Functional connectivity in mild traumatic brain injury
Abstract
Objectives: Research suggests that the majority of mild traumatic brain injury (mTBI) patients exhibit both cognitive and emotional dysfunction within the first weeks of injury, followed by symptom resolution 3–6 months postinjury. The neuronal correlates of said dysfunction are difficult to detect with standard clinical neuroimaging, complicating differential diagnosis and early identification of patients who may not recover. This study examined whether resting state functional magnetic resonance imaging (fMRI) provides objective markers of injury and predicts cognitive, emotional, and somatic complaints in mTBI patients semiacutely (<3 weeks postinjury) and in late recovery (3–5 month) phases. Methods: Twenty-seven semiacute mTBI patients and 26 gender, age, and education-matched controls were studied. Fifteen of 27 patients returned for a follow-up visit 3–5 months postinjury. The main dependent variables were spontaneous fluctuations (temporal correlation) in the default-mode (DMN) and fronto-parietal task-related networks as measured by fMRI. Results: Significant differences in self-reported cognitive, emotional, and somatic complaints were observed (all P < 0.05), despite normal clinical (T1 and T2) imaging and neuropsychological testing results. Mild TBI patients demonstrated decreased functional connectivity within the DMN and hyper-connectivity between the DMN and lateral prefrontal cortex. Measures of functional connectivity exhibited high levels of sensitivity and specificity for patient classification and predicted cognitive complaints in the semi-acute injury stage. However, no changes in functional connectivity were observed across a 4-month recovery period. Conclusions: Abnormal connectivity between the DMN and frontal cortex may provide objective biomarkers of mTBI and underlie cognitive impairment. Hum Brain Mapp, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Amongst neuropsychiatric disorders, mild traumatic brain injury (mTBI) offers a unique opportunity for examining transient disruptions in cognitive and emotional functioning and their neuronal correlates in a human model. Deficits in cognitive functioning during the semiacute (i.e., first 3 weeks) injury phase are followed by full recovery in the majority of patients 3–6 months postinjury [Belanger et al.,2005,2007]. Several studies have used functional magnetic resonance imaging during various cognitive challenges to investigate putative biomarkers of this cognitive dysfunction following mTBI [McAllister et al.,1999,2001; Mayer et al.,2009; Smits et al.,2009]. However, it is increasingly recognized that a majority (∼60–80%) of the brain's resources are expended to maintain homeostasis at rest [Raichle and Mintun,2006]. Although electrophysiological studies indicate abnormal slow-wave activity in mTBI during passive mental activity [Huang et al.,2009; Lewine et al.,2007], an examination of functional connectivity (e.g., characterization of temporal coherence between different regions) in mTBI has not been conducted.
Animal work suggests that the resting brain (i.e., absence of experimentally evoked activity) is characterized by spontaneous neuronal fluctuations that synchronously occur over spatially distributed networks [Raichle and Mintun,2006]. Resting state fluctuations can be measured with the blood oxygen level dependent (BOLD) response in humans and appear to be organized into distinct networks that mirror activity evoked across a variety of cognitive challenges [Smith et al.,2009]. The majority (60–80%) of the brain's resources are expended to maintain homeostasis [Raichle and Mintun,2006], suggesting that resting state networks (RSN) may be particularly vulnerable to injury following mTBI. A recent study suggests that functional connectivity can be used to measure cortical reorganization following peripheral nerve injury in rodent models [Pawela et al.,2010].
The default-mode network (DMN) is a well-studied human RSN that likely mediates a variety of passive mental activities [Buckner et al.,2008]. The primary nodes of the DMN include the rostral anterior cingulate gyrus (rACC), posterior cingulate gyrus (PCC), superior temporal/supramarginal gyrus (SMG), and ventromedial prefrontal cortex, with the rACC and PCC serving as central hubs [Buckner et al.,2008]. DMN activity parametrically varies with task difficulty [Binder et al.,1999] and is predictive of attentional lapses during cognitively demanding tasks [Eichele et al.,2008; Weissman et al.,2006]. In addition, spontaneous BOLD activity in the DMN is negatively (i.e., anticorrelated) correlated with activity in the lateral prefrontal cortex and inferior parietal lobes [Fox et al.,2005], two regions implicated in a range of higher-order cognitive processes [Cabeza and Nyberg,2000]. This anticorrelated relationship between DMN and fronto-parietal task related network (TRN) has been observed during passive mental activity [Fox et al.,2005] and during cognitive tasks [Binder et al.,1999], suggesting that the two networks may act in conjunction to produce states of high (increased TRN activity coupled with decreased DMN activity) or low (increased DMN activity coupled with decreased TRN activity) attentiveness to external events. To date, functional connectivity studies have primarily been conducted in severely injured [Nakamura et al.,2009] or minimally conscious [Boly et al.,2008,2009; Vanhaudenhuyse et al.,2010] patients, with all results indicating decreased connectivity within the DMN following injury and one study indicating that functional connectivity improved with recovery [Nakamura et al.,2009].
