Hippocampal but not amygdalar volume affects contextual fear conditioning in humans
Abstract
Both animal and human studies have identified a critical role of the hippocampus in contextual fear conditioning. In humans mainly functional magnetic resonance imaging has been used. To extend these findings to volumetric properties, 58 healthy participants underwent structural magnetic resonance imaging and participated in a differential fear conditioning paradigm with contextual stimuli. Ratings of emotional valence, arousal, and contingency as well as skin conductance responses (SCRs) were used as indicators of conditioning. Twenty-nine participants with the smallest hippocampal volumes were compared with 29 persons with the largest hippocampal volumes. Persons with larger hippocampal volume (especially on the right side) learned to discriminate between two conditioned contexts, whereas those with small hippocampal volumes did not, as indicated by SCRs. Further analyses showed that these results could not be explained by amygdalar volumes. In contrast, the participant answers on the self-report measures were not significantly influenced by hippocampal or amygdalar, but by total brain volume, suggesting a role of cortical structures in these more cognitive evaluation processes. Reanalysis of the self-report data using partial hippocampal volumes revealed a significant influence of the posterior but not anterior subvolumes, which is in accordance with theories and empirical findings on hippocampal functioning. This study shows the relevance of hippocampal volume for contextual fear conditioning in healthy volunteers and may have important implications for anxiety disorders. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Learning about the association of aversive events and environmental stimuli that predict such events is essential for survival. Pavlovian fear conditioning is one important type of associative learning where an originally neutral stimulus or context is presented several times together with an unconditioned threat stimulus (US) and subsequently becomes a conditioned stimulus or context (CS), which elicits conditioned fear or anxiety responses (CR). Whereas conditioned cues will evoke phasic fear responses, contexts will lead to sustained anxiety responses [Marks,1987]. Therefore, contextual fear conditioning constitutes an important mechanism for the understanding of anxiety disorders [Bouton et al.,2001], for example posttraumatic stress disorder [Grillon and Morgan,1999; Grillon et al.,1998].
Considerable evidence from lesion studies in rodents suggests a role of the hippocampal formation in contextual fear conditioning [Selden et al.,1991; Philips and LeDoux,1992]. Some authors theorized that the function of the hippocampal formation is the binding of different aspects constituting a context [e.g., Maren,2001; Rudy et al.,2002]. Those aspects can be cues comprising the physical context itself or temporal characteristics of the context, but also internal states like hunger [e.g., Bouton,2002,2004 for reviews]. Interestingly, the effects of physical and temporal context change are additive, i.e., lead to enhanced context conditioning [Rosas and Bouton,1998; Rosas et al.,2001; Westbrook et al.,2000] as do USs which are unpredictable [Grillon et al.,2006].
In humans, several functional magnetic resonance imaging (fMRI) or positron emission tomography studies reported hippocampal activations during the acquisition of contextual fear conditioning [Alvarez et al.,2008; Hasler et al.,2007; Lang et al.,2009; Marschner et al.,2008]. In contrast to earlier classical cue conditioning reports, these studies used unpredictable USs and complex or temporally changing contextual stimuli to ensure context conditioning and detected activation of the hippocampal region.
Despite these recent advances, none of the above mentioned studies investigated a potential connection between human contextual fear conditioning and hippocampal volume. Therefore, we tested the hypothesis that differential contextual fear conditioning depends on hippocampal volume in healthy volunteers. Skin conductance responses (SCRs) and verbal ratings during and after conditioning were recorded in persons with small versus large hippocampal volumes. We hypothesized that volunteers with larger hippocampal volumes would show better acquisition of context conditioning as indicated by the SCRs or verbal ratings.
The hippocampus can be divided into an anterior and posterior part based on anatomical connectivity [Duvernoy,1998], i.e., white matter fiber tracts, as opposed to cytoarchitecture. While animal lesion studies suggest a role of the dorsal hippocampus, the equivalent to the posterior hippocampus in humans, in contextual fear conditioning [e.g., Philips and LeDoux,1992], human imaging studies reported peak activation voxels located in the anterior [Alvarez et al.,2008; Lang et al.,2009] as well as the posterior hippocampus [Hasler et al.,2007; Lang et al.,2009; Marschner et al.,2008]. A recent review [Fanselow and Dong,2010] indicated potential contributions of anterior as well as posterior hippocampus to complex processes such as context conditioning and underlined the importance to address these zones separately. Therefore, we investigated if anterior or posterior subvolumes of the hippocampus could explain SCRs or verbal ratings better than the entire hippocampal volume. To control for potential influences of stress on hippocampal volumes [Bremner,1999], childhood trauma and chronic stress were examined. Additionally, intelligence and verbal memory performance were assessed to assure comparability between participants with small versus large hippocampal volumes.
