A cross-modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis
Abstract
Recent anatomical and electrophysiological evidence in primates indicates the presence of direct connections between primary auditory and primary visual cortex that constitute cross-modal systems. We examined the intrinsic functional connectivity (fcMRI) of putative primary auditory cortex in 32 young adults during resting state scanning. We found that the medial Heschl's gyrus was strongly coupled, in particular, to visual cortex along the anterior banks of the calcarine fissure. This observation was confirmed using novel group-level, tensor-based independent components analysis. fcMRI analysis revealed that although overall coupling between the auditory and visual cortex was significantly reduced when subjects performed a visual perception task, coupling between the anterior calcarine cortex and auditory cortex was not disrupted. These results suggest that primary auditory cortex has a functionally distinct relationship with the anterior visual cortex, which is known to represent the peripheral visual field. Our study provides novel, fcMRI-based, support for a neural system involving low-level auditory and visual cortices. Hum Brain Mapp, 2008. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Historically, sensory cortex has been characterized as hierarchical with association cortex providing the linkage between sensory systems [Todd, 1912]. More recently there is tract tracing, electrophysiology, and neuroimaging evidence for direct connections between low-level sensory cortex [Brosch et al., 2005; Cappe and Barone, 2005; Clavagnier et al., 2004; Falchier et al., 2002; Foxe et al., 2002; Ghazanfar et al., 2005; Johnson and Zatorre, 2006; Kayser et al., 2005; Laurienti et al., 2002; Lehmann et al., 2006; Martuzzi et al., 2007; Nir et al., 2006; Rockland and Ojima, 2003; Rockland and Van Hoesen, 1994; Schroeder and Foxe, 2005; Tanabe et al., 2005; Watkins et al., 2006]. The cross-modal results across neuroimaging experiments generally fall into two classes in which low level sensory interactions can be attributed to (1) indirect interactions because of the influence of association cortex and (2) direct connections between sensory cortices.
The majority of neuroimaging studies involving multisensory experiments demonstrate that association cortex drives the interactions between sensory cortex. Posterior association and frontal lobe cortex appear to influence low-level multisensory processing in functional imaging studies requiring attention to a sensory stimulus [Calvert et al., 2000; Macaluso et al., 2000] and high cognitive load [Crottaz-Herbette et al., 2004; Johnson and Zatorre, 2007], respectively. For example, Macaluso et al. [2000] demonstrated that activity in multisensory parietal lobe cortex was selectively correlated with activity in occipital cortex when tactile stimuli were observed to elicit increased occipital responses to a visual stimulus, suggesting that parietal lobe cortex was responsible for the occipital cortex responses to tactile stimuli. In addition, cross-modal inhibition between sensory cortices has been observed when the cognitive load is high (e.g., a two-back working memory task), suggesting the occurrence of top-down inhibitory control by executive function systems [Crottaz-Herbette et al., 2004].
Evidence that association cortex and prefrontal cortex drive low-level multisensory interactions does not preclude, however, that there are direct interactions between low-level sensory cortex. Evidence for direct connections between low-level sensory cortices has been reported in anatomical tracing and electrophysiological studies. Anatomical tracer studies of nonhuman primates demonstrate the existence of direct pathways between primary sensory areas [Cappe and Barone, 2005; Clavagnier et al., 2004; Falchier et al., 2002; Rockland and Ojima, 2003; Rockland and Van Hoesen, 1994]. For example, neurons in nonhuman primate auditory cortex have projections that terminate in the anterior but not posterior regions of primary visual cortex [Falchier et al., 2002; Rockland and Ojima, 2003]. In addition, electrophysiological studies demonstrate cross-modal processing in low-level sensory cortex at time periods well before association cortex could possibly respond to a stimulus and drive the response [Giard and Peronnet, 1999].
