Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli
Abstract
Prior neuroimaging studies support the hypothesis that anticipation, an important component of anxiety, may be mediated by activation within the insular and medial prefrontal cortices including the anterior cingulate cortex. However, there is an insufficient understanding of how affective anticipation differs across anxiety groups in emotional brain loci and networks. We examined 14 anxiety positive (AP) and 14 anxiety normative (AN) individuals completing an affective picture anticipation task during functional magnetic resonance imaging (fMRI). Brain activation was examined across groups for cued anticipation (to aversive or pleasant stimuli). Both groups showed greater activation in the bilateral anterior insula during cued differential anticipation (i.e., aversive vs. pleasant), and activation on the right was significantly higher in AP compared to AN subjects. Functional connectivity showed that the left anterior insula was involved in a similar network during pleasant anticipation in both groups. The left anterior insula during aversive and the right anterior insula during all anticipation conditions coactivated with a cortical network consisting of frontal and parietal lobes in the AP group to a greater degree. These results are consistent with the hypothesis that anxiety is related to greater anticipatory reactivity in the brain and that there may be functional asymmetries in the brain that interact with psychiatric traits. Hum Brain Mapp, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Altered anticipation of future aversive events is a key aspect of anxiety disorders [Eysenck,1997; Grillon,2008]. Panic disorder, social phobia, generalized anxiety disorder, and posttraumatic stress disorder (PTSD) can be conceptualized as altered learning states characterized by exaggerated prediction errors due to an overgeneralization, which is followed by an exaggerated reaction to uncontrollable or unpredictable stressors [Mineka and Zinbarg,2006].
The insula, among other areas, has been suggested as a potentially critical biomarker for the detection of pathological anticipatory anxiety [Paulus and Stein,2006]. In a prior functional imaging study [Simmons et al.,2004], we examined anticipation of aversive images (i.e., spiders and snakes) in healthy volunteers and found anticipation-related activation within the right insula. Furthermore, we observed greater insula activity in nonclinical subjects with high-trait anxiety [Simmons et al.,2006] as well as in patients with PTSD [Simmons et al.,2008]. In a similar study, Nitschke and colleges displayed aversive and pleasant pictures to healthy volunteers to study the anticipatory stage and image presentation. They found anticipation-related activation in ventral and dorsal ACC, bilateral insula, and bilateral amygdala [Nitschke et al.,2006].
Several studies have found that negative anticipation and emotional processing is associated with strong right lateralized activation, particularly among psychiatric populations [Giesecke et al.,2005; Simmons et al.,2004,2008; Sommer et al.,2008; Strigo et al.,2008]. This finding has been linked to current theories of forebrain emotional asymmetry [Davidson et al.,2004] that have been extended to the left and right anterior insula on neuroanatomical grounds [Craig,2005]. This model suggests that the right anterior insula is associated with negative emotions focused on the exertion of energy, whereas left anterior insula activity is associated with positive emotions and the preservation of energy [Craig,2005]. This model also suggests that the neural networks recruited during negative anticipation, where energy must be exerted, may recruit broader networks than when energy is preserved and that individuals with a greater propensity for negative emotions will use these broader networks when engaging the right anterior insula.
Advances in statistical analysis in functional brain imaging provide a way to quantify the degree to which individuals or tasks engage a neural network [Friston et al.,1993]. These techniques can help to identify whether anxiety positive (AP) individuals (i.e., individuals with high trait anxiety which may express itself as one of a spectrum of anxiety disorders) exhibit dysfunction within affective circuits. This approach has been successful at finding differences in neural networks in related populations. Lanius and colleagues [2004] performed a connectivity analysis with a group of PTSD subjects where greater blood oxygenation level-dependent (BOLD) response in the ACC was seen during recall of traumatic events in contrast to nontraumatized controls. This study indicated differential functional connectivity between the MPFC and the insula, frontal, and parietal lobes such that separate subregions were differentially engaged between groups [Lanius et al.,2004]. These investigators showed in a follow-up study that PTSD subjects relative to healthy comparison subjects showed greater connectivity between the ACC and several regions including the right insula and the right middle frontal gyrus during script-driven imagery [Lanius et al.,2005]. Taken together, these findings suggest that individuals with anxiety disorders, thus far shown primarily in PTSD populations, differentially engage an extended cortical network during emotional processing that includes regions of the insula, MPFC, DLPFC, and parietal lobe.