For the amygdala, the core region of fear processing [LeDoux,2003] in the mammalian brain, the above mentioned fMRI studies in humans yielded conflicting results. While two studies found amygdalar activations during context conditioning [Alvarez et al.,2008; Lang et al.,2009] in line with work in animals [Fanselow and Dong,2010], others did not [Hasler et al.,2007; Marschner et al.,2008]. Hence, we additionally assessed amygdalar volumes in our sample to investigate its role in context conditioning.
MATERIALS AND METHODS
Participants
Sixty-seven healthy persons (22 females) participated in the study. They were recruited from training schools for rescue workers who have a heightened risk to develop PTSD, but were not traumatized at the time of the study [Clohessy and Ehlers,1999] in the context of a longitudinal study on predictors of posttraumatic stress disorder (PTSD). Persons with a current Axis I/II mental disorder, including substance dependence or abuse, as determined by the German version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV [SCID I/II; Wittchen et al.,1997], were excluded from the study.
Participants not responding to the skin conductance measurement (five) or with missing self-report data (four) were excluded, resulting in 58 persons (20 females). Based on a median-split of the individual hippocampal volumes (method: see below) two groups with relatively small vs. large hippocampal volumes were identified. The participant with the hippocampal volume on the median was added to the smaller of the two groups to reach the same sample size (sHCV vs. lHCV, N = 29 each). Further details on the entire sample and the two groups are listed in Table I.
| Total sample (N = 58) | sHCV group (N = 29) | lHCV group (N = 29) | Inferential statistics sHCV vs. lHCV | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Age (years) | 21.64 | 2.57 | 21.97 | 2.21 | 21.45 | 2.23 | t(1,56) = 0.71, P = 0.48 |
| Sex: female/male | 20/39 | 10/19 | 10/19 | χ2 (1) = 0.00, P = 1.00 | |||
| Education (years) | 12.29 | 1.81 | 12.54 | 1.64 | 12.14 | 1.94 | t(1,55) = −0.31, P = 0.76 |
| Intelligencea | 114.61 | 13.34 | 114.86 | 14.83 | 114.39 | 12.21 | t(1,54) = 0.97, P = 0.34 |
| Memoryb | |||||||
| Immediate recall | 60.91 | 8.93 | 60.79 | 8.64 | 61.55 | 9.05 | t(1,55) = −0.96, P = 0.34 |
| Delayed recall | 13.58 | 2.15 | 13.40 | 2.00 | 13.92 | 2.23 | t(1,48) = −0.61, P = 0.54 |
| Difference short | 1.23 | 1.75 | 1.48 | 1.55 | 1.03 | 1.94 | t(1,54) = 0.49, P = 0.63 |
| Difference long | 0.73 | 1.86 | 0.96 | 1.33 | 0.50 | 2.30 | t(1,47) = 0.29, P = 0.77 |
| Childhood traumac | 7.56 | 1.85 | 7.78 | 2.24 | 7.35 | 1.37 | t(1,55) = 1.13, P = 0.27 |
| Trait-Anxietyd | 35.82 | 8.57 | 35.39 | 7.44 | 36.39 | 9.79 | t(1,54) = 0.37, P = 0.71 |
| Chronic stresse | 1.35 | 0.53 | 1.32 | 0.53 | 1.39 | 0.54 | t(1,56) = −0.42, P = 0.68 |
| US-intensity | 7.12 | 0.59 | 7.12 | 0.60 | 7.12 | 0.55 | t(1,56) = −0.20, P = 0.85 |
| US-unpleasantness | 6.86 | 1.03 | 6.81 | 1.03 | 6.87 | 1.04 | t(1,51) = −0.09, P = 0.93 |
| US-SCR (μS) | 0.26 | 0.16 | 0.23 | 0.14 | 0.30 | 0.18 | t(1,51) = 0.46, P = 0.65 |
- sHCV, relatively small hippocampal volume; lHCV, relatively large hippocampal volume.
- a Culture Fair Intelligence Test (CFT-20).
- b California Verbal Learning Test (CVLT); difference short, difference between immediate free recall and short delayed free recall; difference long, difference between immediate free recall and long delayed free recall.
- c Childhood Trauma Questionaire (CTQ).
- d State-Trait-Anxiety Inventory (STAI).
- e Trier Inventory for Assessment of Chronic Stress (TICS); US, Unconditioned Stimulus; SCR, Skin Conductance Response.
Written informed consent was obtained prior to the study, which was approved by the Ethics Committee of the Medical Faculty Mannheim of the University of Heidelberg. The study conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki, sixth revision, 2008).
Neuropsychological and Clinical Assessment
To assure comparability between the groups neuropsychological assessments were conducted. Intelligence was screened with the German version of the Culture Fair Intelligence Test [CFT-20, Weiß, 1998]. Memory performance was assessed by the California Verbal Learning Test [CVLT, Ilmberger,1988]. During this test, participants have to learn a verbally presented list A (16 items), which is repeated five times. After each trial immediate free recall is assessed. Thereafter a different list B (16 items) is presented once and recall is tested. Next to this interference trial, free and cued (categories given) recall of list A is assessed again. After a 20-min break, long delayed free and cued recall, as well as recognition of items from list A out of a longer list containing words from list B and distractors, are tested. Analyses of the CVLT focused on two measures [Koehler et al.,1998]: immediate recall performance (sum of correctly recalled items from List A on Trials 1–5, maximum of 80) and delayed recall performance (correct items after short delay, maximum of 16). Additionally, two measures of hippocampus-dependent memory performances were assessed [Van Petten,2004]: The difference between immediate and short delayed free recall as well as the difference between immediate and long delayed free recall.
