo (repetition time [TR] = 3500 ms, echo time [TE] = 24/120 ms; echo train length [ETL] = 5; flip angle [FA] = 150°, 44 contiguous, 3-mm-thick, axial slices with a matrix size = 256 × 256 and a field of view [FOV] = 240 mm2) to check for MRI lesions/abnormalities (all scans were normal); (b) 3D T1-weighted fast field echo (FFE; TR = 25 ms, TE = 4.6 ms, FA = 30°, FOV = 230 mm2, matrix = 256 × 256, slice thickness = 1 mm, 220 contiguous axial slices, in-plane resolution = 0.89 × 0.89 mm2); and (c) T2*-weighted single-shot echo planar imaging (EPI) sequence for RS fMRI (TR = 3000 ms, TE = 35 ms, FA = 90°, FOV = 240 mm2; matrix = 128 × 128, slice thickness = 4 mm, 200 sets of 30 contiguous axial slices, parallel to the AC-PC plane). Total acquisition time of RS fMRI was about 10 min. During scanning, subjects were instructed to remain motionless, to close their eyes and not to think anything in particular. All subjects reported that they had not fallen asleep during scanning, according to a questionnaire delivered immediately after the MRI assessment.
VBM Analysis
On 3D FFE images, intracranial volumes (ICV) were calculated using the Structural Imaging Evaluation of Normalized Atrophy (SIENAx) software [Smith et al., 2002]. VBM was performed using statistical parametric mapping (SPM8) and the Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) registration method, as described elsewhere [Ashburner, 2007]. Briefly, after segmentation of 3D FFE images into GM, white matter (WM) and cerebrospinal fluid (CSF), GM maps were normalized to the GM population-specific template generated from the complete image set using DARTEL [Ashburner, 2007]. Spatially normalized images were then modulated to ensure preservation of the overall amount of GM tissue and smoothed with a 8-mm full-width at half maximum kernel.
Analysis of Intrinsic FC of RS Networks
Using SPM8, RS fMRI scans were realigned to the first one of each session with a six degree rigid-body transformation to correct for minor head movements. None of the subjects was excluded from the analysis because of motion, since the maximum cumulative translation was <1.5 mm and the maximum rotation was <0.3° for all of them. Data were then normalized to the SPM8 default EPI template using a standard affine transformation, through a data subsampling with a resolution of 3 × 3 × 4 mm3 [Calhoun et al., 2001] and smoothed using a 3D 6-mm Gaussian kernel. Linear detrending and band-pass filtering between 0.01 and 0.08 Hz were performed using the REST software (http://resting-fmri.sourceforge.net/) to partially remove low-frequency drifts and physiological high-frequency noise.
After these pre-processing steps, RS FC was assessed by means of ICA performed using the GIFT software [Calhoun et al., 2001] and following three main steps: (i) data reduction, (ii) ICA, and (iii) back reconstruction. First, individual subjects' data were reduced to a lower dimensionality by using a principal component analysis. Then, RS fMRI data from all subjects were concatenated and reduced to one group. The independent group components were estimated using the Infomax approach [Bell and Sejnowski, 1995] and were used to compute spatial maps and temporal profiles of the individual subject components (back reconstruction). The number of independent group components was 41, a dimension determined using the minimum description length criterion [Calhoun et al., 2001]. Statistical reliability of IC decomposition was tested using the ICASSO toolbox [Himberg et al., 2004], and running Infomax 10 times with different initial conditions and bootstrapped data sets. To obtain voxel values comparable across subjects, individual functional maps were converted to z scores before entering group statistics.
Visual inspection of spatial patterns and time courses of the estimated ICs allowed to remove components clearly related to artifacts, such as CSF pulsation (around the fourth ventricle or the brain), or motion-related artifacts (nine components excluded). Then, a systematic process was applied to inspect and select the components of interest from the remaining components. The association of each component spatial map with a priori probabilistic maps of GM, WM, and CSF in the Montreal Neurological Institute (MNI) space contributed to identify those components with a signal change correlated to the GM. Components with a high correlation (r > 0.10) with CSF or WM, or with a low correlation (r < 0.05) with the GM, were excluded (14 components excluded). In addition, to identify ICs with potential functional relevance, a frequency analysis of IC time courses was performed to detect those with a high (50% or greater) spectral power at a low frequency (between 0.01 and 0.05 Hz; seven components further excluded). The spatial patterns of these ICs were sorted out on the basis of their matching with relevant RSNs found in previous studies [Damoiseaux et al., 2006, 2008].