In addition to measures of functional connectivity, diffusion tensor imaging (DTI) can be used to estimate the integrity of white matter tracts both within and between these two networks [Johansen-Berg and Rushworth,2009]. The rACC and PCC are anatomically connected [Aralasmak et al.,2006; van den Heuvel et al.,2009] via the bilateral cingulum bundles (CB). Additionally, there is evidence [Lehericy et al.,2000; Schmahmann et al.,2008] that the ACC and prefrontal cortex may be connected via the anterior limb of the internal capsule (ALIC), through the anterior corona radiata (ACR), and/or by the external capsule (EC). The superior longitudinal fasciculus (SLF) has also been reported to connect the ACC and the inferior parietal lobule, the prefrontal cortex with the inferior parietal lobule, and the ACC with the prefrontal cortex [Aralasmak et al.,2006; Hua et al.,2009; van den Heuvel et al.,2009].
This study examined disruptions in functional connectivity (i.e., temporal correlations in spontaneous BOLD response) between the DMN and TRN following mTBI and whether connectivity normalized as a function of recovery. We also examined how functional connectivity relates to subjective and objective psychological and cognitive deficits as well as measures of white matter integrity (DTI).
METHODS
Participants
Twenty-seven patients with mTBI (15 females; 27.15 ± 7.38 years old; 13.22 ± 2.39 years of education) and 26 sex, age, and education-matched (15 females; 27.12 ± 7.32 years old; 13.96 ± 2.44 years of education) healthy controls (HC) participated in the current study. All mTBI patients were recruited from the University Emergency Room. Inclusion criteria for the mTBI group were based on the American Congress of Rehabilitation Medicine, including a Glasgow Coma Score of 13–15 (at first contact with medical staff), loss of consciousness (if present) limited to 30 min in duration, and posttraumatic amnesia (if present) limited to 24 h. mTBI and HC participants were excluded if there was a prior history of neurological disease, major psychiatric disturbance, additional closed head injuries with more than 5 min loss of consciousness, learning disorder, ADHD, or a history of substance or alcohol abuse/dependence. One male patient and one male HC were identified as outliers (above three standard deviations) on head motion parameters from their respective cohorts and were excluded from further analyses. Informed consent was obtained from all participants according to institutional guidelines at the University of New Mexico.
Five of the mTBI subjects were being prescribed medications for pain related to the accident at the time of their visit, and one patient was taking a prescribed antidepressant (venlafaxine). Patients were evaluated both clinically (mean day postinjury = 11.32 ± 4.56) and with brain imaging (mean day postinjury = 11.50 ± 5.35) within 21 days of injury (see Supporting Information Table I). The maximum allowed time between clinical and imaging sessions was 1 week, although it was typically much shorter (mean days between sessions = 1.56 ± 1.78 for mTBI patients). One mTBI patient and one HC were not able to complete neuropsychological testing due to scheduling difficulties during their first visit. Fifteen mTBI patients (57.69%) and 19 HC (76.00%) returned for a follow-up visit 3–5 months (mTBI = 109.87 ± 12.19; HC = 114.5 ± 12.8 days) post initial assessment.
Primary reasons for visit two attrition included participant's inability to schedule second visit (9 mTBI; 1 HC) or an inability to contact participants (2 mTBI; 2 HC). In addition, 3 HC were not eligible for follow-up at the time of the current summary. Therefore, a total of 15 mTBI patients (10 females; 27.47 ± 8.52 years old; 13.73 ± 2.81 years of education) and 15 sex, age, and education matched (10 females; 28.20 ± 8.17 years old; 14.47 ± 2.23 years of education) HC were used for all longitudinal analyses.
Clinical Assessment and Imaging Protocol
Composite indices were calculated for attention, working memory, processing speed, executive function, memory, emotional status, somatic complaints, and cognitive complaints [Mayer et al.,2009]. The Wechsler Test of Adult Reading (WTAR) and the Test of Memory and Malingering provided estimates of overall premorbid intellectual functioning and effort, respectively.
All images were collected on a 3 Tesla Siemens Trio scanner. Foam padding and paper tape were used to restrict motion within the scanner. High-resolution T1-weighted anatomic images were acquired with a five-echo multiecho MPRAGE sequence [TE (echo time) = 1.64, 3.5, 5.36, 7.22, and 9.08 ms, TR (repetition time) = 2.53 s, TI (inversion time) = 1.2s, 7° flip angle, number of excitations (NEX) = 1, slice thickness = 1 mm, field of view (FOV) = 256 mm, and resolution = 256 × 256]. T2-weighted images were collected with a fast spin echo sequence [TE = 77.0 ms, TR = 1.55 s, flip angle 152°, NEX = 1, slice thickness = 1.5 mm, FOV = 220 mm, matrix = 192 × 192, and voxel size = 1.15 × 1.15 × 1.5 mm3]. Functional connectivity BOLD data was collected using a single-shot, gradient-echo echoplanar pulse sequence [TR = 2,000 ms; TE = 29 ms; 150 measurements; flip angle = 75°; FOV = 240 mm; matrix size = 64 × 64] with 33 contiguous axial 4.55-mm-thick slices for whole-brain coverage (voxel size: 3.75 × 3.75 × 4.55 mm). Two DTI scans (b = 800 s/mm2) were acquired using a twice-refocused spin echo sequence with 30 diffusion gradients and the b = 0 experiment repeated five times [72 interleaved slices; TE = 84 ms; TR = 9.0 s; 90° flip angle; NEX = 1; slice thickness = 2.0 mm; FOV = 256 × 256 mm; matrix size = 128 × 128; voxel resolution = 2 mm3].