To investigate potential clinical associations of hippocampal volume with chronic stress or anxiety symptoms, the trait version of the State-Trait-Anxiety Inventory [STAI; Laux et al.,1981], the Childhood Trauma Questionnaire [CTQ, Bernstein et al.,2003] and the Trier Inventory for Assessment of Chronic Stress [TICS; Schulz and Schlotz,1999] were administered.
EXPERIMENTAL PROCEDURES
Unconditioned Threat Stimulus
The US was an electrical stimulus applied at the right thumb by a cupric (copper) electrode, delivered by an electrical stimulus generator (Digitimer, DS7A, Welwyn Garden City, UK). Each participant received three series of increasingly painful stimuli (50 ms bursts, 12 Hz), starting with a mild stimulus until the participant indicated it as “painful” (pain threshold) and then further until the pain became unbearable (pain tolerance). This procedure was repeated three times and the values of the last two trials were averaged. During conditioning, we delivered stimuli that were 80% above pain threshold. Participants were asked to rate the intensity and unpleasantness of the pain on a Likert scale ranging from 0 (not painful or unpleasant) to 10 (extremely painful or extremely unpleasant). For the experiment we used stimulation intensities that were rated at least 7 on average. All ratings are shown in Table I.
Contextual Fear Conditioning
The procedure was identical to the one used by Lang et al. [2009], where functional imaging data on a partly overlapping subsample (N = 12) of the longitudinal study were reported. Briefly, the conditioning protocol consisted of initial habituation, early and late acquisition and extinction. In accordance with several previous studies [e.g., Barrett and Armony,2009], two colors (orange and blue) were used to represent two different contexts (CS+/−), that were perceived like a surrounding space because they illuminated the entire visible portion of the scanner and thus “surrounded” the participant. Additionally, the colors were slowly blended in and, after having reached their full spectrum for several seconds, passed into the next color to reinforce the feeling of context. The colors designated as CS+ were counterbalanced across participants and the sequence of CS+/− was pseudo-randomized. Stimuli were projected into the magnetic resonance tomograph via a mirror system, thus realizing a surround color, i.e., an actual context.
During habituation the CSs were presented 10 times for 3−12 s in random order. The US was delivered 10 times during the interstimulus interval (4–12 s) and lasted 2.9 s. During acquisition colors were blended in until they reached their full spectrum after 3–4 s. After additional 3–12 s the colors were blended off and passed into the next color. The colors had a slow onset to reinforce the feeling of context, and the color gradients were presented to produce a more complex processing of the stimuli. CS+ was paired with the US (electric shock) in 50% of the trials; CS− was never paired with the shock. US onset was randomized over the time course of the CS+ to maximize unpredictability, which produces greater context conditioning [Bouton,1994; Grillon and Davis,1997; Grillon et al.,2006]. The two acquisition phases consisted of five CS+ without US (CS+ unpaired), five CS+ with US (CS+ paired), and 10 CS− (safe condition). In the extinction phase the two colors (10 CS+ unpaired, 10 CS−) were presented for 3–12 s each.
Participants were uninformed about the CS-US contingency and were told to passively view the stimuli. After each conditioning phase, participants verbally rated emotional valence and arousal of the CSs (1 = very calm to 9 = very arousing, 1 = very pleasant to 9 = very unpleasant) as well as the CS-US contingency (1 = no CS-US contingency to 9 = perfect CS-US contingency). The self-report scales were based on the Self-Assessment Manikin [Bradley and Lang,1994], a reliable and valid measure that is frequently used in emotion research, but were presented acoustically since the contexts were displayed in a continuous fashion. Communication was realized via headphones with attached microphones.
Structural Magnetic Resonance Imaging
Data were acquired with a 1.5 Tesla Magnetom VISION whole body MR-scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a head volume coil. To obtain images for the volumetric analyses, we used a three-dimensional spoiled gradient (FLASH, TR 15 ms, TE 5 ms, FOV 256 × 256 mm2, 170 sagittal slices, voxel size 0.86 × 0.86 × 1 mm3). Additionally, an interleaved T-2 weighted Turbo Spin Echo sequence was acquired (TE 54ms, TR 7,280 ms, FoV 220 × 220 mm2, matrix 256 × 256 × 1, slice thickness 2 mm, flip angle 180°, gap 2 mm, voxel size 0.86 × 0.86 × 4 mm3). Each image was resampled to a 1 mm3 voxel size and T-2 weighted images were reoriented to match the T-1 weighted images. Using the MRIcro free software package [Rorden and Brett,2000], raw image data were converted to BRAINS2 [Magnotta et al.,2002] readable format, where manual tracing was conducted for hippocampus and amygdala. Two trained operators who where unaware of the psychophysiological data of the participants outlined the structures in native space to avoid the distortions intrinsic to normalization.