Together, the findings from functional and anatomical studies of cross-modal connections suggest there are locally specific regions of low-level sensory cortex that engages in cross-modal processing through direct connections with other low-level sensory regions, as well as influences through association cortex. In particular, the tract tracing studies suggest that functional connectivity should be most prominent between medial Heschl's gyrus and anterior calcarine regions.
fcMRI is a functional-imaging method that can be used to examine the strength of functional connectivity between two anatomical regions during tasks and when subjects are not given a specific task or when there is not attention demanding task. fcMRI has been used to examine tonically active sensory, motor, and cognitive networks observed in resting state data [Biswal et al., 1997; Greicius et al., 2003]. These studies have highlighted robust coupling between homologous regions across the cerebral hemispheres [Salvador et al., 2005]. For example, left and right hemisphere auditory cortices demonstrate highly correlated patterns of activity [van de Ven et al., 2004]. In this fcMRI study we used regions-of-interest (ROIs), defined by each individual's unique anatomy, to identify the functional pathways associated with low-level primary auditory cortex during the resting state. In addition to characterizing auditory pathways in resting state data, we asked whether auditory and visual cortex would exhibit positively correlated activity when there was no specific task, and whether these regions would exhibit negatively correlated activity when subjects were engaged in a visual task.
MATERIALS AND METHODS
Participants
Thirty-two Stanford University students (17 female) were recruited for functional imaging tasks over a 4-year period (mean age 21.12, ±2.10 years). Fourteen of these participants also performed a visual perception task. These studies were approved by the Stanford University Institutional Review Board and these tasks were undertaken with the understanding and written assent and/or consent of each subject.
fMRI Paradigms
During a resting state scan participants were instructed to lay quietly with their eyes closed. The length of the scan was 4 min. The visual processing task consisted of the presentation of a static black-and-white radial checkerboard pattern and a “flashing” checkerboard, in which the white and black patterns were inverted with an 8 Hz frequency. The visual stimulus angle subtended by the checkerboard was 18.9°. Presentation of the static and flashing stimuli alternated every 20 s for six cycles. In both conditions, subjects were instructed to passively view the checkerboard. The total length of the task was 4 min.
Image Acquisition
Functional images of the adults were acquired on a 3T General Electric Signa scanner by using a standard General Electric whole-head coil. The scanner runs on an LX platform, with gradients in “miniCRM” configuration (35 mT/m, SR 190 mT per m/s) and has a Magnex (Concord, CA) 3-T 80-cm magnet. The adults were scanned with the following spiral pulse sequence: repeat time = 2,000 ms; echo time = 30 ms; flip angle 80°; field of view 200 × 200 mm2; and a matrix size of 64 × 64 [Glover and Lai, 1998]. Twenty-eight axial slices (4-mm thick, 0.5-mm skip) parallel to the anterior commisure-posterior commisure line were acquired. To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquiring functional MRI data [Kim et al., 2002]. To aid in the localization of functional data, high-resolution T1-weighted spoiled grass gradient recalled 3D MRI images were collected on a 1.5-T or a 3T General Electric Signa scanner with the following sequence parameters: 124 coronal slices, 1.5-mm thickness; no skip; repeat time, 11 ms; echo time, 2 ms; and flip angle, 15°. The images were reconstructed as a 124 × 256 × 256 matrix with a 1.5-, 0.9-, 0.9-mm spatial resolution.
Image and fcMRI Analyses
SPM2 (http://www.fil.ion.ucl.ac.uk/spm) was used for image preprocessing. Images were corrected for movement using least-square minimization without higher-order corrections for spin history and normalized to MNI coordinate space. Images were then resampled to 2 mm isotropic resolution using sinc interpolation and smoothed with a 4-mm Gaussian kernel to decrease spatial noise. Prior to data analysis, scaling and filtering steps were performed across all brain voxels. The scaled waveform of each brain voxel was then filtered by using a bandpass filter (0.0083/s < f < 0.15/s) to reduce the effect of low-frequency drift and high-frequency noise.
To perform the fcMRI analyses, time series data were extracted from each voxel within structural ROIs representing putative primary auditory and visual cortex. The intensity of all the voxels within an ROI were then averaged at each time point to create a single time series over the course of the resting state scan. The resulting time series, representing the average intensity of all voxels in the ROI, was then used as a covariate of interest in a whole-brain regression analysis. Whole-brain gray matter and white matter average time series were included as covariates to identify brain regions that exhibited correlated activity that was independent of global fluctuations in signal. Contrast images corresponding to the ROI regressor were determined individually for each subject and entered into a second-level, single-sample t-test analysis (height threshold of FDR P < 0.05 and an extent threshold of P < 0.01) to determine the brain areas that showed significant functional connectivity across subjects.