Analysis of Intrinsic Brain Connectivity Among RS Networks
The ICA algorithm assumes that the time courses of brain areas belonging to the same IC are synchronous [Calhoun et al., 2004]. Although RS components are spatially independent, significant temporal correlations exist between them. We explored such temporal associations by analyzing the time series of the RSNs of interest using the FNC toolbox (http://mialab.mrn.org), and computing a constrained maximal lagged correlation between component time courses, as previously described [Jafri et al., 2008]. The maximum possible lag between time courses was set at 6 s. The time courses from all ICs of interest for all subjects were first interpolated, using a low-pass temporal interpolation implemented in Matlab (The MathWorks, Natick, MA) and then resampled to 250 ms bins to enable detection of sub-TR hemodynamic delay differences between subjects [Calhoun et al., 2000; Jafri et al., 2008]. The maximal lagged correlation was then examined among all pair-wise combinations between components. In other words, we examined the correlation between two IC time courses, A and B, when B is circularly shifted from −6 to +6 s around A. The maximal absolute correlation value and the corresponding lag were saved for each time course pair.
Statistical Analysis
SPM8 and ANCOVA were used to assess significant gender effects on regional GM volume, including age and ICV as nuisance variables. To exclude misclassified pixels from the statistical analysis, GM maps were thresholded at 0.2.
Significant within-group FC for each RSN with potential functional relevance was assessed using SPM8 and a one-sample t test. Gender effects on RS FC in each network were estimated using ANCOVA models, including age as nuisance variable and explicitly masking the results with the corresponding binarized RSN mask, which was extracted using the Marsbar toolbox [Brett, 2002]. The presence of laterality effects was assessed by extracting, for each network, the average z scores of RS FC of each hemisphere. Then, a two-sample t test for paired data was used to compare z scores of the left (L) and right (R) hemisphere for each gender, and a 2 × 2 factorial ANOVA accounting for repeated measures was used to assess the presence of gender x hemisphere interactions.
To determine whether observed group differences resulted from regional GM volumes, we re-analyzed each contrast after adding voxel-wise GM probability maps, derived from the VBM analysis, as covariates using the Biological Parametric Mapping (BPM) toolbox [Casanova et al., 2007]. For such an analysis, GM and RSN maps were registered to the MNI space and resampled to equalize voxel sizes (to 2 × 2 × 2 mm3) and image dimensions (to 91 × 109 × 91 voxels) across functional and structural data. For all the analyses run with SPM, results were assessed at a threshold of P < 0.05 corrected for multiple comparisons (family-wise error [FWE]). Results were also tested at P < 0.001 (uncorrected for multiple comparisons) and a cluster extent of k = 10 voxels. The identification of regions in the MNI space was performed using the Anatomical Automatic Labeling (AAL) atlas [Tzourio-Mazoyer et al., 2002] and the Brodmann area (BA) atlas [Lancaster et al., 2000; Maldjian et al., 2003].
Pair-wise correlations and lag values obtained for each subject with the FNC toolbox were averaged for the two groups for descriptive purposes. A Shapiro-Wilk test was used to assess the normality of the distribution of the obtained correlation coefficients. Since the assumptions of normality was verified on our data, parametric tests were used to perform statistical analysis of FNC. One-sample t tests were used to define significant within-group correlations for each gender, separately. Significant gender effects on FNC were then assessed with two-sample t-tests. Since such between-group comparison was performed on correlation coefficients and not on spatial maps, FNC results were assessed at a P < 0.05, false-discovery rate (FDR) corrected for multiple comparisons, as implemented in the FNC toolbox [Jafri et al., 2008], and not with the FWE correction, which applies only on comparisons between images.
RESULTS
VBM
Compared to women, men had higher GM volume of the L vermis of the cerebellum. The comparison in the opposite direction showed that women had a higher GM volume of the L superior frontal sulcus, R middle cingulate cortex, L precuneus, and R posterior cingulate cortex (Table I and Fig. 1).
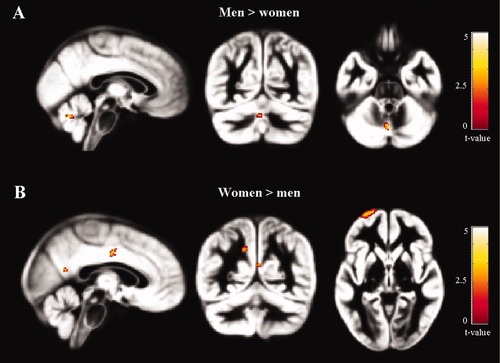
Voxel-based morphometry results showing regions of gray matter volume difference, superimposed on the customized GM template, between men and women (P < 0.001 uncorrected). A: Men>women; B: Women>men. Images are in neurological convention.
| Gender effect | Brain region | MNI space coordinates x y z | t-Value | Cluster extent |
|---|---|---|---|---|
| Men > women | L cerebellum (vermis) | −2 −65 −30 | 3.65 | 26 |
| Women > men | L superior frontal sulcus (BA11) | −26 70 −1 | 4.4 | 283* |
| R middle cingulate cortex (BA23) | 4 −8 37 | 3.6 | 22 | |
| L precuneus | −11 −55 38 | 3.5 | 51 | |
| R posterior cingulate cortex (BA30) | 3 −55 18 | 3.4 | 18 |
- L, left; R, right; MNI, Montreal neurological Institute; BA, Brodmann area.
- * P < 0.05, family-wise error corrected.