In neuroimaging studies on aversive anticipation, we and others have argued that individuals with psychiatric disorders, such as PTSD or depression [Simmons et al.,2008,2009a; Strigo et al.,2008,2010], often spend energy focusing on the affective fear experience rather than adaptive preparation before the experience of an emotional event. This processing style has been suggested in prior literature [Beck and Clark,1997]. This suggests that not only does this experience induce a greater level of reactivity but it may in fact call upon an entirely different neural network. This network may include both modulatory control regions to control reactivity as well associative regions that are responsive to differential aspects of the experience. The existence of differential activation networks may be indicative of a general mechanistic difference in processing for those who are more prone to mental disorders.
The aim of this study was to examine if there is a greater difference in the neural circuitry involved in anticipating aversive compared to pleasant visual stimuli for AP (i.e., defined on the basis of trait measures anxiety) versus anxiety normative (AN) individuals. Although some of the key differences in anticipation in anxious individuals were inspected in a prior study [Simmons et al.,2006] in this study, we extend this by focusing (1) on the contrast between aversive and pleasant anticipation and (2) thorough examination of the differences within the connective anticipatory network between groups. We hypothesized that AP subjects would show greater insular activation than AN subjects during aversive versus pleasant stimuli anticipation. In addition, we predicted that the right insula would recruit a larger network than the left insula, and aversive anticipation more than pleasant anticipation, in AP compared to AN individuals. Support for these hypotheses would link a specific neural substrate, the insular cortex, with a psychological process, anticipation, to suggest a possible vulnerability marker for individuals at elevated risk for anxiety disorders.
MATERIALS AND METHODS
Subjects
Participants
This study was approved by the University of California San Diego (UCSD) and San Diego State University (SDSU) Institutional Review Boards, and all subjects provided written informed consent to participate. Initially, ∼3,000 undergraduate SDSU students were screened using the Spielberger Trait Anxiety Questionnaire [Spielberger et al.,1983]. Subjects who scored high in trait anxiety (in the upper 15th percentile of the distribution) and those who had normative levels of trait anxiety (from the 40th to 60th percentile of the distribution) were selected for further screening. Subjects were excluded from the AP group if they did not carry a current diagnosis of general anxiety disorder (GAD) or social anxiety disorder (SAD). Of these, 14 healthy AP subjects [11 females and 3 males, age 19.4 years ± 2.2 (range, 18–26) with an average education level of 13.7 ± 1 years (range, 13–15)] and 14 healthy AN subjects [8 females and 6 males, age 18.6 years ± 0.8 (range, 18–20) with an average education level of 13.4 ± 0.5 years (range, 13–16)] were interested, available, and passed scanner safety screening and thus completed scanning sessions. There were no significant differences between groups in these demographic variables. This sample was nonoverlapping with prior work with an anxious student population [Simmons et al.,2006; Stein et al.,2007].
All subjects underwent the Structured Clinical Interview for DSM-IV [First et al.,1997] to identify anxiety and mood disorders. AP subjects could have a current DSM-IV diagnosis of mood and/or anxiety disorder but were excluded if they were currently seeking, or had ever sought, treatment for their anxiety or mood symptoms. All 14 members of the AP group carried a current acute diagnosis, 3 subjects had GAD, 2 subjects had SAD, 4 subjects had both GAD and SAD, 4 subjects had GAD, SAD and major depressive disorder (MDD), and 1 subject had SAD and MDD. AN subjects were healthy and did not meet criteria for any DSM-IV disorder, even at a subthreshold level. None of the subjects had taken any psychotropic medications in the prior 12 months. Subjects consumed less than 400 mg of caffeine daily. All subjects gave their informed, written consent, and performed an anticipatory anxiety task during functional magnetic resonance imaging (fMRI).
Stimulus and Apparatus
The task combined a continuous performance task (CPT) during fMRI similar to a task described elsewhere [Simmons et al.,2008,2009b] with the occasional presentation of affective stimuli (see Fig. 1). During the CPT, subjects were asked to press a LEFT button whenever they saw a circle and a RIGHT button whenever they saw a square on the screen. During the baseline CPT, all shapes were blue with the color changing as a cue before image presentation. Stimuli were presented at a visual angle of 4° at a rate of 0.5 Hz. Simultaneously, a 250-msec long 500 Hz tone was presented at a rate of 2 Hz. Thirty-four picture stimuli were drawn from the International Affective Picture System [Lang et al.,1998] (IAPS). Subjects were instructed that a stimulus would indicate whether an aversive (n = 17; mean valence = 7.4, mean arousal = 5.0 on standardized IAPS ratings) or pleasant (n = 17; mean valence = 2.3, mean arousal = 6.3) image was going to be presented on the screen for 2 s. The combination of a low-pitch tone and a green circle or square predicted a pleasant picture 8 s in advance and a high-pitch tone combined with a red circle or square predicted an aversive picture 8 s in advance. As the contrasts were primarily between-group, the potential biasing of the cue-color association was similar for both groups and was expected to have minimal effects on the observed group differences. The total duration of the task was 580 s. Each subject completed a 4-min practice run before the imaging session to ensure sufficient strength of association between the cues and the stimulus. Behavioral data were collected and scored for accuracy and latency of response during CPT. No response from the subjects was required when a picture stimulus was presented on the screen.