Volumetric Analysis of the Hippocampus
Manual volumetric measurements were performed by one rater (S.P.) following the anatomical guidelines published by Malykhin et al. [2007]. These guidelines permit parcellation of the hippocampus into three subdivisions (head, body, and tail) based on anatomical connectivity [Duvernoy,1998]. Images were smoothed and reoriented along the longitudinal axis of the hippocampus with the resample function of BRAINS2. First, hippocampal volumes were outlined in each relevant sagittal plane to enhance segmentation accuracy in critical coronal slices. Based on this region of interest (ROI), a coronal ROI was outlined to assess hippocampal volume, always beginning at the posterior end of the hippocampus. The resulting three-dimensional shape was visually inspected with the surface interface and adjusted if necessary. The final ROI was transformed into a mask to calculate hippocampal volume. Additionally, final outlines were divided into three subdivisions (head, body, and tail) based on anatomical landmarks [see Fig. 1; Malykhin et al.,2007]. Intra-rater reliability was assessed by analyzing the first 10 brains three times, twice in the beginning and once again after the entire sample was segmented. Intraclass correlation coefficients were 0.98 at both time points suggesting high stability. Additionally, 10 brains were manually outlined by a second rater (R.C.) to assess inter-rater reliability. An intraclass correlation coefficient of 0.92 suggested high reliability.
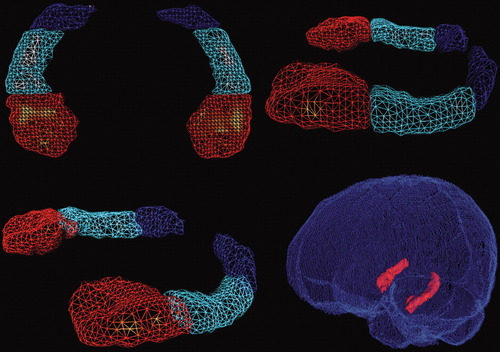
Three-dimensional rendering of left and right hippocampus of one participant and within the respective brain. Note that different colors indicate substructures of the hippocampus: Red, hippocampal head; light blue, hippocampal body; dark blue, hippocampal tail.
Volumetric Analysis of the Amygdala
The manual tracing protocol carried out by a trained operator (R.C.) was developed on the basis of previously published standardized guidelines [Nacewicz et al.,2006; Pruessner et al.,2002; Szeszko et al.,2004]. The coronal plane was set parallel to the line passing through the anterior and posterior commissure, in order to achieve the best orientation for manual tracing of the amygdala [Brierley et al.,2002]. First, the ventro-caudal surface of the amygdala was disentangled from the head of the hippocampus in the sagittal section, using both T1- and T2-weighted images. In the coronal plane, the superior border was defined by a straight line drawn from the dorsolateral aspect of the optic tract to the fundus of the circular sulcus of the insula. The amygdala was traced from posterior to anterior sections, constantly checking the sagittal and axial views. Once the tracing was completed, each amygdala was refined through plane-by-plane comparison and compared with ex vivo atlas sections [Talairach and Tournoux,1993]. As for the hippocampus, intra-rater reliability suggested very high stability with intra-class coefficients of 0.98 for the right and 0.99 for the left amygdala. Inter-rater reliability was assessed for 10 brains manually outlined by a second rater (S.P.) yielding an intra-class correlation coefficients of 0.88.
Total Brain Volume Measurement
We additionally assessed total brain volume (sum of gray and white matter) to conduct head-size corrections [Van Petten,2004 for a review], thereby assuring comparability between groups. The total brain volume of each participant was calculated from the T1-weighted images using the brain extraction tool [BET; Smith,2002] and the automated segmentation tool [FAST; Zhang et al.,2001] from the FMRIB software library (http://www.fmrib.ox.ac.uk/fsl/).
Skin Conductance Response (SCR) and Self-Report Data
The SCRs were recorded from two electrodes placed on the thenar and hypothenar eminence of the participants' right hand using a sampling rate of 16 Hz and a VarioPort recording system (BECKER MEDITEC, Karlsruhe, Germany). Data analysis was performed using EDA-PARA software (F. Schäfer, Wuppertal, Germany) and followed the guidelines of Fowles et al. [1981]. Trials were visually inspected for artifacts. SCR amplitudes were quantified as the maximum response in the time window of 1–4 s (First Interval Response, FIR) and 5–9 s [Second Interval Response, SIR; Prokasy and Ebel,1967] after stimulus onset and were measured in microSiemens (μS). SCR amplitudes below 0.05 μS were classified as zero responses. SCR data were normalized using a logarithmic [ln (1 + SCR)] transformation. Extreme cases were excluded from the analyses (cut-off 3SDs; 2.7% of the CS+/− trials). All CS+/− trials of one phase were averaged.