RS fMRI
The analysis of RS fMRI detected 11 spatial maps of potentially relevant RSNs, including those related to sensorimotor areas (sensorimotor RSN I and II), primary and secondary visual cortical areas, primary and secondary auditory areas, default mode network (DMN) [Raichle and Snyder, 2007], executive control network (ECN) [Seeley et al., 2007], salience processing network (SN) [Seeley et al., 2007], attention function network [Fox et al., 2005], and working memory network (WMN; Fig. 2). All these components were stable across multiple runs of IC decomposition (stability index, assessed with ICASSO, ranged from 0.92 to 0.98).
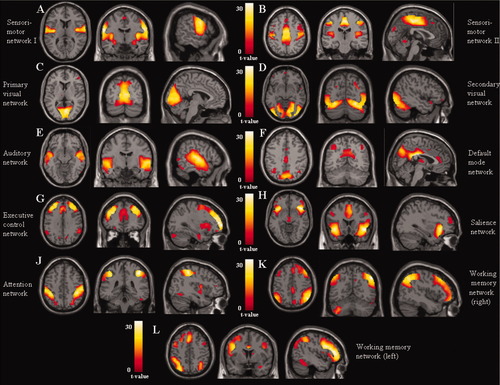
Spatial maps of potentially functional relevant resting state networks from the cohort of healthy subjects (t contrast thresholded for positive values; ANCOVA models, P < 0.05 family-wise error corrected for multiple comparisons): (A and B) sensorimotor networks; (C) primary visual network; (D) secondary visual network; (E) auditory network; (F) default mode network; (G) executive control network; (H) salience network; (J) attention network; (K) right fronto-parietal working memory network; and (L) left fronto-parietal working memory network. Images are in neurological convention.
Gender-Related Differences of FC Within RSNs
Table II reports the average L and R z scores of RS FC in each RSN. Consistently with the right-handedness of our subjects, a significantly higher intrinsic FC was found in the L than in the R hemisphere within all sensory and motor RSNs, as well as within the SN (P ranging from <0.001 to 0.004), whereas the FC within the ECN did not show any significant lateralization effect. This asymmetric distribution of RS FC did not differ between genders. Differences in the L–R distribution of RS FC between genders were detected in the DMN and the attention networks, with a more asymmetric distribution of RS FC in women than in men. No significant gender x hemisphere interaction was found for any of the RSNs examined.
| RSNs | Males | Females | p† | ||||
|---|---|---|---|---|---|---|---|
| L average z score (SD) | R average z score (SD) | p* | L average z score (SD) | R average z score (SD) | P* | ||
| Sensorimotor I | 0.75 (0.21) | 0.65 (0.19) | <0.001 | 0.65 (0.19) | 0.58 (0.19) | 0.004 | 0.31 |
| Sensorimotor II | 0.22 (0.12) | 0.10 (0.08) | <0.001 | 0.21 (0.13) | 0.13 (0.10) | <0.001 | 0.16 |
| Primary visual network | 0.70 (0.22) | 0.42 (0.18) | <0.001 | 0.65 (0.22) | 0.43 (0.17) | <0.001 | 0.08 |
| Secondary visual network | 0.51 (0.15) | 0.19 (0.11) | <0.001 | 0.54 (0.17) | 0.21 (0.11) | <0.001 | 0.71 |
| Auditory network | 0.53 (0.14) | 0.42 (0.16) | <0.001 | 0.52 (0.11) | 0.43 (0.14) | 0.002 | 0.37 |
| Default mode network | 0.34 (0.14) | 0.34 (0.12) | 0.66 | 0.30 (0.11) | 0.38 (0.14) | 0.002 | 0.08 |
| Executive control network | 0.10 (0.06) | 0.09 (0.09) | 0.31 | 0.07 (0.08) | 0.11 (0.08) | 0.20 | 0.77 |
| Salience network | 0.37 (0.08) | 0.20 (0.09) | <0.001 | 0.37 (0.11) | 0.18 (0.10) | <0.001 | 0.83 |
| Attention network | 0.31 (0.11) | 0.28 (0.10) | 0.20 | 0.30 (0.10) | 0.23 (0.08) | 0.002 | 0.22 |
| R working memory network | 0.21 (0.08) | 0.64 (0.09) | <0.001 | 0.22 (0.07) | 0.63 (0.08) | <0.001 | 0.72 |
| L working memory network | 0.34 (0.07) | 0.02 (0.08) | <0.001 | 0.34 (0.07) | 0.01 (0.07) | <0.001 | 0.65 |
- L, left; R, right; SD, standard deviation.
- * Two-sample t-test for paired data.
- † Gender x hemisphere interaction, 2 × 2 ANOVA for repeated measures.