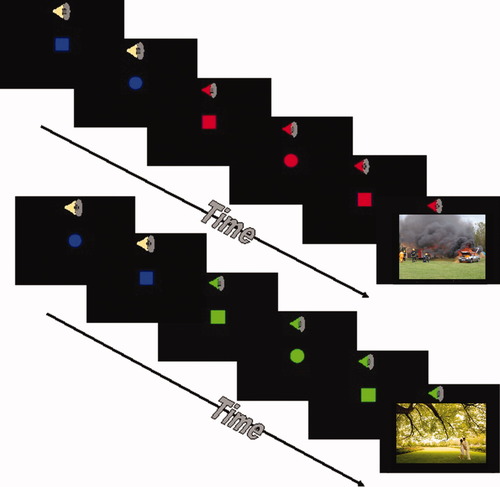
Anticipatory anxiety task. The fMRI task combined a continuous performance task (CPT) with the interspersed presentation of affective stimuli (IAPS). Subjects were asked to press the left or right button on a touch pad based on the shape on the screen. Subjects were instructed before the task that a switch from a blue to a green shape accompanied by a low tone would indicate that a positive image was going to appear on the screen. In contrast, a switch from a blue to a red shape accompanied by a high-tone signaled an impending negative image. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Response accuracy and response latency were obtained for the CPT during: (1) baseline = blue shapes, medium pitch tone, (2) anticipation of a pleasant or positive image = green shapes, low-pitch tone, and (3) anticipation of an aversive or negative image = red shapes, high-pitch tone. To examine the behavioral effect of anticipation, we examined the difference between behavioral measures during the aversive and pleasant anticipation. Repeated measures analysis was performed with both absolute reaction time and response accuracy during each condition as the repeated measure and group differences were examined.
Image Acquisition
During the task, an fMRI run sensitive to BOLD contrast was collected for each subject using a 3 Tesla GE scanner (T2 * weighted echo planar imaging, TR = 2,000 ms, TE = 32 ms, FOV = 250 × 250 mm3, 64 × 64 matrix, 30 2.6-mm axial slices with a 1.4-mm gap, 290 scans). fMRI acquisitions were time-locked to the onset of the task. During the same experimental session, a high-resolution T1-weighted image (SPGR, TI = 450 ms, TR = 8 ms, TE = 4 ms, flip angle = 12°, FOV = 256 × 256, 1-mm3 voxels) was obtained for anatomical reference.
Data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package [Cox, 1996]. Preprocessed time series data for each individual were analyzed using a multiple regression model. Regressors of interest included five regressors that were constructed to quantify the neural substrates contributing to the different components of the task: (1) the CPT, measuring the baseline performance during the processing of the CPT, (2) pleasant, capturing the anticipation of a pleasant (positive) image, (3) aversive, capturing the anticipation of an aversive (negative) image, (4) the positive image phase, which assesses the processing of pleasant (positive) stimuli, and (5) the negative image phase, which assesses the processing of aversive (negative) stimuli. In addition, five nuisance regressors were entered into the linear regression model: three movement-related regressors used to account for residual motion (in the roll, pitch, and yaw direction), and regressors for baseline and linear trends used to eliminate slow signal drifts. Subsequently, a simple contrast was constructed for task effect on an individual subject level for differential anticipation (i.e., aversive versus pleasant). A Gaussian filter with full width-half maximum 6 mm was applied to the voxel-wise percent signal change data to account for individual variations in the anatomical landmarks. Data of each subject were normalized to Talairach coordinates.