Functional Magnetic Resonance Image Processing
Data from the extended resting task (e.g., functional BOLD connectivity analyses) were spatially registered in both two- and three-dimensional space to minimize effects of head motion, temporally interpolated to correct for slice-time acquisition differences, and despiked using the AFNI software package [Cox,1996]. A regression analysis was then conducted on individual subjects' time-series to remove potential sources of noise (physiological and machine-based) from the data based on established methodologies [Fox et al.,2005]. Briefly, individual anatomical images (i.e., T1) were first segmented into maps of white matter, gray matter, and cerebral spinal fluid (CSF); the resultant CSF and white matter masks were then used to obtain an average time-series for these tissues during the extended resting state run. Next, all six movement parameters, the region of interest (ROI)-based time-series for CSF and white matter, a constant term, and a linear term were entered into a linear regression against the extended resting state time-series. A global gray matter term was not entered into the regression to minimize likelihood of increased anticorrelations [Fox et al.,2009; Murphy et al.,2009]. The residual time-series data were then transformed into a standardized coordinate space [Talairach and Tournoux,1988].
Based on previous studies, the “seeds” for functional analyses were placed within the rACC and PCC to determine connectivity with the DMN [Fox et al.,2005; Uddin et al.,2009], whereas the inferior parietal lobule and lateral prefrontal cortex were used as seeds to define the TRN [Fox et al.,2005]. Specifically, 12-mm spheres for the connectivity analyses were generated based on voxels exhibiting maximal DMN activity in the rACC (0, 49, 9) and PCC (0, −47, 33) in an independent sample of 42 HC subjects [Franco et al.,2008] (http://icatb.sourceforge.net/gift/gift_startup.php). Twelve millimeter spheres for the right inferior parietal lobule (37, −50, 40) and right lateral prefrontal cortex (33, 23, 5) were generated based on a previous study involving mTBI patients during an attention paradigm [Mayer et al.,2009]. Averaged individual residualized time-courses from these spheres were used as the primary regressor in four separate whole-brain BOLD connectivity analyses. Resultant Pearson's correlation coefficients were then converted to z-scores using Fisher's method, blurred using a 10-mm Gaussian kernel, and contrasted across the two groups. Statistical maps were corrected at P < 0.05 using both parametric and spatial thresholds determined by 10,000 Monte Carlo simulations [Forman et al.,1995]. Clusters that did not exhibit absolute connectivity coefficients greater than .05 for either group were also excluded from further processing.
Diffusion Tensor Imaging
The methods used to calculate fractional anisotropy (FA), axial, and radial diffusivity (RD) are similar to our previous publication on a subset (∼75%) of this cohort [Mayer et al.,2010]. Based on a literature review [Aralasmak et al.,2006; Hua et al.,2009; Lehericy et al.,2004; Schmahmann et al.,2008; van den Heuvel et al.,2009], several of the major white-matter tracts implicated in connecting the primary nodes of the DMN and TRN, including the CB, SLF, ACR, ALIC, and EC, were directly compared across the two groups. In addition, relationships between functional connectivity and white matter integrity were also examined.
RESULTS
Neuropsychological and Clinical Measures
Increases in emotional (t1,47 = −2.8, P < 0.05), cognitive (t1,48 = −4.1; P < 0.001), and somatic (t1,48 = −4.3; P < 0.001) complaints were observed for mTBI patients (see Table I). Despite educational matching, HC achieved higher estimates of premorbid intellectual functioning (WTAR; t1,47 = 2.3, P < 0.05), resulting in the use of WTAR as a covariate in all subsequent analyses. A MANCOVA indicated that the effect of diagnostic group was not significant (P > 0.10) for composite indices of attention, working memory, memory, processing speed, and executive functioning.