Statistical Analyses
Comparisons of volumetric measures as well as neuropsychological and clinical assessments between the two groups were performed using t-tests for independent samples. Total brain volume was entered in all following analyses as covariate to control for differences in head-size between individuals [Van Petten,2004]. To control for possible influences of neuropsychological and clinical variables on the hippocampal volumes of the entire sample (sum of left and right hippocampus), partial correlation analyses were conducted across all participants. SCRs and self-report data were analyzed separately. To control for baseline differences, Bonferoni-corrected t-tests for dependent (within) and independent (between groups) measures were conducted for the habituation phase. Data from acquisition and extinction were entered into analyses of variance for repeated measures (ANOVARMs). CS-type (CS+/−) and conditioning phase (early and late acquisition and extinction) served as within- and group (sHCV, lHCV) as between-subjects factors. To decompose the observed effects in the planned contrasts, follow-up t-tests were conducted. Whenever the assumption of homogeneity of variances was violated, we applied the Greenhouse-Geisser adjustment and corrected degrees of freedom are reported. To investigate if observed effects were continuous, we additionally conducted regression analyses for the entire group and each phase. Differential SCRs to CS+/− were entered as dependent and raw hippocampal volumes and total brain volume as independent variables in a backward regression analysis for each conditioning phase.
To explore possible effects of hippocampal substructures on SCR data or verbal ratings, the repeated measures ANOVAs were rerun using the volumes of the anterior (head) or posterior (body + tail of left and right side) hippocampal volume as basis for the factor group. Furthermore, laterality was investigated by reanalyzing SCR and self-report data based on left or right hippocampal volume only.
As significant group effects might also be explained by (for example) amygdalar volumes, we additionally calculated an ANOVARM model that included amygdalar volumes in the two hippocampal volume groups as covariate and allowed for interaction between amygdalar volume and group membership. For all statistical analyses we used the Predictive Analytic Software (PASW, SPSS Inc., Chicago IL) for windows, version 18.0.1.
RESULTS
Hippocampal Volumes, Partial Volumes, and Total Brain Volumes
Mean volumetric data, volumes of hippocampal head, body, and tail as well as total brain volumes for the entire sample and the two extreme groups are shown in Table II. Total brain volume in the entire sample corresponds to values reported by other groups [e. g. Lenroot and Giedd,2010; Leonard et al.,2008].
| Total sample (N = 58) | sHCV group (N = 29) | lHCV group (N = 29) | Inferential statistics sHCV vs. lHCV | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| TBV | 1215.64 | 101.62 | 1186.24 | 110.85 | 1248.83 | 81.47 | t(1,56) = −2.45, P < 0.05 |
| Raw Volumes | |||||||
| HC, right | 2.73 | 0.27 | 2.52 | 0.15 | 2.95 | 0.18 | t(1,56) = −10.11, P < 0.001 |
| HC, left | 2.61 | 0.29 | 2.41 | 0.20 | 2.82 | 0.22 | t(1,56) = −7.29, P < 0.001 |
| HC, total | 5.35 | 0.51 | 4.93 | 0.27 | 5.77 | 0.33 | t(1,56) = −10.65, P < 0.001 |
| Amygdala, righta | 1.75 | 0.18 | 1.68 | 0.17 | 1.83 | 0.16 | t(1,52) = −3.32, P < 0.005 |
| Amygdala, left | 1.69 | 0.19 | 1.60 | 0.17 | 1.77 | 0.18 | t(1,52) = −3.39, P < 0.005 |
| Amygdala, total | 3.44 | 0.33 | 3.28 | 0.31 | 3.59 | 0.28 | t(1,52) = −3.84, P < 0.005 |
| Partial Volumes | |||||||
| Anterior HC, right | 1.28 | 0.30 | 1.13 | 0.28 | 1.43 | 0.25 | t(1,56) = −4.32, P < 0.001 |
| Anterior HC, left | 1.22 | 0.26 | 1.11 | 0.22 | 1.33 | 0.25 | t(1,56) = −3.38, P < 0.005 |
| Posterior HC, right | 1.46 | 0.27 | 1.39 | 0.28 | 1.53 | 0.26 | t(1,56) = −1.92, P = 0.06 |
| Posterior HC, left | 1.39 | 0.29 | 1.30 | 0.28 | 1.49 | 0.29 | t(1,56) = −2.57, P < 0.05 |
- TBV, total brain volume; sHCV, relatively smallest hippocampal volume; lHCV, relatively largest hippocampal volume; HC, Hippocampus.
- a Amygdala volumes were assessed for 54 participants only, 27 in each group.
Neuropsychological and Clinical Assessments
The two groups showed no significant differences for measures of intelligence (CFT-20), traumatic experiences during childhood (CTQ), the reported level of chronic stress (TICS), or memory measures (CVLT, see Table I).