Voxel-wise percent signal change data for the whole brain were entered into two independent samples t-tests to assess: (1) task effect (i.e., AP + AN during aversive vs. pleasant anticipation) and (2) group effect (i.e., AP versus AN during aversive vs. pleasant anticipation). A threshold adjustment method based on Monte–Carlo simulations was used to guard against identifying false positive areas of activation [Forman et al.,1995]. A region of interest (ROI) based analysis was performed on the bilateral insula, dorsal lateral prefrontal cortex (DLPFC) medial prefrontal cortex (including dorsal anterior cingulate; MPFC), and amygdala. The insula mask included the entire insula cortex, and all masks were based on the AFNI version of the talairach atlas [Talairach and Tournoux,1998]. The ROI analysis was performed using volume-dependent clustering thresholds based on the total volume of the ROI using AFNI function alphasim. This was done in two ways; first, all regions where taken together as a collective brain mask set, this resulted in a minimum cluster size across regions of 768 μl. To ensure that the reported regions were not biased toward larger ROI structures individual calculations were performed, these resulted in minimum clusters sizes of 128 μl for the amygdala ROIs, 256 μl for the insula, 320 μl for the DLPFC, and 512 μl for the MPFC. However, no regions were included in these individually obtained volumes that did not satisfy the collective cluster thresholds. Connectivity and image-processing analysis, described below, were performed on the whole brain data. A prior voxel-wise probability of P < 0.05 in a cluster of 1,408 μl resulted in an a posteriori probability of P < 0.05. Finally, the average percent signal difference was extracted from regions of activation that were found to survive this threshold/cluster method. All analyses for the behavioral data were carried out with SPSS 12.0. To determine whether variance in neural activation for group or task clusters could be explained by the behavioral performance during CPT or by psychological variables, we correlated percent signal change during anticipation and image processing phases with behavioral performance (i.e., response latency and response selection) and response to psychological measures.
Asymmetry Analysis
To test if regions differed significantly between groups based on laterality, we extracted percent signal change for the contralateral region for all areas that showed group differences and performed a repeated measures ANOVA with group as a between subjects factor and side (original versus contralateral) as a within subjects factor. The interaction variable of group by side was used to determine the degree of group difference in laterality.
Functional Connectivity Analysis
Functional connectivity analysis was performed on both AP and AN subjects. The connectivity results are reported for (1) aversive anticipation, (2) pleasant anticipation, and (3) combined anticipation (i.e., aversive + pleasant). We used functional connectivity method based on the psychophysiological interaction method introduced by Friston et al. [1997] and adapted for AFNI (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html. Before conducting the functional connectivity analysis, the individual raw signal datasets were (1) band-pass filtered (0.009 < ƒ < 0.08), (2) corrected for slice-dependent time shifts, (3) corrected for interleaved acquisition, (4) corrected for rigid body head motion, and (5) warped to conform to the Talairach atlas [Lancaster et al.,2000]. Individual time courses in these processed raw signal datasets were extracted for the seed ROIs, namely bilateral anterior insula. These were defined based on the main effects of task (Table I). Data points were censored if they differed by more than two standard deviation from the average echoplanar signal for the given seed ROI. We then used AFNI function “3dTfitter” to remove hemodynamic delay from these insula (right, left) time courses. The resultant signal in insula (right, left) was then multiplied by the anticipation regressor (aversive, pleasant, and combined) thereby creating six interaction time courses, which were then each convolved with γ-variate hemodynamic using the AFNI function “waver.” Six regression models were run thereafter to examine connectivity of right and left insula during aversive, pleasant, and combined anticipation. Interaction time courses were used as regressors of interest. Six nuisance regressors controlling for baseline differences, linear drift, head movement (roll, pitch, and yaw), and fluctuations in white matter signal were added to each regression model. The resulting correlation coefficient for each time course of interest was calculated for each voxel. This provided correlation maps for the time course in the seeded ROIs and the time course from all other brain voxels as a function of anticipation. The Fisher Z transforms of these correlation maps were then warped to conform to the Talairach atlas [Lancaster et al.,2000], and a Gaussian blur of 6 mm FWHM was applied to allow for group comparisons of the Fisher Z transforms in right and left insula during aversive, pleasant, and combined anticipations using independent two-sample t-tests. This assessed differences in functional connectivity in right and left insula during aversive, pleasant, and combined anticipations between AP and AN groups. In addition, to test if the left and right anterior insula differed with regard to the degree of difference in network recruitment a paired voxelwise t-test was performed contrasting between group differences in the difference score between left and right Fisher Z scores. The resulting voxel-based data was cluster thresholded using Monte–Carlo simulations as described in greater detail in the primary analysis. The mean Fisher Z transformations are provided in all figures and tables concerning the connectivity analysis.