Table III and Figure 3 summarize the differences between genders in RS FC in the sensory (i.e., sensorimotor, visual and auditory) and cognitive (i.e., DMN, ECN, SN, attention, and WMN) networks. For all networks, significant between-group differences were found (Table III, where the results of the between-group comparisons adjusted for GM volume are also presented). Apart from the attention network, significant gender effects on FC within RSNs changed when adjusting for GM volume, as follows: (1) the analysis of sensory networks was only marginally affected by GM volume correction (an improvement in the detection of gender-related differences was found only in the primary visual network); (2) the analysis of cognitive networks (apart from the attention one) significantly improved by the use of regional GM volume as a covariate, since the majority of between-group differences were detected only after GM volume correction, or the significance level increased after such a correction.
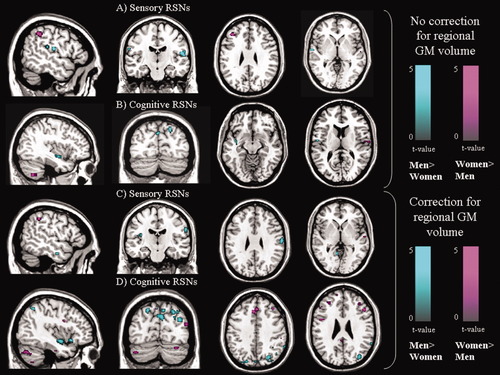
Illustrative pictures showing significant gender effects on resting state functional connectivity for all potential brain networks with functional relevance. (A and B): gender differences detected without correction for regional GM volume for sensory (A) and cognitive (B) RSNs; and (C and D): gender differences detected with correction for regional GM volume for sensory (C) and cognitive (D) RSNs (P < 0.001, uncorrected, cluster extent 10 voxels). Images are in neurological convention.
| RSNs | Gender effect | Brain region | MNI space coordinates x y z | No-BPM correction | BPM correction | |||
|---|---|---|---|---|---|---|---|---|
| t-Values | k | t-Values | k | |||||
| Sensory networks | Sensorimotor I | Men>women | R postcentral gyrus (BA48) | 62 −12 18 | 3.7 | 64 | 4.5* | 130 |
| L insula (BA48) | −36 −10 14 | 4.3 | 30 | 3.3 | 10 | |||
| L STG (BA42) | −54 −28 18 | 3.9 | 36 | – | – | |||
| L IFG ((BA38) | −26 16 −24 | 3.7 | 12 | – | – | |||
| Women>men | R MTG (BA21) | 58 0 −24 | 4.5* | 31 | – | – | ||
| L MTG (BA22) | −58 −12 −2 | 3.7 | 20 | 4.4* | 15 | |||
| Sensorimotor II | Men>women | L postcentral gyrus (BA48) | −54 −16 16 | 4.1 | 50 | 3.5 | 10 | |
| L insula (BA13) | −42 −20 18 | 3.9 | 13 | 3.7 | 13 | |||
| Women>men | L IPL (BA40) | −54 −46 46 | 4.3* | 56 | 3.6 | 21 | ||
| R cerebellum crus I | 34 −54 −32 | 3.7 | 14 | – | – | |||
| Primary visual network | Men>women | R cerebellum 8 | 32 −68 −48 | 3.8 | 38 | – | – | |
| R lingual gyrus (BA17) | 8 −54 2 | 1.3 | – | 3.8* | 31 | |||
| Women>men | – | – | – | – | – | – | ||
| Secondary visual network | Men>women | L STG (BA48) | −46 −4 −12 | 4.5* | 41 | – | – | |
| L postcentral gyrus (BA48) | −60 −14 18 | 3.9 | 11 | – | – | |||
| Women>men | – | – | – | – | – | – | ||
| Auditory network | Men>women | L insula (BA13) | −18 18 48 | 3.6 | 13 | 3.6 | 11 | |
| Women>men | L MFG (BA10) | −38 36 28 | 3.8 | 40 | – | – | ||
| Cognitive networks | Default mode network | Men>women | R cuneus (BA18) | 2 −76 36 | 3.7 | 15 | 4.2* | 41 |
| R STG (BA42) | 60 −28 16 | 3.6 | 15 | – | – | |||
| Women>men | R precuneus | 8 −70 56 | – | – | 4.2* | 31 | ||
| Dorsal ACC (BA32) | 0 34 42 | – | – | 3.9 | 19 | |||
| Executive control network | Men>women | R MOG (BA39) | 36 −78 28 | – | – | 4.4* | 47 | |
| R SPL (BA7) | 26 −72 42 | – | – | 4.4* | 52 | |||
| Women>men | L ITG | −50 −8 −30 | 4.5* | 15 | 5.1* | 72 | ||
| L insula (BA48) | −44 −20 −2 | 3.9 | 15 | – | – | |||
| R Temporal pole (BA38) | 28 12 −24 | – | – | 4.8* | 38 | |||
| R MFG (BA9) | 38 14 54 | – | – | 3.7 | 28 | |||
| Salience network | Men>women | L postcentral gyrus | −54 −8 14 | 3.9 | 22 | – | – | |
| L insula (BA13) | −40 −4 −12 | 3.5 | 21 | 4.0* | 26 | |||