No significant correlations between hippocampal volumes (with TBV partialled out) of the entire sample and the measures of intelligence (CFT-20; r44 = −0.12; P = 0.43), traumatic experiences during childhood (CTQ; r44 = −0.14; P = 0.36) or the reported level of chronic stress (TICS; r44 = 0.28; P = 0.06) could be found. The same was true for the different measures of verbal memory performance (CVLT): immediate (r44 = 0.02; P = 0.91) and delayed recall (r44 = 0.04; P = 0.77), difference between immediate and short (r44 = −0.13; P = 0.39) or long delayed recall (r44 = −0.10; P = 0.50).
Habituation
No significant effects within or between the two extreme groups were detectable for the FIRs, SIRs or self-report data, indicating no significant differences in the baseline of any of these measures.
Hippocampal Volume and Conditionability
Skin conductance responses
For the FIR, no significant effects of group, CS-type or phase were detectable. For the SIR, we found a significant main effect of group (F1, 55 = 5.91; P < 0.05), but not of CS-type, phase or CS-type × phase interaction, suggesting that successful conditioning depended on group membership. The significant group × CS-type interaction (F1, 55 = 5.62; P < 0.05) indicated that the CS+/− differentiation was modulated by group membership. Further, the significant group × phase interaction (F2, 110 = 5.74; P < 0.005) indicated that the SCRs in the particular phases depended on group. The significant group × CS-type × phase interaction (F1.6, 88.3 = 6.32; P < 0.01) showed successful CS+/− differentiation in the lHCV group for early (t28 = 2.32; P < 0.05) and late acquisition (t28 = 3.39; P < 0.005) but not during extinction. In contrast, for the sHCV group higher SCRs to CS− than to CS+ were detectable during early acquisition (t28 = −2.38; P < 0.05), while no significant CS+/− differentiation was observed for late acquisition. During extinction SCRs were significantly higher to CS+ than to CS− (t28 = 2.46; P < 0.05). Further, we observed higher SCRs to CS+ in the lHCV group compared with the sHCV group during acquisition (early: t56 = −2.96; P < 0.005, late: t56 = −2.57; P < 0.05) but lower SCRs during extinction (t56 = 2.28; P < 0.05; see Fig. 2).
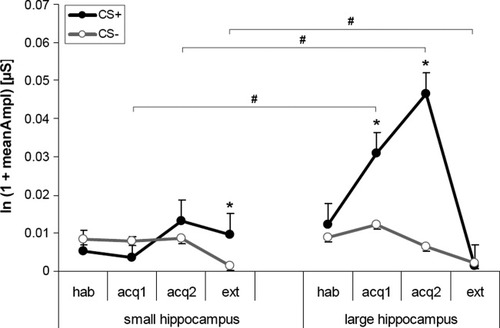
Skin conductance responses (SCR; means and standard errors of the means) during the second interval (5–9 s after stimulus onset) for the two groups during context conditioning; SIR, second interval response; hab, habituation phase; acq1, early acquisition phase; acq2, late acquisition phase; ext, extinction phase. # indicate significant between, * within group t-tests with P < 0.05.
Regression analyses for early acquisition revealed that hippocampal volume predicted differential SCRs to CS+/− (b = 0.26, t56 = 2.02, P < 0.05) and explained a significant proportion of the variance in the differential SCRs to CS+/− (R2 = 0.07, F1, 58 = 4.08, P < 0.05). A similar result was observed for the late acquisition phase (b = 0.27, t56 = 2.12, P < 0.05; R2 = 0.07, F1, 58 = 4.50 P < 0.05), while no significant results were detectable for extinction.
Self-report data
We found no significant main or interaction effects for group based on hippocampal volume in the measures of emotional valence, arousal or contingency (Supporting Information Fig. S1 on the journal website).
Hippocampal Subvolumes and Conditionability
Skin conductance responses
For the anterior or posterior portion of left and right hippocampus, we found no significant effect of group, CS-type or phase.
However, using groups based on right hippocampal volume we found a significant group × phase interaction (F2, 108 = 4.05; P < 0.05), indicating that the SCRs in each phase are influenced by group. The interaction of group × CS-type (F1, 54 = 3.30; P = 0.08) tended to be significant. The significant group × phase × CS-type interaction (F2, 108 = 6.03; P < 0.01) indicated successful CS+/− differentiation in the lHCV group during late acquisition (t28 = 3.25; P < 0.005) and for the sHCV group during extinction (t28 = 2.45; P < 0.05). Additionally, we found higher SCRs to CS+ in the lHCV group compared with the sHCV group during late acquisition (t56 = −2.39; P < 0.05) and the opposite pattern during extinction (t56 = 2.39; P < 0.05). Using groups based on left hippocampal volume we found a significant main effect of group (F1, 54 = 5.30; P < 0.05), but not of CS-type or phase. Further, group × phase (F2, 108 = 2.76; P = 0.07) as well as group × phase × CS-type (F2, 108 = 2.81; P = 0.08) tended to be significant.