| Location | Side | Vol (mm3) | x | y | z | t-value |
|---|---|---|---|---|---|---|
| Insula | Right | 1,024 | 34 | 23 | 9 | 5.90 |
| Left | 896 | −31 | 16 | 13 | 3.52 | |
| DMPFC | Right/Left | 1,088 | 8 | 32 | 23 | 3.06 |
| DLPFC | Right | 19,072 | 37 | 23 | 35 | 6.37 |
| Left | 6,848 | −31 | 38 | 29 | 4.40 | |
| 1,856 | −23 | −1 | 50 | 3.75 | ||
| 896 | −31 | 16 | 13 | 3.52 |
RESULTS
Behavioral Measures
AP subjects did not differ from AN subjects on response latency [F(22,1) = 1.27, NS] or response accuracy [F(22,1) = 1.51, NS] during the performance of the CPT regardless of the phase examined, that is, baseline, aversive anticipation, and pleasant anticipation (see Supporting Information Fig. 1).
fMRI Data: Task-Related Activation Differences
Task effects
Both AP and AN subjects showed significant activation in the bilateral anterior insula, DLPFC, and dorsal medial prefrontal cortex (DMPFC) during the cued differential anticipation contrast (i.e., anticipation of aversive minus anticipation of pleasant images; Fig. 2A; Table I) in a one-sample voxel based ROI analysis.
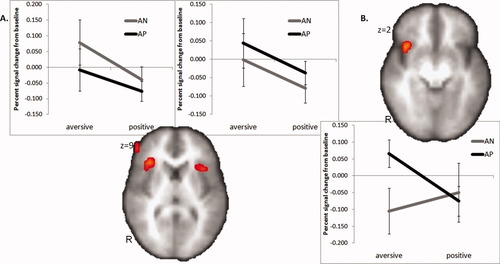
Significant activation in the anterior insula for task (A) and group (B) effects during anticipation of aversive stimuli versus pleasant stimuli. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Group effects
AP subjects showed significantly greater activation than AN subjects in the right anterior insula and left DLPFC during the cued differential anticipation contrast (Fig. 2B; Table II) in a two-sample voxel-based ROI analysis. There were no regions of significant clusters that were stronger for the AN than the AP group. The amygdala ROI showed no significant task or group effects during differential anticipation contrast. Likewise, the DMPFC/ACC showed no significant group effects. The difference that was found within the right anterior insula region did not overlap with the anterior insula regions found in the task effects (see Supporting Information Fig. 2). Activation during the image phase is presented in the supplementary documents (Supporting Information Table I and Fig. 3). To better depict the subpopulations within the AP group, percent signal changes were extracted for the GAD positive (n = 11) and SAD positive groups (n = 11; see Supporting Information Table II). In addition, the group contrast of the combined anticipatory effects was also extracted (see Supporting Information Table III).
| Location | Side | Vol (mm3) | x | y | z | t-value |
|---|---|---|---|---|---|---|
| Insula | Right | 768 | 38 | 17 | −2 | 3.18 |
| DLPFC | Left | 1664 | −40 | 11 | 38 | 4.75 |
| 768 | −20 | 11 | 53 | 3.40 |
Group asymmetry analysis
For any areas of group differences, the contralateral region was inspected and contrasted as specified earlier. Group-by-side interactions were significant for the left DLPFC regions, when compared with the same region on the right [F(1,26) = 14.627, P = 0.001; and F(1,26) = 10.217, P = 0.004, respectively] as well as for the right insula when compared with the same region on the left [F(1,26) = 4.520, P = 0.043].
Combined anticipatory connectivity
The combined anticipatory connectivity analysis with left anterior insula as the seed voxel region showed significantly stronger correlations for the AP compared to AN group with the left inferior frontal gyrus and cerebellum. Connectivity analysis with the right anterior insula as the seed voxel region showed significantly stronger correlations for the AP compared to AN group with a wider network, such as the left inferior frontal, DLPFC, parietal lobe, and cerebellum (Fig. 3; Supporting Information Table IV). The differences in the connectivity maps between right and left insula were contrasted and compared between groups (Supporting Information Table V and Fig. 5). This analysis confirmed that the right insula showed numerous areas with significantly greater connectivity to the right than the left insula in the AP compared to AN group.