| Women>men | R MTG (BA21) | 56 −32 0 | 3.5 | 13 | – | – | ||
| Dorsal ACC (BA32) | −6 28 40 | – | – | 3.9* | 29 | |||
| Attention network | Men>women | – | – | – | – | – | – | |
| Women>men | R STG (BA22) | 66 −28 20 | 3.7 | 19 | 3.7 | 12 | ||
| R working memory network | Men>women | R STG | 66 −24 10 | 3.9 | 19 | – | – | |
| L IPL (BA7) | −40 −58 52 | – | – | 3.5 | 11 | |||
| Women>men | L cerebellum lobule VIII | −38 −58 −52 | 4.6* | 48 | 4.3* | 26 | ||
| R IFG (BA48) | 48 24 28 | – | – | 4.2* | 38 | |||
| L working memory network | Men>women | L SPL (BA7) | −20 −78 46 | 4.1 | 27 | 4.3* | 60 | |
| Women>men | R cerebellum lobule VIII | 32 −42 −46 | – | – | 3.8 | 14 | ||
| L cerebellum lobule VII | −32 −40 −38 | – | – | 3.5 | 20 | |||
| R insula (BA38) | 40 10 −2 | – | – | 3.4 | 14 | |||
- Brain regions with a significant between-gender difference with and without BPM correction are reported in light black, those with a significant between-gender difference only when BPM correction was not applied are reported in shaded entries, and those with a significant between-gender difference after BPM correction are reported in bold. For regions with a significant between-gender difference with and without BPM correction, the reported MNI coordinates, t values and cluster extents are those disclosed by the analysis carried out without BPM correction.
- RSN, resting state network; MNI, Montreal neurological institute; BPM, biological parametric mapping; L, left; R, right; BA, Brodmann area; MTG, middle temporal gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobe; ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; ACC, anterior cingulate cortex; MOG, middle occipital gyrus; SPL, superior parietal lobule; ITG, inferior temporal gyrus.
- * P < 0.05, family-wise error corrected.
Differences of FC Among RSNs
Results of FNC analysis are shown in Table IV, which reports significant correlations among RSNs in men and women, separately, and significant differences of correlations between genders. This analysis revealed that there was a significant connectivity among all sensory (i.e., sensorimotor, visual, and auditory) networks in both genders. Similarly, both genders had a significant connectivity among all cognitive (i.e., DMN, ECN, SN, attention, and WMN) networks. In line with previous reports [Fox et al., 2005], the DMN and the parietal attention network were significantly anticorrelated, both in men (r = −0.10, P = 0.04) and in women (r = −0.16, P < 0.001). A negative correlation was also found between the attention network and R WMN in women only (r = −0.10, P = 0.005).
| RSN pair: correlation | Men | Women | P-Value** | |||
|---|---|---|---|---|---|---|
| Between | And | R | P-Value* | R | P-Value* | |
| Sensorimotor I | Sensorimotor II | 0.47 | <0.001 | 0.43 | <0.001 | 0.50 |
| Primary visual network | 0.40 | <0.001 | 0.28 | <0.001 | 0.07 | |
| Secondary visual network | 0.42 | <0.001 | 0.30 | <0.001 | 0.06 | |
| Auditory network | 0.49 | <0.001 | 0.47 | <0.001 | 0.51 | |
| DMN | 0.09 | 0.06 | 0.09 | 0.01 | 0.96 | |
| SN | 0.21 | <0.001 | 0.05 | 0.28 | 0.07 | |
| Attention network | 0.38 | <0.001 | 0.24 | <0.001 | 0.08 | |
| L WMN | 0.22 | <0.001 | 0.13 | 0.002 | 0.15 | |
| Sensorimotor II | Primary visual network | 0.39 | <0.001 | 0.30 | <0.001 | 0.21 |
| Secondary visual network | 0.40 | <0.001 | 0.33 | <0.001 | 0.34 | |
| Auditory network | 0.51 | <0.001 | 0.42 | <0.001 | 0.06 | |
| DMN | 0.14 | 0.005 | 0.14 | 0.001 | 0.92 | |
| SN | 0.20 | <0.001 | 0.02 | 0.68 | 0.008 | |
| Attention network | 0.34 | <0.001 | 0.26 | <0.001 | 0.29 | |
| Primary visual network | Secondary visual network | 0.63 | <0.001 | 0.61 | <0.001 | 0.67 |