Anatomical Regions of Interest
To maintain a consistent coordinate space for the structural and functional MRI datasets, the structural MRI scans were normalized to the MNI template using affine and nonlinear transformations in SPM2. ROIs representing the left and right putative primary auditory cortex (A1) were created based on anatomical landmarks from each participant's normalized structural MRI. The term putative is used in describing these ROIs because we do not have histological confirmation that the ROIs cover or are limited to koniocortex. The first medial appearance of Heschl's gyrus was used to define the medial boundary of the A1 anatomical ROI. The lateral boundary was defined by the most lateral position of Heschl's gyrus as it is viewed in the coronal plane of section where there is a clear distinction between the insula and Heschl's gyrus. MRIcro was used to demarcate Heschl's gyrus in the sagittal plane of section [Rorden and Brett, 2000]. When a sulcus intermedius indented the crown of Heschl's gyrus and created two gyri, both gyri were included in the ROI. Complete duplications of Heschl's gyrus (separate extra gyrus) were not included in the ROI. Supplementary materials Figure 1 shows that the A1 masks correspond to the Te1.0 and Te1.1 regions [Morosan et al., 2005]. ROIs for the putative left and right V1 also were created based on anatomical landmarks for each participant. Here again, the term putative is used to denote that we did not have histological confirmation of the boundaries for primary visual cortex. MRIcro was used to demarcate the upper and lower bank of the calcarine fissure in sagittal plane of section. The lateral boundary was defined by the sagittal section in which the calcarine fissure was no longer continuous from its anterior to posterior end. Supplementary materials Figure 1 shows that that the V1 ROI correspond to 60% probability maps of Brodmann area 17 (http://www.bic.mni.mcgill.ca/cytoarchitectonics) [Amunts et al., 2000]. The cytoarchitectonic probability maps were not used because the probability maps extended into nonauditory cortex among individual subjects, presumably because of the high degree of sulcal/gyral variability in this region. High probability masks (e.g., 90%), which were more constrained to the sulcal/gyral features of auditory and visual cortex were composed of too few voxels to have confidence that a reliable resting state fMRI signal could be obtained.
Steps also were taken to reduce the potentially confounding BOLD signal from white matter. The normalized structural images were segmented using the MNI a priori gray, white and CSF images in SPM2. Each normalized gray matter image was then multiplied by the anatomical ROI to create a gray matter-specific ROI for the left and right putative A1 and V1 regions.
The resulting left and right A1 ROIs exhibited a left greater than right structural asymmetry that is consistent with previous observations of structural asymmetries in Heschl's gyrus (Left A1 ROI:
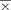 = 0.94 cc, sd = 0.31; Right A1 ROI:
= 0.94 cc, sd = 0.31; Right A1 ROI: 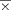 = 0.71 cc, sd = 0.25; paired t-test t = 4.12, P < 0.001) [Leonard et al., 1998; Penhune et al., 1996]. There was no hemispheric difference in left and right V1 ROI volumes (Left V1 ROI:
= 0.71 cc, sd = 0.25; paired t-test t = 4.12, P < 0.001) [Leonard et al., 1998; Penhune et al., 1996]. There was no hemispheric difference in left and right V1 ROI volumes (Left V1 ROI: 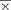 = 2.88 cc, sd = 1.18; Right V1 ROI:
= 2.88 cc, sd = 1.18; Right V1 ROI: 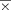 = 2.64 cc, sd = 1.01; paired t-test t = 1.16, ns). Individual variation in these anatomical ROIs did not significantly predict individual variation in the strength of auditory and visual associations (cluster t-scores) that are presented later.
= 2.64 cc, sd = 1.01; paired t-test t = 1.16, ns). Individual variation in these anatomical ROIs did not significantly predict individual variation in the strength of auditory and visual associations (cluster t-scores) that are presented later.