| Demographica | mTBI | HC | P value | Cohen's d | ||
|---|---|---|---|---|---|---|
| Mean | SD (±) | Mean | SD (±) | |||
| Age | 27.15 | 7.37 | 27.12 | 7.32 | P > 0.10 | 0.00 |
| Education | 13.22 | 2.39 | 13.96 | 2.44 | P > 0.10 | 0.31 |
| HQ | 80.31 | 35.39 | 84.69 | 33.22 | P > 0.10 | 0.13 |
| Neuropsych | ||||||
| WTAR | 49.20 | 8.54 | 54.71 | 8.24 | P < 0.005 | 0.66 |
| TOMM | 53.44 | 7.41 | 52.58 | 11.00 | P > 0.10 | −0.09 |
| Attentionb | 50.81 | 6.85 | 50.94 | 6.87 | P > 0.10 | 0.14 |
| Memoryb | 49.55 | 7.72 | 52.13 | 7.73 | P > 0.10 | 0.35 |
| WMb | 49.90 | 6.66 | 49.44 | 6.67 | P > 0.10 | −0.07 |
| PSb | 46.34 | 8.77 | 47.73 | 8.79 | P > 0.10 | 0.16 |
| EFb | 46.88 | 7.23 | 46.13 | 7.25 | P > 0.10 | 0.10 |
| Sx Severity | ||||||
| Emotional | 50.44 | 9.27 | 43.96 | 7.00 | P < 0.05 | −0.79 |
| NBSI-Som | 6.80 | 5.55 | 1.60 | 2.25 | P < 0.01 | −1.23 |
| NBSI-Cog | 7.32 | 5.65 | 2.28 | 2.56 | P < 0.001 | −1.15 |
- a Demographic values calculated from full sample, including outliers.
- b Means, standard deviations, and effect sizes for neuropsychological indices reported following correction for WTAR as covariate at 51.90.
- Note: HQ, handedness quotient; WM, working memory; PS, processing speed; EF, executive function; WTAR, Wechsler Test of Adult Reading; TOMM, Test of Memory Malingering; Sx, symptom; NBSI, Neurobehavioral Symptom Inventory.
Clinical Data for Visit Two
There were no differences (P > 0.10) in premorbid intelligence, levels of effort, or neuropsychological indices for returning (n = 15) versus nonreturning (n = 12) patients.
Three 2 × 2 [time (visit 1, visit 2) × diagnosis (HC, mTBI)] mixed measures ANOVAs examined potential changes in self-report measures as a function of time. Results indicated an effect of group for somatic (F1,28 = 25.9; P < 0.001) and emotional (F1,28 = 4.9; P < 0.05) complaints, with mTBI self-reporting more symptoms than HC. The group by visit interaction term was significant (F1,28 = 6.6; P < 0.05) for cognitive complaints, with mTBI (t1,14 = 3.2; P < 0.01) reporting fewer cognitive complaints at visit 2 (3.0 ± 2.8) compared to visit 1 (mean = 6.9 ± 4.4).
Structural Imaging Data
T1- and T2-weighted images were deemed to be free of trauma-related pathology (i.e., noncomplicated mTBI patients) by a neuroradiologist blinded to diagnosis.
Motion Parameter Analyses
The group effect was not significant (P > 0.10) on a MANOVA examining differences in head motion (three translational and three rotational parameters) following rigid body correction. However, several univariate measures suggested increased motion in the mTBI group (roll: F1,49 = 7.1; P < 0.05; yaw: F1,49 = 5.6; P < 0.05; displacement in right–left plane: F1,49 = 5.4; P < 0.05).
BOLD Functional Connectivity Analyses
WTAR was used as a covariate for voxel-wise connectivity analyses. For both rACC and PCC seeds (see Fig. 1; Supporting Information Table II), BOLD connectivity was greater for HC compared to mTBI patients within the right SMG (BAs 22/39) and superior frontal gyrus (BAs 9/10) of the DMN. HC also demonstrated higher functional connectivity within the PCC (BAs 23/24/29/30/31/7) for the rACC seed and within the rACC (BAs 33/24/32) for the PCC seed. In addition, anticorrelations with the rACC seed were greater for HC compared to mTBI patients within the bilateral inferior parietal lobule (BA 40), a central node of the TRN. In contrast, greater connectivity between the rACC and the bilateral prefrontal cortex (BAs 10/46/13/44/45) was observed for mTBI patients compared to HC.
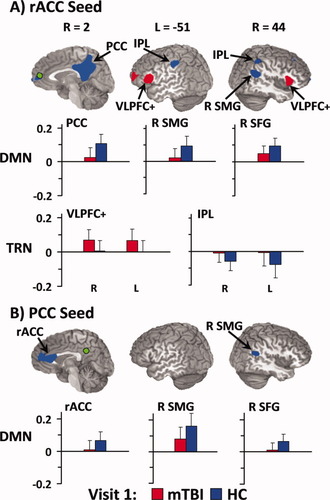
Regions demonstrating group differences in functional connectivity for the rostral anterior cingulate (rACC; Panel A, green coloring) and posterior cingulate (PCC; Panel B, green coloring) seeds during visit 1. Red coloring indicates regions where the absolute measure of functional connectivity (i.e., correlation or anticorrelation) was greater for mild traumatic brain injury patients (mTBI), whereas blue refers to regions where connectivity was greater for healthy controls (HC). The graphs depicting connectivity coefficients (error bars = one standard deviation) follow an identical color scheme and are grouped according to whether selected regions are from the default-mode [DMN; PCC, right supramarginal gyrus (R SMG), and right superior frontal gyrus (R SFG)) or task-related (TRN; ventral lateral prefrontal cortex and insula (VLPFC+), and bilateral inferior parietal lobule (IPL)] networks. Coordinates for slice locations are presented according to the Talairach atlas (R, right; L, left), and cluster volumes are presented in supplementary tables.