| Auditory network | 0.49 | <0.001 | 0.40 | <0.001 | 0.10 | |
| DMN | 0.17 | <0.001 | 0.19 | <0.001 | 0.71 | |
| ECN | 0.14 | 0.003 | 0.02 | 0.65 | 0.07 | |
| SN | 0.32 | <0.001 | 0.17 | <0.001 | 0.06 | |
| Attention network | 0.25 | <0.001 | 0.09 | 0.06 | 0.08 | |
| L WMN | 0.23 | <0.001 | 0.12 | 0.004 | 0.08 | |
| Secondary visual network | Auditory network | 0.47 | <0.001 | 0.31 | <0.001 | 0.003 |
| DMN | 0.19 | <0.001 | 0.17 | <0.001 | 0.65 | |
| ECN | 0.15 | 0.009 | −0.06 | 0.09 | <0.001 | |
| SN | 0.23 | <0.001 | −0.005 | 0.91 | <0.001 | |
| Attention network | 0.32 | <0.001 | 0.16 | 0.002 | 0.06 | |
| R WMN | 0.16 | 0.008 | −0.08 | 0.07 | <0.001 | |
| L WMN | 0.28 | <0.001 | −0.008 | 0.86 | <0.001 | |
| Auditory network | DMN | 0.36 | <0.001 | 0.19 | <0.001 | 0.006 |
| ECN | 0.22 | <0.001 | 0.12 | 0.001 | 0.08 | |
| SN | 0.53 | <0.001 | 0.39 | <0.001 | 0.001 | |
| Attention network | 0.29 | <0.001 | 0.18 | <0.001 | 0.08 | |
| R WMN | 0.27 | <0.001 | 0.06 | 0.14 | <0.001 | |
| L WMN | 0.30 | <0.001 | 0.21 | <0.001 | 0.12 | |
| DMN | ECN | 0.24 | <0.001 | 0.21 | <0.001 | 0.46 |
| SN | 0.29 | <0.001 | 0.15 | <0.001 | 0.10 | |
| Attention network | −0.10 | 0.04 | −0.17 | <0.001 | 0.25 | |
| R WMN | 0.41 | <0.001 | 0.32 | <0.001 | 0.06 | |
| L WMN | 0.14 | <0.001 | 0.08 | 0.03 | 0.31 | |
| ECN | SN | 0.54 | <0.001 | 0.49 | <0.001 | 0.17 |
| Attention network | 0.10 | 0.02 | 0.07 | 0.06 | 0.41 | |
| R WMN | 0.32 | <0.001 | 0.24 | <0.001 | 0.09 | |
| L WMN | 0.29 | <0.001 | 0.25 | <0.001 | 0.51 | |
| SN | Attention network | 0.11 | 0.003 | 0.06 | 0.15 | 0.40 |
| R WMN | 0.41 | <0.001 | 0.31 | <0.001 | 0.06 | |
| L WMN | 0.29 | <0.001 | 0.28 | <0.001 | 0.84 | |
| Attention network | R WMN | 0.05 | 0.19 | −0.10 | 0.005 | 0.005 |
| L WMN | 0.41 | <0.001 | 0.30 | <0.001 | 0.008 | |
| R WMN | L WMN | 0.48 | <0.001 | 0.47 | <0.001 | 0.85 |
- Significant gender effects on functional network connectivity are highlighted in bold. Note that, since coefficients are symmetrical (i.e., given two RSNs A and B, correlation between A and B is the same as that between B and A), they were reported only once.
- DMN, default mode network; ECN, executive control network; SN, salience network; WMN, working memory network; L, left; R, right; n.s., not significant.
- * One-sample t-test, P-value false-discovery rate corrected for multiple comparisons.
- ** Two-sample t-test, P-value FDR corrected for multiple comparisons.
The comparison between genders showed higher FNC in men than in women between the auditory and the secondary visual RSN (P = 0.003). Compared to women, men also had an increased FNC between sensory and cognitive networks. In particular, they showed increased connectivities between: (a) the sensorimotor network II and the SN (P = 0.008), (b) the secondary visual network and the ECN, SN, R WMN, and L WMN (P < 0.001, for all the comparisons), (c) the auditory network and the DMN (P = 0.006), SN (P = 0.001) and R WMN (P < 0.001), and (d) the attention network and the L WMN (P = 0.008; Fig. 4). The same comparison in the opposite direction showed an increased negative correlation in women than in men between the attention network and the R WMN (P = 0.005). Figure 4 summarizes the results of the between group comparisons of FNC. No significant differences between men and women were found in the lag of correlation among RSNs.
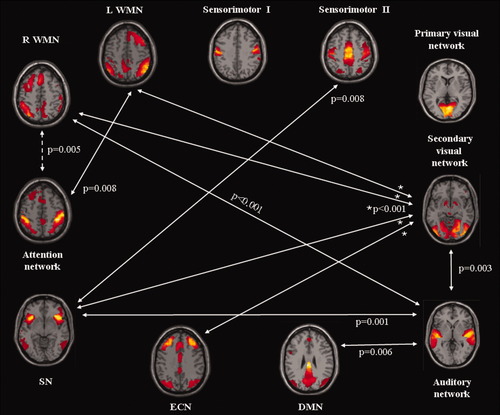
Diagram showing RSNs with a significant gender effects on pair-wise connectivity, as assessed with functional network connectivity analysis. Continue arrows indicate stronger network connectivity in men as compared to women; dotted arrow indicates weaker network connectivity in men as compared to women.