Self-report data
Analyses of the arousal, valence and contingency ratings with groups based on anterior hippocampal volume revealed no significant influence of group on any self-report measures.
In contrast, using groups based on posterior hippocampal volume, arousal yielded a significant group × phase × CS-type interaction (F2, 110 = 3.51; P < 0.05) suggesting that successful arousal involved the posterior hippocampus, although follow-up tests revealed no significant differences between the groups. For emotional valence, we observed a trend towards significance for group (F1, 55 = 3.03; P = 0.09) and a significant group × phase × CS-type interaction (F2, 110 = 6.15; P < 0.005). Again we could not detect any significant differences between the groups. Analysis of the contingency ratings revealed only a trend towards a significant effect of the group × CS-type × phase interaction (F1.5, 80.0 = 3.17; P = 0.06).
Using groups based on right or left hippocampal volumes did not reveal any significant main or interaction effects of group.
Amygdalar and Total Brain Volume and Conditionability
Skin conductance responses
Adding amygdalar volumes to the model and allowing for interaction with group membership showed no significant main (F1, 47 = 0.59; P = 0.45) or interaction effects with phase (F2, 94 = 0.26; P = 0.77), CS-type (F1, 47 = 0.68; P = 0.41) or phase × CS-type (F1.4, 67.4 = 0.07; P = 0.87).
For total brain volume as covariate, we found no significant main or interaction effect with phase or CS-type for the analyses based on hippocampal or amygdalar volumes.
Self-report data
Using groups based on amygdalar volume, we found no significant influence of group on any of the self-report measures.
However, for arousal a nearly significant CS-type × covariate effect (F1, 55 = 4.01; P = 0.05) suggested an influence of total brain volume. For emotional valence, we observed significant interactions of covariate × phase (F2, 110 = 5.28; P < 0.01) and covariate × CS-type (F1, 55 = 8.90; P < 0.005), suggesting a role of total brain volume but not hippocampal or amygdalar volumes for the ratings of emotional valence. Analysis of the contingency ratings revealed a significant main effect of total brain volume (F1, 55 = 9.29; P < 0.005), again suggesting that successful CS+/− differentiation in all conditioning phases depended not on hippocampal or amygdalar, but total brain volumes. The significant interaction of covariate × CS-type (F1, 55 = 5.67; P < 0.05) and the trend towards significance for covariate × phase (F1.3, 70.9 = 3.17; P = 0.07) further underlined the role of other cortical areas for the cognitive stimulus evaluation.
DISCUSSION
The aim of the present study was to investigate a potential role of hippocampal and amygdalar volumes on contextual fear conditioning in humans. The results provide evidence for the involvement of hippocampal, but not amygdalar structures in the different measures of contextual fear conditioning. Healthy volunteers with lHCV showed successful differential conditioning and extinction. The SCRs in participants with sHCV indicated no significant differential conditioning during acquisition (although the increase from early to late acquisition suggests slower learning) and reached differential CS+/− responses only during extinction. Further analyses revealed that this effect on SCRs was mainly driven by the right hippocampus. In contrast, analyses of the self-reports showed no significant differences between the groups, but a significant impact of total brain volume. However, the posterior subvolume of the hippocampus influenced our measures of self-report, whereas the anterior subvolume did not.
Lesion studies in rodents implicated the hippocampus in contextual fear conditioning [Fanselow,2000, Rudy et al.,2002; Selden et al.1991). Analyses of temporal gene transcription in the amygdala and the hippocampus of the rat during fear conditioning support this notion [Barot et al.,2009]. Furthermore, imaging studies in humans found hippocampal activations during contextual fear conditioning [Alvarez et al.,2008; Hasler et al.2007; Lang et al.,2009; Marschner et al.,2008]. Hence, the present study is in line with and extends the major findings from animal and human imaging research.
The seemingly conflicting results between SCRs and self-report measures could be related to several factors: First, in earlier studies hippocampal volume as potentially mediating variable has not been assessed [Soeter and Kindt,2010; Tabbert et al., 2010]. Second, different cortical areas subserve autonomic responses and stimulus evaluation. For example, lesions of limbic structures, which lead to diminished or absent autonomic responses such as SCRs, leave correct stimulus evaluation intact [e.g., Slomine et al.,1999; Soussignan et al.,2005]. Our finding that total brain volume influenced differences in the self-reports but not the SCRs supports this interpretation. Third, SCRs are subject to substantial habituation, which, to our knowledge, has not been reported for self-reports so far. Fourth, the SCRs reflect a mean value of the ongoing learning process; whereas self-report data were recorded at the end of each phase, thereby constituting the final self-reported outcome of the learning process. This might also contribute to the observed deviations. Fifth, subvolumes of the hippocampus might be differentially involved in contextual fear conditioning. Our observation that specifically the posterior but not the anterior part of the hippocampus is involved in stimulus evaluation is in accordance with theories [e.g., Fanselow and Dong,2010] and empirical findings on hippocampal functioning. Several lines of evidence suggest that the anterior part is predominantly involved in anxiety-related processes, whereas the posterior part is associated with aspects such as spatial orientation or explicit memory [e.g., Pentkowski et al.,2006; Philips and LeDoux,1992; Quinn et al.,2008; Selden et al.1991]. Additionally, Bonne et al. [2008] found reduced posterior hippocampal volumes (i.e., body and tail of the hippocampus) in persons suffering from PTSD compared with healthy controls [but see Vythilingam et al.,2005]. In the present study, the use of anterior or posterior hippocampus yielded specific results for the self-reports, but not for the SCRs. Possible reasons for that are manifold; one conceivable explanation might be the functional dissociation for encoding and retrieval of different hippocampal substructures during the acquisition and extinction of contextual fear conditioning [Hunsaker and Kessner,2008]. With the current design, dissociation of these two processes was not possible.