Connectivity with the right lateral prefrontal cortex seed was greater for mTBI patients within the bilateral medial superior parietal lobule (BA 7), right frontotemporal cortex (BAs 38/47/13/28/34), and striatum compared to HC (Fig. 2; Supporting Information Table III). In contrast, connectivity with the right lateral prefrontal cortex seed was greater for HC than mTBI patients within the left medial and superior frontal gyrus (BAs 8/9).
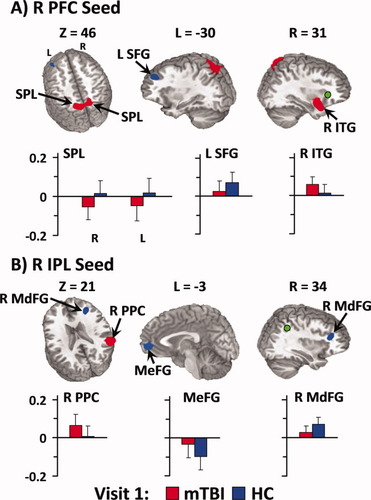
Regions demonstrating group differences in functional connectivity for the right lateral prefrontal cortex (R PFC; Panel A, green coloring) and right inferior parietal lobule (R IPL; Panel B, green coloring) seeds during the first visit. Red coloring indicates regions where the absolute measure of functional connectivity (i.e., correlation or anticorrelation) was greater for mild traumatic brain injury patients (mTBI), whereas blue refers to regions where connectivity was greater for healthy controls (HC). The graphs depicting connectivity coefficients (error bars = one standard deviation) follow an identical color scheme. Connectivity coefficients from the R PFC seed are depicted for the bilateral superior parietal lobule (SPL), left superior frontal gyrus (L SFG), and right inferior temporal gyrus (R ITG). The R IPL results include the right posterior parietal cortex (R PPC), bilateral medial frontal gyrus (MeFG), and right middle frontal gyrus (R MdFG). Coordinates for slice locations are presented according to the Talairach atlas (R, right; L, left; Z, axial view), and cluster volumes are presented in supplementary tables.
Functional connectivity results from the right IPL seed (Fig. 2; Supporting Information Table III) indicated greater anticorrelations for HC than mTBI patients within the ventromedial prefrontal cortex of the DMN (BAs 10/32) as well as greater correlations with right middle frontal gyrus. Finally, mTBI patients exhibited greater connectivity between the right IPL seed and the posterior parietal cortex (BAs 13/22/39/40).
Binary logistical regression was then performed to determine if mTBI patients and HC could be classified on the basis of functional connectivity indices for the DMN, lateral prefrontal cortex (rACC seed), IPL (rACC seed), and superior parietal lobule (right prefrontal cortex seed). Specifically, a weighted average (based on number of voxels per ROI) of functional connectivity within the DMN was created by combining clusters from the rACC seed (HC > mTBI; ROI = PCC, right SMG, and right superior/medial frontal gyrus) and the rACC cluster from the PCC seed (HC > mTBI). Likewise, weighted functional connectivity averages were also created for clusters within lateral prefrontal cortex (mTBI > HC, rACC seed; ROI = bilateral prefrontal cortex), inferior parietal lobule (HC > mTBI, rACC seed; ROI = bilateral IPL), and the superior parietal lobule (mTBI > HC, right prefrontal seed; ROI = bilateral SPL). These combined networks were then used to investigate the relationship between functional connectivity and clinical measures. Results indicated that estimates of premorbid intelligence (Wald = 4.9; P < 0.05) were able to discriminate between HC (64% accuracy) and mTBI patients (65.4%) when it was the only variable in the regression. BOLD connectivity measures improved classification accuracy (HC = 88%; mTBI = 80.8%), with group differentiation primarily achieved by differences in functional connectivity in DMN (Wald = 5.6; P < 0.05) and bilateral superior parietal lobule (Wald = 4.5; P < 0.05).
Three hierarchical multiple regressions (premorbid intelligence entered first) were conducted to determine whether functional connectivity within these same networks predicted cognitive, emotional and somatic complaints. The overall model for cognitive complaints was significant (F4, 44 = 2.7; P < 0.05; R2 change = 0.19), with connectivity within the IPL being correlated with cognitive symptoms at a trend level (t = 1.8; P = 0.08; r = 0.37). Of interest, a negative correlation of the same magnitude (r = −0.36) was observed between measures of DMN connectivity and self-reports of cognitive deficits. Although the overall model for somatic and emotional complaints was not significant (P > 0.10), a similar relationship was observed between measures of DMN connectivity and self-reports of emotional deficits (r = −0.38).