DISCUSSION
This study applied a whole-brain, unbiased analysis to investigate gender-related differences of RS FC and FNC in a large sample of young healthy individuals, spanning a narrow range of ages. One of the main strengths of the study is the assessment of FC at RS, which allowed to avoid confounding effects related to active fMRI paradigms, including between-subject differences in task performance, as well as differences in task complexity, stimulus presentation modalities [Clements et al., 2006], and stimulus-related emotional contents [Canli et al., 2002]. Furthermore, differently from previous RS FC investigations [Allen et al., 2011; Biswal et al., 2010; Tian et al., 2011; Weissman-Fogel et al., 2010], the influence of brain morphology on fMRI findings was also investigated. Finally, the assessment of a large group of young healthy individuals with a narrow age range allowed us to minimize possible confounding factors due to maturation, aging and presence of brain lesions.
Previous structural MRI studies consistently demonstrated gender-specific differences in brain morphology, with an increased cortical thickness in temporal, parietal, and frontal cortices in women [Chen et al., 2007; Good et al., 2001; Luders et al., 2005, 2009], and an increased volume of the mesial temporal lobes, entorhinal cortex and cerebellum in men [Good et al., 2001]. In line with these results, we found an increased GM volume in fronto-parietal regions and in the cingulate cortex in women versus men, whereas the opposite comparison showed a higher cerebellar volume in men. The discussion of the possible role of morphological differences of these regions among genders is beyond the scope of this study, where regional GM volumes were derived only to assess their influence on dissimilarities of function between men and women.
One of the most intriguing findings of our study is the demonstration that the correction for regional GM volume has a “network-related” effect: we found that, for the sensory networks, differences of function between men and women were for the most part detected without a GM correction (results of between-group comparison improved after GM correction only in the primary visual network). On the contrary, for cognitive networks, differences among genders were detected, for the most part, only after correcting for GM volume. These results suggest that anatomical differences of brain structure might influence activity within cognitive-related networks but not within sensory networks. Such a finding might help explaining the correspondence found between abnormalities of function and structure within large-scale brain neuronal networks recently described in several neurodegenerative disorders [Seeley et al., 2009] characterized by selective and specific patterns of cognitive dysfunction. The use of a voxel-wise GM covariate when performing statistical assessment of intrinsic FC is not novel [Oakes et al., 2007] and it has already been shown to have an impact when contrasting different groups of individuals [Wang et al., 2011; Zhou et al., 2010]. As a consequence, these results have important implications for future RS studies, because they show that GM correction might have an influence not only in studies of patients with neurological conditions, in whom gender distribution and topography of GM damage may vary considerably, but also when investigating healthy controls, in whom only subtle differences of GM volume distribution are expected.
In line with previous reports [Liu et al., 2009; Tian et al., 2010], the analysis of functional lateralization showed a strong asymmetry of RS FC almost in all networks and for both genders. Consistently with the right-handedness of our subjects, FC of the L hemisphere was on average higher than that of the R hemisphere. As previously reported [Liu et al., 2009; Tian et al., 2010], we found that gender effects on laterality are modest and restricted to a few RSNs. Since our finding of a stronger asymmetry of RS FC in women than in men disagrees with the results of Liu et al. [2009], who found a stronger functional asymmetry in men than in women, further studies are needed to clarify gender-related lateralization effects.
In agreement with previous reports [Allen et al., 2011; Biswal et al., 2010], our analysis of gender-related differences of RS FC revealed that, for all networks with potential functional relevance identified by ICA, significant between-gender differences do exist. Regions with significant gender effects seem to be consistent across previous studies [Allen et al., 2011; Biswal et al., 2010] and our own. Clearly, the interpretation of these differences could contribute to the understanding of between-gender variability, which is known to occur when performing several active tasks, as well as the patterns of abnormalities detected in neurologic and psychiatric diseases with a gender prevalence (e.g., all forms of depression, anxiety disorders, eating disorders, somatization disorders, and borderline disorders are diagnosed more frequently in women, whereas drug and alcohol abuse and antisocial personality disorders are seen more often in men [Riecher-Rossler, 2010]).
The stronger FC at rest in several regions of both visual networks in men as compared to women, including areas within the dorsal and the ventral visual streams [Goodale and Westwood, 2004; Milner and Goodale, 2008], which are involved in processing information about structure and spatial location of objects, might be related to the greater ability of men in performing visuo-spatial tasks [Wegesin, 1998]. Likewise, the higher FC seen in men in regions of the sensorimotor network, such as the postcentral gyrus and the insula (a multimodal convergence area [Mesulam, 1985] playing an important role in integrating information related to sensorimotor, allostatic/homeostatic, emotional and cognitive functions [Craig, 2002; Critchley et al., 2004]) might reflect a different peripheral arousal enhancement between genders.