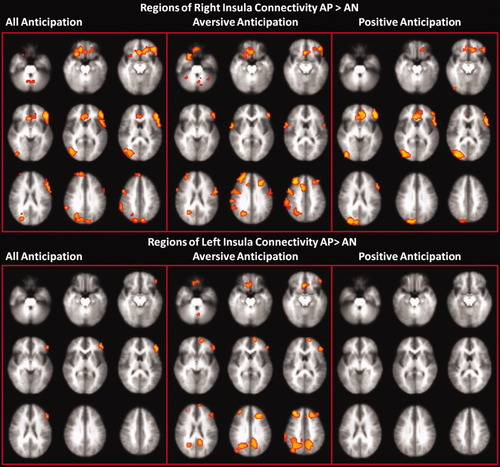
Group differences in functional connectivity of the right and left insula during combined (aversive + pleasant), aversive, and pleasant anticipatory periods (z = −26, −18, −10, −2, 6, 14, 22, 30, 38). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Conditional anticipatory connectivity
The aversive anticipatory connectivity analysis with both left and right anterior insula as the seed voxel region showed significantly stronger correlations with the posterior cingulate, parietal lobe, right inferior prefrontal gyrus, and ventral medial prefrontal gyrus in the AP compared to AN group. Connectivity analysis for the pleasant anticipation with the right anterior insula as the seed voxel showed more connected areas such as the ventral medial frontal gyrus, left inferior prefrontal gyrus, and right precuneus in the AP compared to AN group. No regions showed significant group difference in the pleasant anticipation connectivity analysis with the left anterior insula seed region (Fig. 3; Table III).
| Condition | Seed | Vol | x | y | z | Region | BA | t-value | ANa | APa |
|---|---|---|---|---|---|---|---|---|---|---|
| Aversive anticipation | ||||||||||
| Right insula | ||||||||||
| 19,008 | −14 | −56 | −38 | Left cerebellum | 2.598 | 0.042 (0.055) | 0.100 (0.052) | |||
| 9,792 | 42 | −42 | 38 | Right inferior parietal lobule | 40 | 2.323 | 0.114 (0.075) | 0.161 (0.065) | ||
| 5,888 | 29 | 39 | 35 | Right middle frontal gyrus | 9 | 2.364 | 0.148 (0.102) | 0.223 (0.120) | ||
| 5,184 | 25 | 4 | 57 | Right middle frontal gyrus | 6 | 2.418 | 0.128 (0.096) | 0.182 (0.081) | ||
| 4,160 | −2 | −61 | 49 | Left precuneus | 7 | 2.291 | 0.114 (0.074) | 0.173 (0.079) | ||
| 3,840 | 4 | 29 | −17 | Right medial frontal gyrus | 25 | 2.611 | 0.045 (0.073) | 0.121 (0.071) | ||
| 3,008 | −43 | 35 | −4 | Left middle frontal gyrus | 47 | 2.386 | 0.076 (0.057) | 0.151 (0.095) | ||
| 2,432 | 47 | 3 | 36 | Right precentral gyrus | 6 | 2.353 | 0.142 (0.095) | 0.198 (0.088) | ||
| 2,368 | 22 | 15 | −26 | Right superior temporal gyrus | 38 | 2.499 | 0.069 (0.072) | 0.143 (0.063) | ||
| 2,176 | −27 | 30 | 33 | Left middle frontal gyrus | 9 | 2.217 | 0.132 (0.094) | 0.219 (0.144) | ||
| 2,176 | 8 | 17 | 39 | Right cingulate gyrus | 32 | 2.186 | 0.148 (0.108) | 0.217 (0.120) | ||
| 2,112 | 33 | −45 | −34 | Right cerebellum | 2.470 | 0.045 (0.052) | 0.107 (0.063) | |||
| 1,728 | 58 | 5 | 11 | Right precentral gyrus | 6 | 2.456 | 0.118 (0.089) | 0.190 (0.086) | ||
| 1,728 | −53 | 3 | 24 | Left inferior frontal gyrus | 9 | 2.311 | 0.097 (0.083) | 0.162 (0.096) | ||
| Left insula | ||||||||||
| 34,368 | 6 | −57 | 42 | Right precuneus | 7 | 2.456 | 0.112 (0.062) | 0.169 (0.070) | ||
| 5,440 | 1 | −46 | −39 | Right cerebellum | 2.360 | 0.034 (0.073) | 0.101 (0.065) | |||
| 5,440 | −18 | 29 | 39 | Left middle frontal gyrus | 8 | 2.205 | 0.143 (0.069) | 0.232 (0.140) | ||
| 2,752 | 3 | 27 | −16 | Right medial frontal gyrus | 25 | 2.534 | 0.066 (0.064) | 0.140 (0.066) | ||
| 2,368 | 52 | −33 | 46 | Right inferior parietal lobule | 40 | 2.536 | 0.102 (0.051) | 0.155 (0.068) | ||
| 2,368 | −29 | 3 | 53 | Left middle frontal gyrus | 6 | 2.218 | 0.125 (0.068) | 0.188 (0.101) | ||
| 2,240 | −46 | 34 | 8 | Left inferior frontal gyrus | 46 | 2.304 | 0.096 (0.056) | 0.177 (0.094) | ||
| 2,240 | 28 | 35 | 37 | Right middle frontal gyrus | 9 | 2.262 | 0.151 (0.086) | 0.239 (0.132) | ||
| 1,664 | −12 | 58 | 8 | Left medial frontal gyrus | 10 | 2.268 | 0.134 (0.062) | 0.209 (0.094) | ||
| Pleasant anticipation | ||||||||||
| Right insula | ||||||||||
| 12,544 | 28 | −81 | 16 | Right middle occipital gyrus | 19 | 2.449 | 0.063 (0.085) | 0.146 (0.107) | ||
| 8,512 | −4 | 34 | −1 | Left anterior cingulate | 24 | 2.483 | 0.027 (0.086) | 0.093 (0.085) | ||
| 6,272 | −48 | 27 | 6 | Left inferior frontal gyrus | 45 | 2.287 | 0.028 (0.121) | 0.116 (0.115) | ||
| Left insula | ||||||||||
| N/A | ||||||||||
- a The connectivity values are the Fisher Z transformations or the r values provided as mean (standard error).