BOLD Functional Connectivity Analyses for Visit Two
There were no significant differences (P > 0.10) in functional connectivity measurements for mTBI patients who returned (n = 15) compared to those who did not (n = 12). Four 2 × 2 [time (visit 1, visit 2) × diagnosis (HC, mTBI)] mixed ANCOVAs examined whether connectivity values changed as a function of recovery following mTBI (i.e., time × group interaction). Results (see Fig. 3) indicated a main effect of diagnosis for the DMN (F1,27 = 12.7; P < 0.001), lateral prefrontal cortex (F1,27 = 10.4; P < 0.005), IPL (F1,27 = 9.1; P < 0.01), and superior parietal lobule (F1,27 = 7.9; P < 0.01); however, the time × diagnosis term was not significant (P > 0.10).
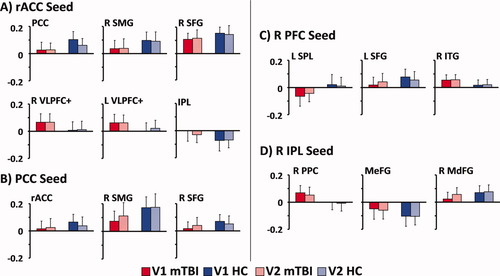
Selected functional connectivity data (error bars = one standard deviation) from the 15 mild traumatic brain injury patients (mTBI; red bars) and 15 matched healthy controls (HC; blue bars) who completed visits 1 (V1; dark-colored bars) and 2 (V2; light-colored bars). The different panels correspond to the different seeds used in the analyses, and abbreviations for regions are identical to Figures 1 and 2.
DTI Results
Results from ANCOVA indicated that FA values (see Fig. 4) were greater for mTBI patients compared to HC within the EC (F1,48 = 4.3; P < 0.05) and ACR (F1,48 = 4.0; P = 0.05). Increased FA was the result of lower RD rather than higher axial diffusivity (AD; P > 0.10), although differences in RD existed only on a trend level for both the EC (F1,48 = 2.8; P = 0.10) and ACR (F1,48 = 3.4; P = 0.07).
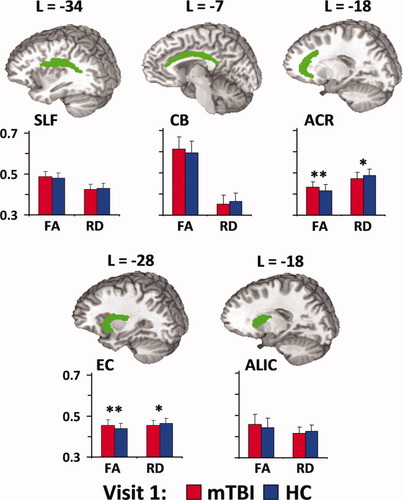
Panel A depicts five white matter tracts that have previously been implicated in connecting different nodes of the DMN and TRN, including the superior longitudinal fasciculus (SLF), cingulum bundle (CB), anterior corona radiata (ACR), external capsule (EC), and anterior limb of the internal capsule (ALIC). Coordinates for tract locations are presented according to the Talairach atlas. Panel B depicts fractional anisotropy (FA) and radial diffusivity (RD) values for both mild traumatic brain injury patients (mTBI; red bars) and healthy controls (HC; blue bars) at visit 1. Double asterisks denote significant group differences, whereas single asterisks denote statistical trends. Error bars equal one standard deviation.
DTI Analyses for Visit Two
There were no significant differences (P > 0.10) in DTI measurements for mTBI patients who returned (n = 15) compared to those who did not (n = 12). Similar 2 × 2 ANCOVAs examined whether connectivity values changed as a function of recovery following mTBI. A significant group × time interaction (F1,27 = 7.6; P < 0.05) was observed for the EC, with follow-up tests indicating a small but significant change in FA (partial normalization) for mTBI subjects at visit 2 (0.446 ± 0.03) compared to visit 1 (0.454 ± 0.022),with no change for HC (P > 0.10). The interaction effect was not significant within the ACR.
Anatomical and Functional Connectivity Analyses
Two multiple regressions investigated the relationship between measures of white-matter integrity (FA) and functional connectivity between the rACC seed and PCC (CB) and the rACC seed and lateral prefrontal cortex (anterior limb of internal capsule, ACR, and EC). Results indicated a negative relationship between FA in the cingulate bundle (r = −0.64) and the degree of functional connectivity (rACC seed) in the PCC for HC (F1,23 = 15.6; P < 0.005; adjusted R2 = 0.38), but not for mTBI patients (P > 0.10). Results from the second regression suggested a trend between connectivity in the lateral PFC and rACC seed with increased FA within the ACR (r = 0.48) for HC only (t = 1.9; P = 0.066).