Given that partial reinforcement schedules usually lead to robust conditioning effects in the SCRs, our finding of no significant CS+/− differentiation during extinction might be explained by a habituation effect. The total length of our paradigm (40 min in total) could explain, why we see nearly no differentiation in the SCRs but still substantially in the self-reports. This interpretation is in line with other studies reporting similar patterns [e.g., Birbaumer et al.,2005]. The decrease in the self-reports suggests a beginning extinction and that effect in the SCRs is most probably overlaid by habituation processes. The use of aversive stimuli as CS itself as in other studies [e.g., Soeter and Kindt,2010] might have prevented such a process.
Regarding this study, one should be aware of several strengths and limitations. Clinical questionnaires were used to show that both groups were comparable with respect to traumatic experiences during childhood or chronic stress levels, which might have an impact on hippocampal volume, thereby influencing memory performance [Bremner,1999]. Furthermore, there was no significant difference between the two groups with respect to intelligence, education or measures of verbal memory performance. Correlation of these variables with hippocampal volumes in the entire sample yielded no significant results. The contingency ratings indicate that the results cannot be attributed to contingency awareness [Tabbert et al.,2006], since all participants were aware of the contingency between CS+ and US and there was no significant difference between the two groups. Additionally, we controlled for total brain as well as amygdalar volumes between the two groups. However, it must be recognized that the quality of manual tracing of the hippocampal volumes is restricted by image acquisition characteristics, such as resolution. The very high intra- and inter-rater reliabilities in this study only indicate the stability of the segmentation, not the anatomical accuracy. Many different protocols [Geuze et al.,2005] with slightly divergent anatomical boundaries of the target structure exist and the resulting volumes are mainly comparable within the measured sample. Comparisons between different studies have to be made with caution. In addition, the border between anterior and posterior hippocampus was based on anatomical landmarks, as opposed to gene expression approaches [Fanselow and Dong,2010], which provide higher accuracy. Another limiting factor might be that smaller hippocampal volumes can be compensated by increased activity. Analysis of the functional data of our participants showed no significant activation differences in the hippocampus between the two groups (results not shown). However, conditioning involves many different structures in the brain (e.g., prefrontal cortex), which we did not analyze here. Further, a more detailed analysis of the SCRs (trial by trial) was not possible due to a technical artifact in the scanner resulting in missing values during acquisition and extinction. However, we did analyze early and late phases during acquisition and (although not reported explicitly) during extinction to evaluate the course of learning. Finally, one has to consider that contextual fear conditioning in unaffected by damage to the hippocampus before but not after conditioning [e.g., Rudy,2009]. Therefore, future research should study the recall of contextual fear.
In sum, we found better acquisition and extinction of contextual fear as indicated by SCRs in healthy participants with lHCV compared to those with sHCV, who may show slower responding. While amygdalar volumes had no detectable influence on any of our measures, we observed that verbal self-reports depended on total brain volume. A reanalysis for the arousal, emotional valence and contingency ratings with groups based on hippocampal subvolumes revealed that self-reports are modulated by the posterior but not anterior hippocampus. Interestingly, structural imaging studies reported diminished hippocampal volumes in anxiety disorders such as PTSD [Bremner et al.,1995; Karl et al.,2006]. Gilbertson et al. [2002] found reduced hippocampal volumes not only in Vietnam veterans suffering from PTSD, but also in their healthy monozygotic twins, suggesting that reduced hippocampal volume might be a vulnerability factor, not only a consequence of PTSD. Given the importance of fear conditioning for the etiology and maintenance of PTSD [Rothbaum and Davis,2003], the finding of impaired context conditioning in healthy participants with sHCV adds to the literature on predictive factors for PTSD, where deficient contextual binding and conditioning have been proposed as important fear-maintaining factors [Bryant, 2003; Wessa and Flor,2007]. Thus, reduced hippocampal volume is not only related to slower autonomic differentiation of contexts but may also negatively influence the attribution of correct contexts, both of which might interfere with trauma extinction and relearning.
Acknowledgements
The help of R. Dinu-Biringer is gratefully acknowledged.