fMRI Data: Brain behavior relationships
To determine whether neural activation differences between the groups were associated with behavioral performance, we extracted percent signal change in anticipation main effects [see Table II and functional connectivity values (see Table III)]. There were no statistically significant correlations between activation differences and response latency or response accuracy. After controlling for multiple comparison, there were no statistically significant within group correlations between activation differences and psychological measures.
DISCUSSION
This investigation yielded two main results. First, AP individuals showed heightened right anterior insula and left DLPFC response during the anticipation of aversive versus pleasant images relative to AN individuals. Second, functional connectivity analyses showed that AP subjects had a larger connective network for the aversive anticipation in the right and left anterior insula as well as during pleasant anticipation with the right anterior insula and no significant difference in the left anterior insula. Taken together, these findings are consistent with many prior studies supporting an important role of the anterior insula in affective anticipation. Furthermore, these results highlight the role that the right anterior insula plays in anticipatory processes related to anxiety via engagement of a wide cortical network.
A growing body of literature has attributed to the insular cortex an important role in anticipatory processing both in healthy and anxious populations [Adolphs et al.,2000; Paulus and Stein,2006]. For example, anticipation of aversive stimulation in healthy [Eisenberger et al.,2003; Price,2002; Simmons et al.,2004] and anxious individuals [Dilger et al.,2003; Rauch et al.,1997; Simmons et al.,2006,2008; Straube et al.,2004a,b; Wright et al.,2003] has been associated with insular cortex activation. In particular, individuals with PTSD have shown altered insula activation [Lanius et al.,2004,2005] or decreased modulatory change in the right anterior insula [Simmons et al.,2009a]. The insular cortex is part of a neural system involved in homeostatic processing of autonomic arousal and visceral changes [Craig,2002; Critchley et al.,2003,2004,2005], signaling executive areas to initiate avoidant behavior [Langs et al.,2000; Starcevic et al.,1993] and altering self-awareness [Karnath et al.,2005]. The insular cortex, MPFC, and amygdala play crucial roles when linking internal physiological states to external cues or events.
Considering the functionality of this circuitry, it is not surprising that the right anterior insular cortex is a heavily recruited region for processing during affect anticipation in highly anxious individuals. In addition, Caria and colleagues [2007] recently showed that when supplied “real-time” feedback about their physiological state, individuals could actively regulate anterior insula activation and that this activation led to the recruitment of numerous prefrontal regions. These observations strongly implicate the insula as part of an interoceptive processing network that also includes the DLPFC and DMPFC. Our functional connectivity analysis suggested that several regions activated in connection with the right anterior insula, including the inferior frontal, ventral medial prefrontal, and superior parietal lobe, a coupling previously found in the context of anticipation [Chua et al.,1999; Simmons et al.,2004,2008] and attention [Cabeza and Nyberg,2000; Gitelman et al.,1999]. Although the amygdala is thought to play an important role in affective anticipation [Nitschke et al.,2006], we did not see activation within this region in our study. One potential reason for this difference may be the inclusion of the CPT in this study. Prior research has found that negative emotions can be attenuated during the performance of a cognitive task [Lavie,1995].