DISCUSSION
Traditional measures of cognitive functioning (i.e., neuropsychological testing) and anatomical imaging (T1- and T2-weighted images) were not sensitive for detecting differences between semiacutely injured mTBI patients and well-matched HC. In contrast, mTBI patients exhibited decreased BOLD connectivity within the DMN, hyper-connectivity between the rACC and lateral prefrontal cortical areas, and hyper-connectivity between the right prefrontal cortex and posterior parietal cortex. Functional connectivity measurements correctly classified patients and controls in 84.3% of the cases and predicted cognitive complaints. Abnormalities in anatomical connectivity were also observed within the ACR and EC, which have been previously implicated in connecting different nodes of the DMN and TRN [Lehericy et al.,2004; Schmahmann et al.,2008]. Finally, a disruption between indices of anatomical and functional connectivity was also observed following mTBI, with FA values from the CB predicting functional connectivity between the rACC and PCC in HC only.
Similar to previous reports in more severely injured patients [Boly et al.,2008,2009; Nakamura et al.,2009; Vanhaudenhuyse et al.,2010], current results indicate a reduced connectivity within the DMN for mTBI relative to their controls, suggesting that decreased DMN connectivity may span the spectrum of trauma-related changes. Reduced connectivity within the DMN and greater connectivity in regions associated with top–down attentional control may provide a physiological substrate for common but poorly understood neuropsychiatric complaints following mTBI: increased distractibility and excessive cognitive fatigue (see McAllister et al. [2006] for review). DMN activity has been related to introspection, self-referential thought, and mind wandering [Kelley et al.,2002; Mason et al.,2007; Raichle et al.,2001], all of which occur in the presence of decreased awareness to the external world. In contrast, the TRN is activated when attentional focus is shifted from the internal to external environment across multiple cognitive tasks [Cabeza and Nyberg,2000], with the lateral prefrontal cortex exerting top–down attentional control to minimize distractibility [Botvinick et al.,2001]. The putative balance between the DMN and TRN appears to be partially disrupted following mTBI, which may result in increased distractibility as mTBI patients try to suppress internal mentations (mediated by the DMN), potentially inducing cognitive fatigue.
Current findings may have implications for previous [Chen et al.,2004; Mayer et al.,2009; McAllister et al.,1999,2001; Smits et al.,2009] and future functional magnetic resonance imaging (fMRI) studies on evoked brain activity following mTBI. The physiological basis for the BOLD signal is the change in blood flow, blood volume, and ratio of deoxyhemoglobin to oxyhemoglobin from baseline to activation states [Raichle and Mintun,2006]. The baseline state is typically defined as an extended period of unconstrained passive mental activity in fMRI [Raichle et al.,2001]. If nodes of the DMN are relatively less active while nodes of the TRN are relatively more active following mTBI, differences in subtraction images may not simply reflect evoked activity. Previous reports of both hypo- and hyperactivation within lateral prefrontal cortex, ACC, and parietal lobes following mTBI could partially be the result of differences in baseline, rather than evoked, activity.
Alterations in functional connectivity (i.e., temporal covariation of the BOLD signal) may result from changes in spontaneous firings of neuronal ensembles, in blood flow, in oxidative metabolism, or a combination of these factors [Fox and Raichle,2007]. A recent EEG study indicated a disruption in temporal coherence based on the electrophysiological response, with mTBI patients exhibiting both decreased long-distance functional connectivity and a departure from small-world network properties [Cao and Slobounov,2010]. The BOLD response is primarily vascular in nature, arising from the need to restore cellular homeostasis following increased neuronal and metabolic activity [Logothetis,2008; Raichle and Mintun,2006]. TBI reduces cerebral perfusion [Soustiel and Sviri,2007], increases reactivity of smooth muscle in the walls of microvessels [Ueda et al.,2006], and decreases the density and diameters of capillaries both at the injury site and diffusively [Park et al.,2009]. Metabolic failure following TBI occurs even in the presence of normal perfusion [Vespa et al.,2007], with a pronounced reduction in oxidative metabolism [Soustiel and Sviri,2007].
Although the majority of these vascular and metabolic findings have been generated in models of severe TBI, it is likely that similar pathophysiological processes occur following mTBI, albeit on a much smaller scale. In a subsample from the current study, a reduction in the combined concentrations of gray matter glutamate and glutamine was observed in the semiacute phase of injury [Gasparovic et al.,2009], which may be reflective of decreased metabolism. More chronic forms of mTBI have also been associated with frank neuronal pathology, including increased slow-wave activity during passive mental activity [Huang et al.,2009; Lewine et al.,2007], which was posited to be a result of diffuse axonal injury [Huang et al.,2009].