Tensor-ICA Replication
Tensor-ICA was performed to examine patterns of temporally correlated activity across subjects [Beckmann and Smith, 2005]. Traditional ICA is used to characterize unique patterns of variance or structure in data without dependence on an explicit generative model. Tensor-ICA is similar but is extended to a group analysis. Unlike the previous approach, tensor-ICA does not depend on a specific seed point or ROI and represents latent signals in the data as the outer product of matrices that characterizes these signals in the spatial, temporal, and subjects domain. The final ICA regression coefficients in the spatial domain are rescaled in units of voxel-wise residual noise variance, so that these maps can be understood as Z-statistics which express the voxel-wise signal-to-noise ratio or the number of standard deviations the regression coefficient is from the background noise. These Z-statistic images are then thresholded using an alternative hypothesis testing approach by fitting a Gaussian/Gamma mixture model to the histogram of Z values. Voxel-wise posterior probabilities are evaluated to determine whether the data fit a Gamma distribution or Gaussian distributed background noise. A voxel is considered as a significant member of an independent component when it exceeds the probability of belonging to the Gaussian background noise class (P > 0.5). Please see Beckman and Smith [ 2004] for additional details.
RESULTS
Intrinsic Auditory Cortex Connectivity
As shown in Figure 1a and Table I, the left A1 ROI exhibited significant correlated activity with the left and right superior temporal gyrus and the medial geniculate nucleus (MGN) [Devlin et al., 2006] (FDR < 0.05, cluster extent < 0.01). In addition, significantly correlated activity was also detected in cortex dorsal to the left and right anterior calcarine fissure, posterior thalamic nuclei (distinct from the medial geniculate nucleus), and lateral occipitotemporal cortex corresponding to area MT+ [Huk et al., 2002]. The right A1 ROI showed a similar connectivity pattern, as shown in Supplementary materials Figure 2a and Supplementary materials Table I.
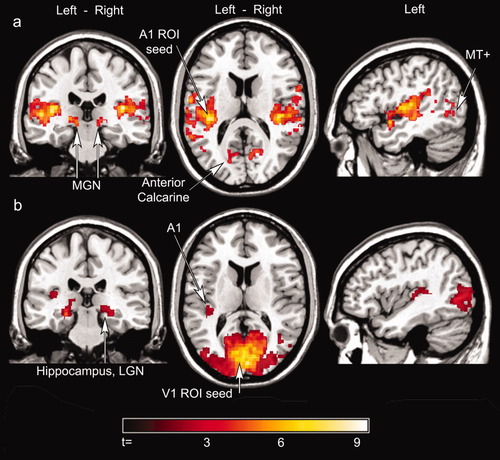
Functional connectivity results-left hemisphere. (a) Activity correlated with A1: Voxels in the anatomically defined left A1 ROI demonstrated significant correlated activity with the MGN, contralateral HG and STG, anterior calcarine, and MT+ while subjects were exposed to scanner noise and rested with their eyes closed. (b) Activity correlated with V1: Voxels from the anatomically defined left V1 ROI exhibited significant correlated activity throughout the visual system and with the left medial Heschl's gyrus. The sagittal sections displayed in (a) and (b) are from the left hemisphere. FDR P < 0.05, cluster extent P < 0.01. The color scale represents the t-scores. A1 = putative primary auditory cortex/Heschl's gyrus; STG, superior temporal gyrus; MGN, medial geniculate nucleus.
| Anatomical regions | Peak coordinate | Cluster extent | Maximum Z-score |
|---|---|---|---|
| (L) Heschl's gyrus, STG, Insula, IFG, globus pallidus, putamen, caudate | −40, −22, 8 | 4,166 | 6.21 |
| (R) Heschl's gyrus, STG, Insula, IFG | 40, −26, 14 | 2,878 | 6.00 |
| (R) Putamen/globus pallidus | 28, −12, −4 | 190 | 5.06 |
| (L) and (R) Posterior thalamus, MGN | −18, −22, 4 | 149 | 5.12 |
| 8, −20, −6 | 112 | 4.03 | |
| (L) and (R) Peripheral V1 | −6, −66, 12 | 108 | 3.41 |
| 10, −66, 10 | 166 | 4.10 | |
| (L) MT+ | −50, −68, −2 | 101 | 3.74 |
- (L) Left hemishpere; (R) right hemisphere; STG, superior temporal gyrus; IFG, inferior frontal gyrus; MGN, medial geniculate nucleus.