A role for insular cortex in both interoceptive and emotional processing has been noted in prior reports [Craig,2002,2005; Paulus and Stein,2006; Phan et al.,2002,2004], and converging evidence supports a valence-dependent asymmetric association of left anterior insula with positive affect and parasympathetic activity, and right anterior insula with negative affect and sympathetic activity. Activation of the left anterior insula is associated with pleasant, “energy enrichment” emotions [Craig,2005; Porges et al.,1994], such as feelings of maternal attachment and romantic love [Bartels and Zeki,2004; Leibenluft et al.,2004], as well as the “chills down the spine” feeling provoked by subjectively pleasing music [Griffiths et al.,2004; Koelsch,2005]. In one recent study, activation of left insula showed a linear relationship with the valence of an IAPS image, that is, the more pleasant a particular image was rated the greater the left insula was activated [Heinzel et al.,2005]. In contrast, right anterior insula is activated by pain [Schweinhardt et al.,2006] and is associated with depression and anxiety [Giesecke et al.,2005; Simmons et al.,2006]. Furthermore, activation of the left/right anterior insula is associated with parasympathetic/sympathetic activity, respectively [Craig,2005; Oppenheimer et al.,1992]. The increased activation in the right insular cortex of anxious individuals may be due to attenuated top-down control via fronto-cortical circuits, a possibility suggested by others [Devinsky et al.,1995]. This notion is consistent with our findings of attenuated functional connectivity between insula and DLPFC in anxious subjects, and by other studies showing activation of frontal circuits during volitional reduction of anterior insula activity when subjects are provided with real-time fMRI feedback [Caria et al.,2007]. However, the significance of the asymmetry has not previously been statistically assessed.
Craig [2005] posits that this asymmetry is due to the lateralization of afferent vagal nerve connections to the insula. Several functional imaging findings have found that the right insula is specifically activated in those subjects having stronger emotional experiences [Paulus et al.,2003; Sander and Scheich,2005; Simmons et al.,2004; Strigo et al.,2008]. The current findings imply that while both the right and left insula are engaged during cued affective anticipation, the right anterior insula is more engaged in anxious individuals and is functionally connected to a much larger network during anticipation than the left insula. Thus, increased activation in the right lateralized insula may be considered a biomarker for anxious individual when performing an emotive task with anticipatory demands.
We speculate that anxious individuals have an increased inertia to adjust to changing emotional conditions, that is, that bottom-up signals of altered interoceptive processing are not sufficiently connected to top-down modulation of this signal. Specifically, although the right insula is anatomically and physiologically connected to areas important for cognitive control, such as the DLPFC, this connectivity may be inefficient in high-trait anxiety individuals, because it does not result in adequate attenuation of insula activation in response to changing emotional cues. Treatment studies with anxiolytic and antidepressant substances provide corroborating evidence for the “ineffective modulation” hypothesis that we suggest here. These posit that the effects of selective serotonin reuptake inhibitors (SSRIs) in affective disorders are likely to be the result of the interaction between serotonin (5-HT) pathways with the cortical and subcortical circuitry responsible for processing of emotional stimuli [Fu et al.,2004]. In fact, our group has recently shown that SSRIs modulate the affective signal produced by emotional probes [Arce et al.,2007] including anticipation [Simmons et al.,2009b].
This study has several limitations. First, we studied the behavior of individuals with a variety of anxiety disorder diagnoses rather than a specific clinical diagnosis, which may limit the generalizability of our findings to any singular anxiety disorder. It should be noted that the classification of high-trait anxiety using the STAI captures those with co-morbid MDD, and thus the data may reflect symptomology that is not exclusive to anxiety disorders. There is, however, growing evidence that several of the anxiety disorders—notably excluding obsessive compulsive disorder, which was not a disorder represented in our study sample—have common abnormalities in emotion processing [Etkin and Wager,2007]. Second, significant neuroimaging differences between groups were observed in the absence of differences in the behavioral task data. One possible explanation for this discrepancy, particularly as far as the response latency data are concerned, could be the repetitive pacing and the relative ease of the task, that is, the button pressing does not directly reflect interoceptive state. Future studies may include a randomized temporal jitter during the CPT to better detect response time differences during anticipation.
To summarize, the current findings support heightened right anterior insula activation in high-trait anxiety individuals during anticipation of negative stimuli. In addition, it appears in high-anxiety individuals that the right anterior insula is engaged in an extensive cortical network and that activation during aversive anticipation recruits more regions than pleasant anticipation does. It will be important to replicate these findings and to evaluate the extent to which these patterns of neural responding are altered with successful treatments.
Acknowledgements
We acknowledge the invaluable help of Scott Matthews, Ryan Pepin, David Leland, and Marc Wittmann.