Current DTI findings indicated increased FA and reduced RD in both the EC and ACR, white-matter tracts that convey information between the primary nodes of the DMN and TRN [Lehericy et al.,2004; Schmahmann et al.,2008]. In addition, current findings indicate a disruption between indices of anatomical and functional connectivity, with FA values from the CB predicting functional connectivity between the rACC and PCC in HC but not in patients. The mechanical forces of mTBI result in the stretching of axons and related supporting structures such as oligodendrocytes [Povlishock and Katz,2005], altering receptor functioning and the balance of intracellular and extracellular ions [Rosenblum,2007; Sotak,2002]. The influx of sodium and calcium ions is followed by increased intracellular and decreased extracellular water (i.e., cytotoxic edema), which may lead to dramatic changes in perpendicular diffusion coefficients and increased FA [Peled,2007].
Previous studies involving unselected semiacutely injured patients have reported both reduced [Arfanakis et al.,2002; Inglese et al.,2005; Lipton et al.,2009; Miles et al.,2008] and increased [Bazarian et al.,2007; Mayer et al.,2010; Wilde et al.,2008] FA, whereas studies on chronic, symptomatic patients typically report reduced FA [Kraus et al.,2007; Niogi et al.,2008]. FA magnitudes are likely influenced by injury severity, symptom severity, and time postinjury [Wilde et al.,2008], but may also be influenced by artifacts such as subject motion. A recent study suggests that head motion increases FA in regions of low anisotropy (i.e., frontal grey matter) and under certain (i.e., a small number of gradient directions) experimental conditions [Tijssen et al.,2009]. Although neither factor applies to this study, more research is needed to determine the impact of head motion on DTI scalars in clinical groups.
McAllister [2006] reported persistent fMRI abnormalities 1 year following mTBI in asymptomatic patients, suggesting that alterations in neural functioning may persist beyond overt clinical symptoms. Despite a significant decrease in the amount of self-reported cognitive symptoms, a smaller cohort of returning patients (n = 15) continued to report increased cognitive, emotional, and somatic symptoms compared to HC. Likewise, functional BOLD connectivity coefficients remained relatively unchanged from visit 1. Collectively, these results suggest that a longer recovery period may be required to fully assess clinical and neurological changes following mTBI. There is some evidence that a second, temporally proximal mTBI may result in additional cognitive deficits compared to a single injury [Wall et al.,2006], and current results may provide a physiological basis for this phenomenon.
Current findings require replication in an independent validation sample to ensure generalizability of results. Specifically, the current sample had a large number of female participants, and recent data [Bazarian et al.,2010] suggests that females may be more likely to experience postconcussive symptoms compared to males. In addition, our returning patients consisted of a relatively small cohort (n = 15), which could have undermined our ability to detect small effect sizes. Future studies should also include an orthopedically injured control group to ensure that current findings are not the result of nonspecific trauma factors such as pain, emotional distress, and/or increased fatigue. However, resting-state paradigms mitigate some of these confounds as they are less susceptible to both fatigue and effort compared to more traditional evoked paradigms. Furthermore, the patterns of DMN abnormalities described in depression, chronic pain, and ADHD [Broyd et al.,2009; Castellanos et al.,2008; Greicius et al.,2004] are markedly different from current results, diminishing the likelihood that current findings are the result of one of these processes. Second, the finding of increased head motion in the mTBI group on several parameters may have influenced BOLD connectivity. However, increased head motion does not readily explain the differential BOLD connectivity coefficients that were observed in lateral prefrontal cortex (mTBI > HC) compared to the DMN (HC > mTBI) or the relative stability of these findings across a 3–5-month period (see Figs. 1 and 3). Finally, our anatomical protocol did not include susceptibility weighted or high-resolution T2*-weighted gradient imaging, which may be more sensitive than conventional MRI for detecting intraparenchymal pathology [Chastain et al.,2009].
Finally, a complex relationship exists between measures of functional connectivity (temporal coherence) and the physiological basis of the BOLD signal [Raichle and Mintun,2006], between measures of diffusion and alterations in white matter structures [Peled,2007], and the multidimensional pathological changes that can occur following trauma (e.g., alterations in cerebral blood flow, oxidative metabolism, neuronal firing, and sodium channels). This is further complicated by limitations within each of the respective imaging modalities (e.g., crossing fibers in DTI research and large vessel effects in fMRI). In spite of these caveats, newer neuroimaging techniques may provide important information regarding correlates of mild trauma in a human injury model, objective markers for difficult differential diagnosis, and a potential mechanism for monitoring recovery [Belanger et al.,2007; Bigler,2008]. To this end, BOLD connectivity indices yielded relatively high levels of both sensitivity (81%) and specificity (88%) for patient classification and were associated with level of cognitive complaints. If replicable, these indices may serve as biomarkers of injury that can be further explored in animal models to determine causality and underlying pathophysiology.
Acknowledgements
Special thanks to Diana South and Cathy Smith for assistance with data collection, to Reyaad Hayek, M.D. for review of anatomical images, and to Gayle Pohl and her students for generous contributions to help fund this study.