To corroborate our findings of auditory-visual cortex coupling, time series data were averaged from an anatomically defined V1 ROI and used as the seed in a parallel fcMRI analysis. As shown in Figure 1b and Table II, the left V1 ROI exhibited significant correlated activity with the left medial Heschl's gyrus (FDR < 0.05, cluster extent < 0.01). Significant correlated activity also was observed in area MT+, a lateral geniculate nucleus, and the hippocampus. The right V1 RO1 showed a similar connectivity pattern, as shown in Supplementary materials Figure 2b and Supplementary materials Table II.
| Anatomical regions | Peak coordinate | Cluster extent | Maximum Z-score |
|---|---|---|---|
| (L) and (R) visual cortex, area MT+/lateral occipitotemporal cortex, parahippocampal gyrus, hippocampus, posterior thalamus, LGN | −4, −84, 8 | 15,207 | 7.75 |
| Medial frontal cortex | 0, 58, −14 | 171 | 4.17 |
| (L) Heschl's gyrus | −40, −22, 6 | 160 | 4.13 |
- (L) Left hemishpere; (R) right hemisphere.
fcMRI maps from the left A1 ROI and left V1 ROI analyses showed overlap within putative primary auditory and visual cortex. Specifically, regions in the medial Heschl's gyrus and cortex just dorsal to the anterior calcarine fissure exhibited significant correlated activity across the two analyses.
Tensor-ICA Replication
Tensor-ICA was performed to confirm the findings from the fcMRI analyses. Thirty independent components were computed, from which we identified a component with strong coupling within the auditory cortex. As shown in Figure 2, this component reflects temporally correlated patterns of activity in the auditory cortex and includes the anterior calcarine cortex, thus confirming findings from the ROI analyses.
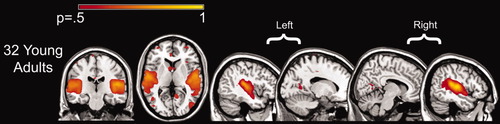
Tensor-ICA maps demonstrate temporally correlated activity between auditory and visual cortex. Significant auditory to anterior calcarine connectivity was present bilaterally. Note the common patterns of connectivity across the tensor-ICA and fcMRI maps presented in Figure 1 and Supplementary Figure 2. The color bar represents the probabilities of correlated activity between 0.5 and 1.
Cross-Modal Connectivity During the Visual Perception Task
Time series data from the flashing checkerboard task were used to test the prediction that auditory and visual cortex would be uncoupled when subjects were engaged in a visual task. Figure 3a,b shows a significant attenuation of correlated activity between auditory and visual cortex when the entire V1 ROI was used to define the visual cortex (left: t = 3.66, P < 0.005; right t = 3.25, P < 0.01), but not when the comparisons were based on activity in the A1 ROI and the anterior calcarine cluster that was coupled with A1 during the resting state (left: t = 1.46, ns; right t = 1.34, ns). The anterior calcarine cluster exhibited positively correlated activity with auditory cortex during the checkerboard task, in contrast to posterior calcarine regions. Figure 3c shows that the attenuation in correlated activity between the A1 ROI and the V1 ROI (including the anterior calcarine regions correlated with A1 during the resting state) was most prominent in the middle and posterior regions of calcarine cortex.