Similar to what reported by Allen et al. [2011], the analysis of cognitive networks showed that, compared to men, women had an enhanced intrinsic FC in several frontal regions, including the cingulate cortex, dorsolateral (DL), prefrontal cortex (PFC), and IFG. All these regions have been shown to be also more active in women than in men in several active fMRI experiments based on working and episodic memory [Goldstein et al., 2005; Piefke et al., 2005] and cognitive control [Christakou et al., 2009] tasks. The anterior cingulum is recruited when selective attention, response selection, monitoring of conflicting responses, error detection and initiation of actions are engaged [Cabeza and Nyberg, 2000]. This region also has a role in cognitive control of emotions [Bush et al., 2000]. The DL PFC is involved in manipulating information in working and episodic memory, and in the integration of emotional and decision-making processes [Bechara et al., 1997]. The increased parietal RS FC in men and the increased prefrontal RS FC in women seem to mirror the results of active fMRI studies performed during complex cognitive tasks [Thomsen et al., 2000], and might reflect the use of different strategies for solving the same cognitive processes. In this context, another factor that should be taken into consideration is the interaction between cognitive and emotional processing occurring among genders [Canli et al., 2002]. We found a stronger insular RS FC in men than in women not only within the sensorimotor network, but also within the SN, a system which is supposed to integrate highly processed sensory with visceral and autonomic information to guide subjects' behavior [Seeley et al., 2007]. In line with this, previous active fMRI investigations described an increased insular activity in men versus women during emotion recognition [Lee et al., 2005], which was interpreted as origin of a higher ability of men to achieve a more efficient integration of visceral responses and emotion arousal for further interaction with cortical regions.
Regions of enhanced RS FC in cognitive-related RSNs in women included also the right IFG and areas in the bilateral temporal lobes, which are likely linked with memory and language functions [Allen et al., 2011; Axmacher et al., 2009; Baxter et al., 2003; Clements et al., 2006; Shaywitz et al., 1995]. Previous fMRI studies of language processing found that women tend to have a more bilateral activation of frontal and temporal regions in comparison to men [Baxter et al., 2003; Kansaku et al., 2000], who, conversely, show a strictly left lateralized pattern of activity. The increased activation of right language regions is in line with the theory proposing a greater bihemispheric representation of language in women versus men [Bookheimer, 2002; McGlone, 1977]. Noteworthy, apart from the bilateral engagement of language-related areas, our analysis did not disclose any peculiar lateralization pattern of brain function according to gender, which was suggested by previous studies. Estrogens are supposed to modulate functional hemispheric lateralization [McEwen et al., 1998] yielding differences in neuropsychological task performance between women and men and variation in cognitive performance across the menstrual cycle in women. Regretfully, our study can not contribute to such a debate since we did not control for hormonal levels in our female subjects.
An higher FC in women than in men was detected not only in the frontal, but also in cerebellar regions of the WMN. An increased activity of the fronto-cerebellar regions was found in women vs. men also during cognitive switching tasks [Christakou et al., 2009], and the authors interpreted this finding as an enhancement of the recruitment of the entire fronto-cerebellar pathway for inhibitory control in women. The analysis of FC between intrinsic networks also showed that, whereas the majority of RSNs were correlated positively one with the other, indicating that these networks are typically comodulated during task performance [Fox et al., 2005], the DMN and the parietal attention network were correlated negatively, indicating that these networks are temporally modulated in an opposite direction [Fox et al., 2005], in both genders. These results are consistent with those of previous observations [Fox et al., 2005; Uddin et al., 2009], which used seed-region FC and identified two anticorrelated RS networks, one commonly activated and the other deactivated during cognitive demanding tasks. Our findings deepen previous results by showing that in young healthy subjects the anticorrelation between the DMN and the attention network is not influenced by gender. Noteworthy, a disruption of the coordination among these two networks has been postulated to contribute to the pathophysiology of schizophrenia and Alzheimer's disease, two conditions which do not have any gender prevalence [Liu et al., 2010; Williamson, 2007; Zhou et al., 2010].
Another intriguing observation resulted from FNC analysis, which disclosed a stronger connectivity in men than in women between sensory and cognitive networks. This is in line with the findings from Allen et al. [2011], who reported a higher inter-network connectivity in men, especially when considering sensory and motor RSNs. In our study, the secondary visual RSN was the one showing the highest number of increased correlations in men than in women, particularly with the SN, ECN and WMNs. Although our investigation was performed using RS fMRI data and not during the performance of any complex task, the finding of enhanced FC in the secondary visual network, which mainly includes associative visual areas, seems to support the notion that men may use mental imagery strategies and visuo-spatial representations to solve cognitive problems [Christakou et al., 2009; Thomsen et al., 2000].
In conclusion, we have shown that the organization of intrinsic brain FC and FNC differ between genders, thus confirming the need for a proper gender matching in fMRI studies of healthy individuals and diseased people. Future studies should attempt to elucidate whether this uneven distribution of brain activity and RS connectivity among genders contributes to the gender-penetrance of several neurological and psychiatric conditions, and if the loss of gender-related brain function might predispose to neurological and psychiatric diseases, as it has been supported in autism spectrum conditions [Baron-Cohen, 2005].