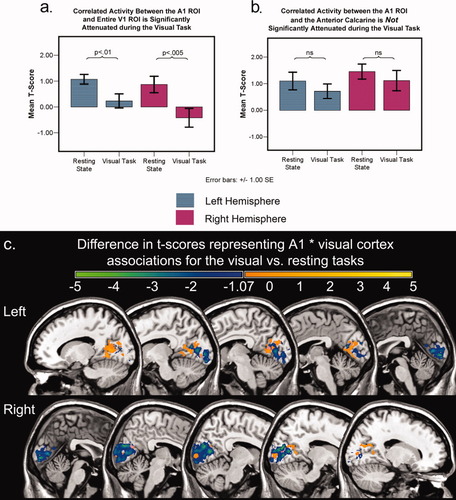
Direct comparisons of correlated activity between auditory and visual cortex during the resting state as compared with the checkerboard tasks. (a) Correlated activity between auditory cortex and the entire V1 ROI: t-scores in the putative primary auditory cortex were derived from the functional connectivity results with the V1 ROI. Correlated activity was significantly reduced when subjects viewed the flashing checkerboard as compared with the resting-state task. (b) Correlated activity between auditory cortex and the anterior calcarine region identified in the resting-state analysis: t-scores in the peripheral V1 were extracted from the functional connectivity results with the Heschl's gyrus ROI. There was no significant difference between the two tasks in this case. (c) The t-score map representing the correlation between the A1 ROI and visual cortex during the resting state task was subtracted from the t-score map representing the correlation between the A1 ROI and visual cortex during the visual task. This comparison was limited to the V1 ROI, as well as the anterior calcarine region significantly correlated with the A1 ROI during the resting state task. The blue-green regions are areas that showed decreases in correlated activity with the presentation of visual stimuli when compared with the resting state task (thresholded for t = −1.07, which represents P < 0.05 for a one-tailed t-test involving 14 subjects). Results for the left and right hemisphere A1 ROI analyses are presented together. Please note the relative decrease in t-score in posterior and middle calcarine regions in comparison with the anterior calcarine regions.
DISCUSSION
Both ROI-based fcMRI analysis and tensor-ICA of resting state data identified a cross-modal network that includes low-level auditory and low-level visual cortices. Our analyses further indicate (1) that functional connectivity of low-level auditory and visual cortex is influenced by perceptual conditions and (2) that auditory cortex exhibits different patterns of coupled activity with anterior and posterior calcarine cortex. Our findings are consistent with anatomical evidence from nonhuman primate tracing studies for a cross-modal system linking low-level auditory cortex with the anterior portion of striate cortex.
One strength of our study was the use of anatomically defined ROIs as seeds in the fcMRI analyses. These ROIs were demarcated on normalized structural images that were coregistered with the normalized functional images. Supplementary Figure 1 shows that these anatomical ROI exhibited a high degree of overlap with the cytoarchitectonic probability maps for these areas [Amunts et al., 2000; Morosan et al., 2005], but had the additional advantage of constraining the analyses to each individual's unique anatomy. The careful delineation of auditory cortex anatomy yielded functional pathways that have not been reported in prior intrinsic functional connectivity studies [Beckmann and Smith, 2005; Damoiseaux et al., 2006; Salvador et al., 2005; van de Ven et al., 2004]. This precision may have allowed us to identify, for the first time, functional connectivity between putative primary auditory cortex and its thalamic gateway, the MGN. The tensor ICA results confirmed correlated activity between auditory and visual cortex. The tensor ICA approach did not, however, capture the thalamic associations seen with the ROI analyses. This may reflect the lack of unique independent components in the MGN.
The anterior calcarine cortex is a termination site for primary auditory cortex fibers in nonhuman primate fiber tracing studies [Clavagnier et al., 2004; Falchier et al., 2002; Rockland and Ojima, 2003; Rockland and Van Hoesen, 1994]. As shown in Figures 1 and 2 and Supplementary Figure 2, medial Heschl's gyrus exhibits significant correlated activity with anterior calcarine cortex. Our functional connectivity results support the premise that a similar auditory–visual pathway exists in humans. Further support for this premise comes from observations of (1) direct fiber connections between auditory and visual cortex noted earlier; (2) reduced functional connectivity of homologous cortical areas in individuals with callosal agenesis [Achard et al., 2006; Quigley et al., 2003]; (3) disrupted auditory localization when visual cortex receives transcranial magnetic stimulation [Lewald et al., 2004]; (4) auditory cortex activity during silent lip reading [Calvert et al., 1997]; and (5) auditory–visual interactions that have been observed in low-level visual cortex within ∼70 ms of stimulus presentation [Giard and Peronnet, 1999].
Our results point to distinct patterns of cross-modal organization within primary visual cortex. Comparison of functional connectivity during the visual perception and resting state tasks revealed that correlated activity between auditory and anterior calcarine cortices was not significantly affected by the checker board stimuli. In contrast, correlated activity between the auditory and posterior calcarine cortex was significantly reduced. Furthermore, during the visual perception task, these regions were inversely correlated, whereas auditory cortex was positively correlated with anterior calcarine cortex. One explanation for these findings is that cortex representing peripheral visual space (at least 30° from the midline) may not have been engaged by our checkerboard stimuli (visual angle of 18.9°), and for this reason did not exhibit a significant decrease in correlated activity with auditory cortex during the visual task. An eyes-open condition or a condition that directed attention to peripheral space may have significantly reduced the strength of correlated activity between auditory and anterior calcarine cortex. Although additional experiments are necessary to test the hypothesis that anterior and posterior calcarine cortex have distinct functional relationships with low-level auditory cortex, the distinct patterns of correlated resting state activity between anterior and posterior calcarine cortex supports the premise that cortex in the medial Heschl's gyrus and anterior calcarine cortex constitute a cross-modal system for stimulus processing; whether this is accomplished through direct or indirect connections remains an open question.
Our data provide novel evidence that the degree of functional connectivity between putative primary sensory regions can be selectively altered during perception, and point to functional hetereogeneity within the putative primary visual cortex with respect to cross-modal integration. The behavioral significance of this differential pattern of coupled network activity involving the medial Heschl's gyrus and anterior calcarine cortex remains to be investigated. Further research is needed to carefully delineate interactions between the primary and auditory cortices in relation to stimuli presented in the peripheral and foveal visual fields. The differential patterns of correlated activity between auditory cortex with anterior calcarine versus posterior calcarine regions, as well as the animal tracing studies demonstrating connections between auditory cortex and anterior calcarine cortex, suggests some intriguing hypotheses. In particular, we hypothesize that the cross-modal network observed in our study reflects a system that facilitates the identification of stimuli in peripheral space. Based on our findings, we predict that auditory stimuli presented to peripheral space will elicit correlated activity in the anterior calcarine cortex.
At present it is not clear whether the observed functional connectivity between low-level auditory and visual cortices reflect direct connections between these regions. It is possible that interactions between these sensory regions reflect top–down modulation from associations areas such as V5/MT [Beauchamp, 2005] or higher order cortical regions such as the ACC [Crottaz-Herbette and Menon, 2006]. Multisensory and/or cross-modal neurons are most frequently observed in association cortex. One explanation for correlated activity between low-level sensory systems is that bidirectional [Peltier et al., 2007] or feedback connections from association cortex mediate this interaction [Calvert et al., 2000; Macaluso et al., 2000, 2005; Schlack et al., 2005]. One candidate association cortex region is V5/MT+, which appears to provide feedback information between auditory and visual cortex. For example, Calvert et al. [1999] demonstrated increased activity in low-level auditory cortex and V5/MT+ when people listened to speech and viewed the speaker's face compared to when people heard only the speech or viewed only the speaker's face. In our study, V5/MT+ was the only association cortex region that was part of the low-level multisensory fcMRI network. We hypothesize that V5/MT+ is capable of modulating signaling between low-level auditory and visual cortices. Using rTMS, a V5/MT+ to V1 feedback pathway has been observed in the primate that is important for visual awareness [Pascual-Leone and Walsh, 2001]. Whether this feedback pathway enhances the signaling of the low-level auditory and visual regions when vision is limited or degraded remains to be examined [Weeks et al., 2000]. Our findings, nevertheless, suggest that rTMS studies of area V5/MT+ when combined with fcMRI studies of resting state data can help to shed light on this important question.
Acknowledgements
The authors thank Gary Glover for helpful discussions related to this project.




